Fabrication and Actuation of Cu-Ionic Polymer Metal Composite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication
2.3. Measurements
3. Results and Discussion
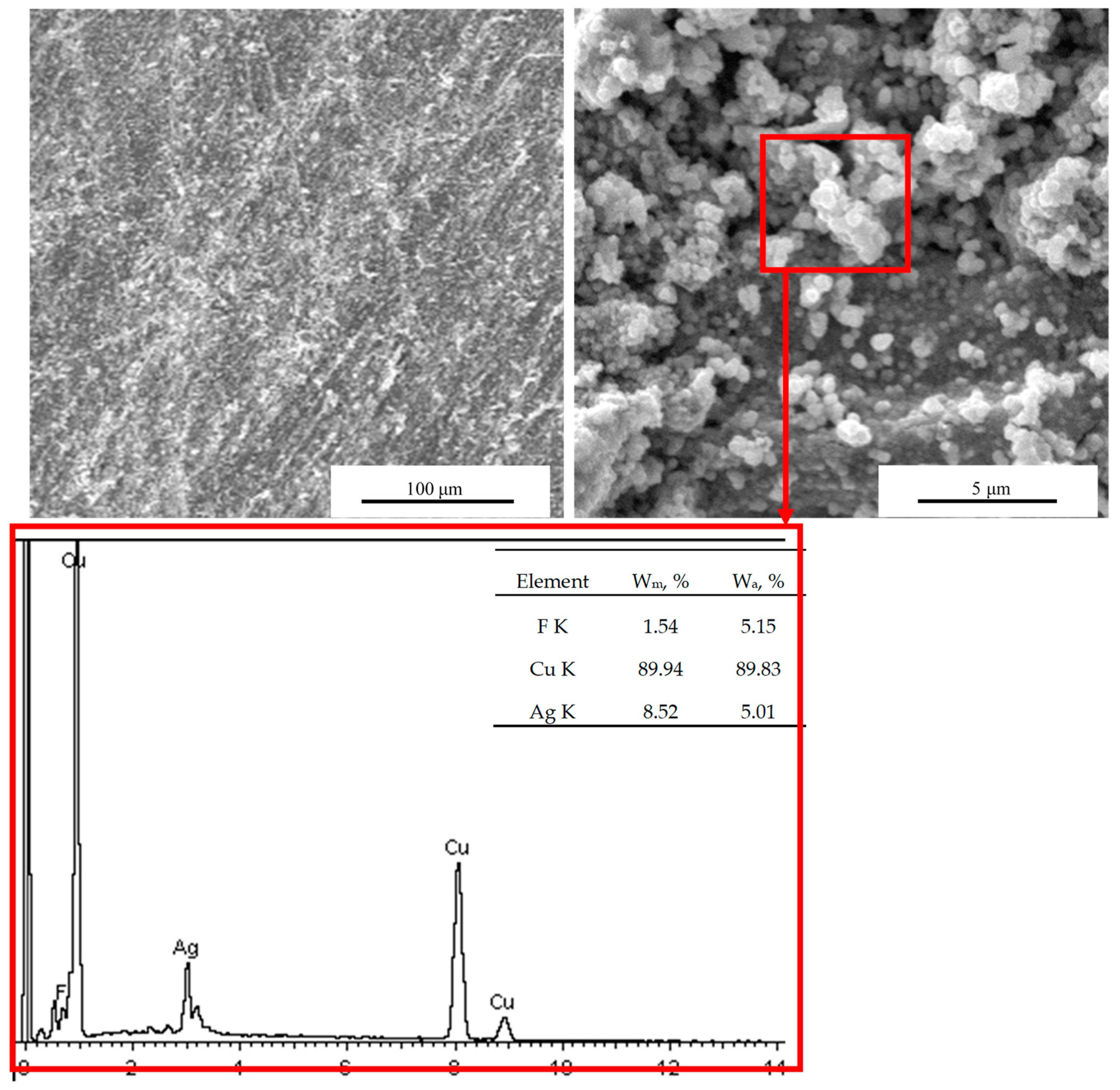
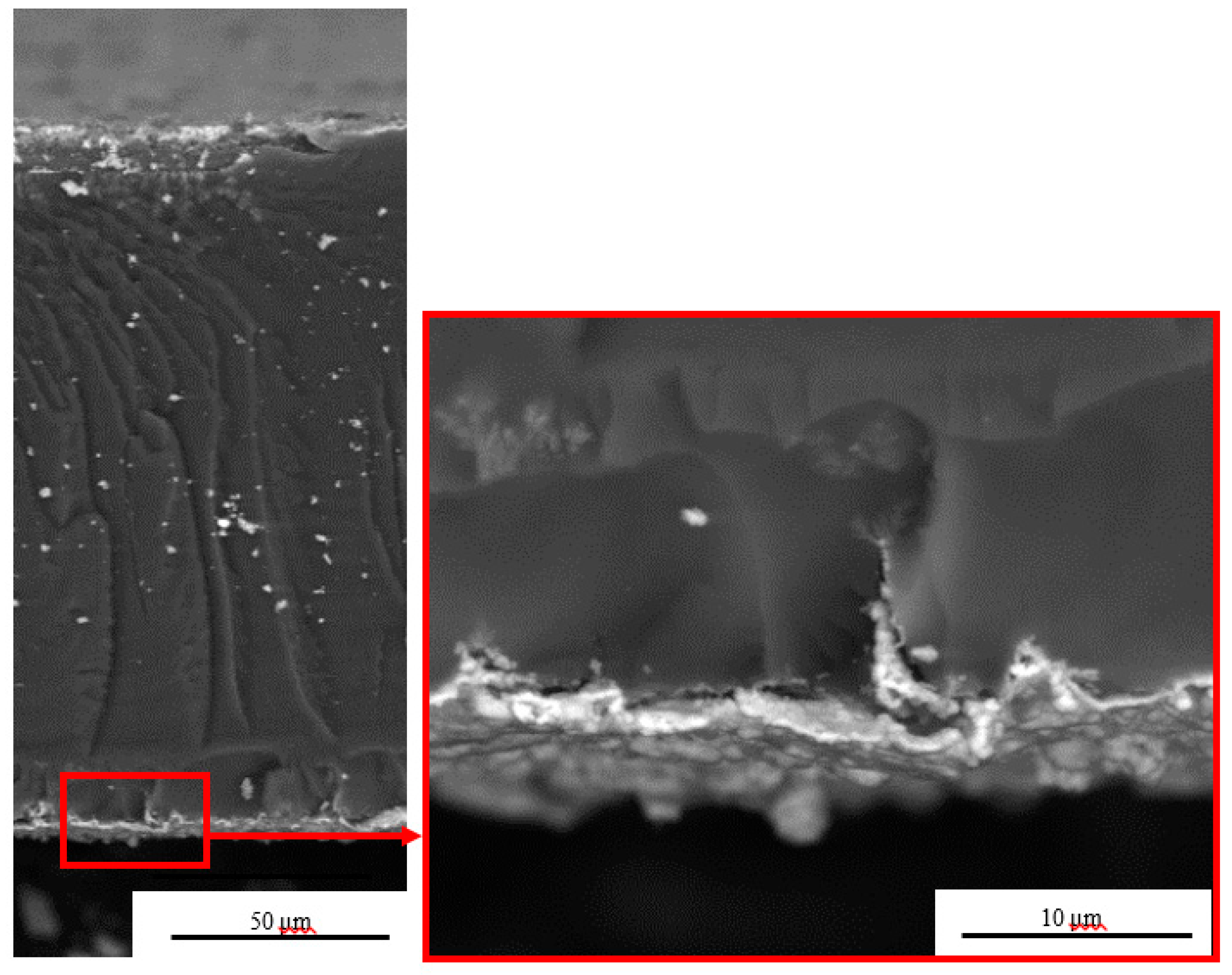
3.1. Morphology
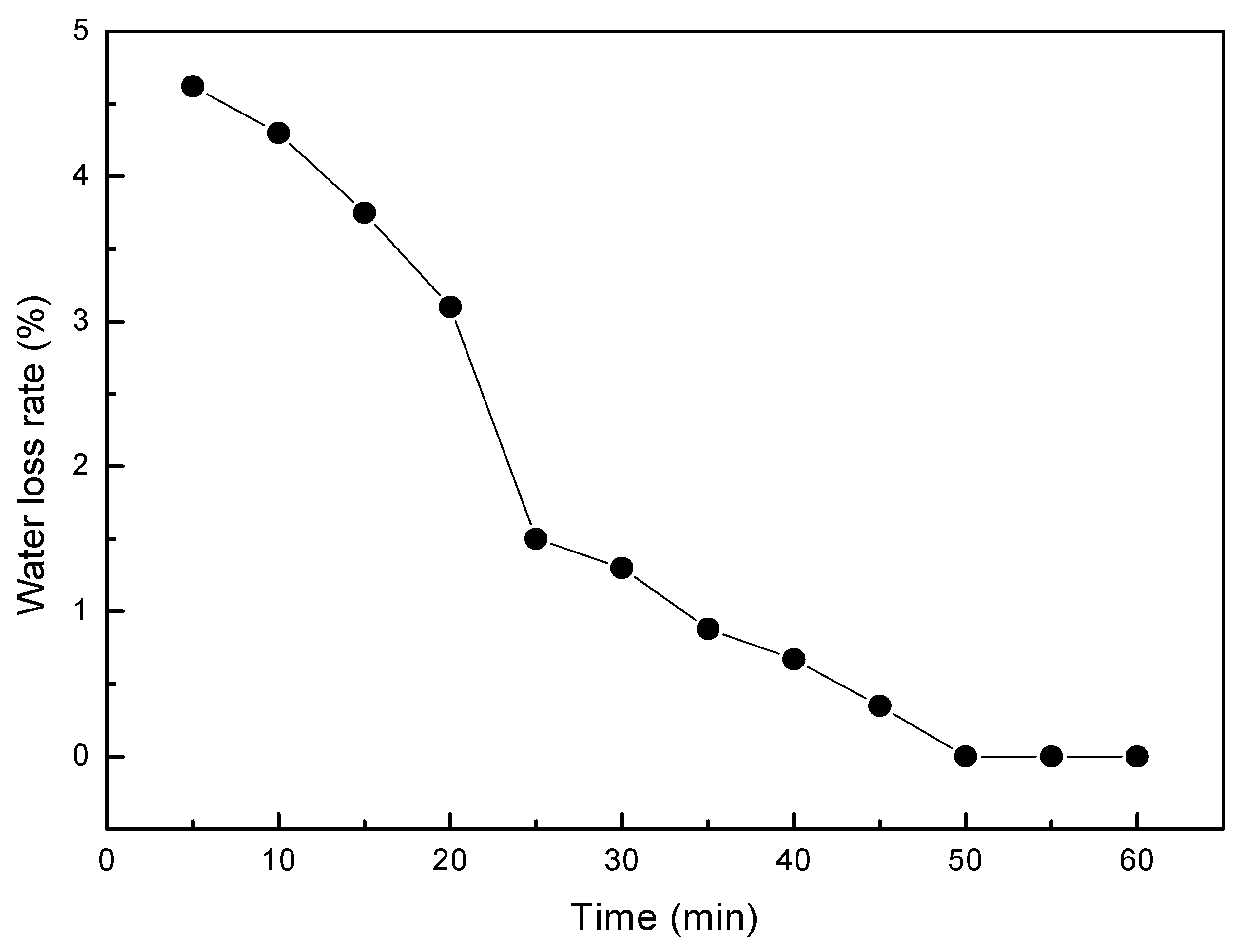
3.2. Water Loss Test
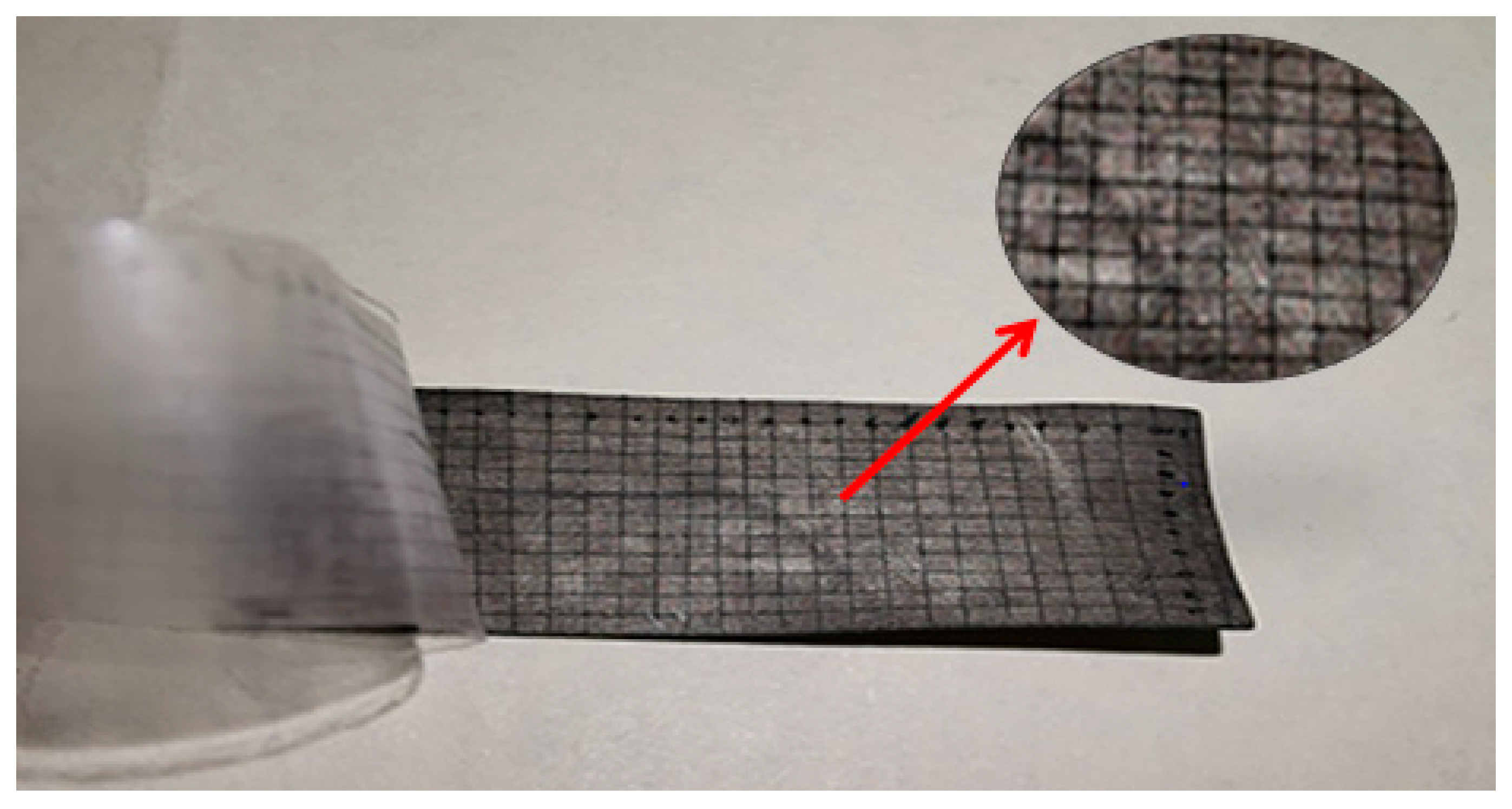
3.3. Adhesive Force
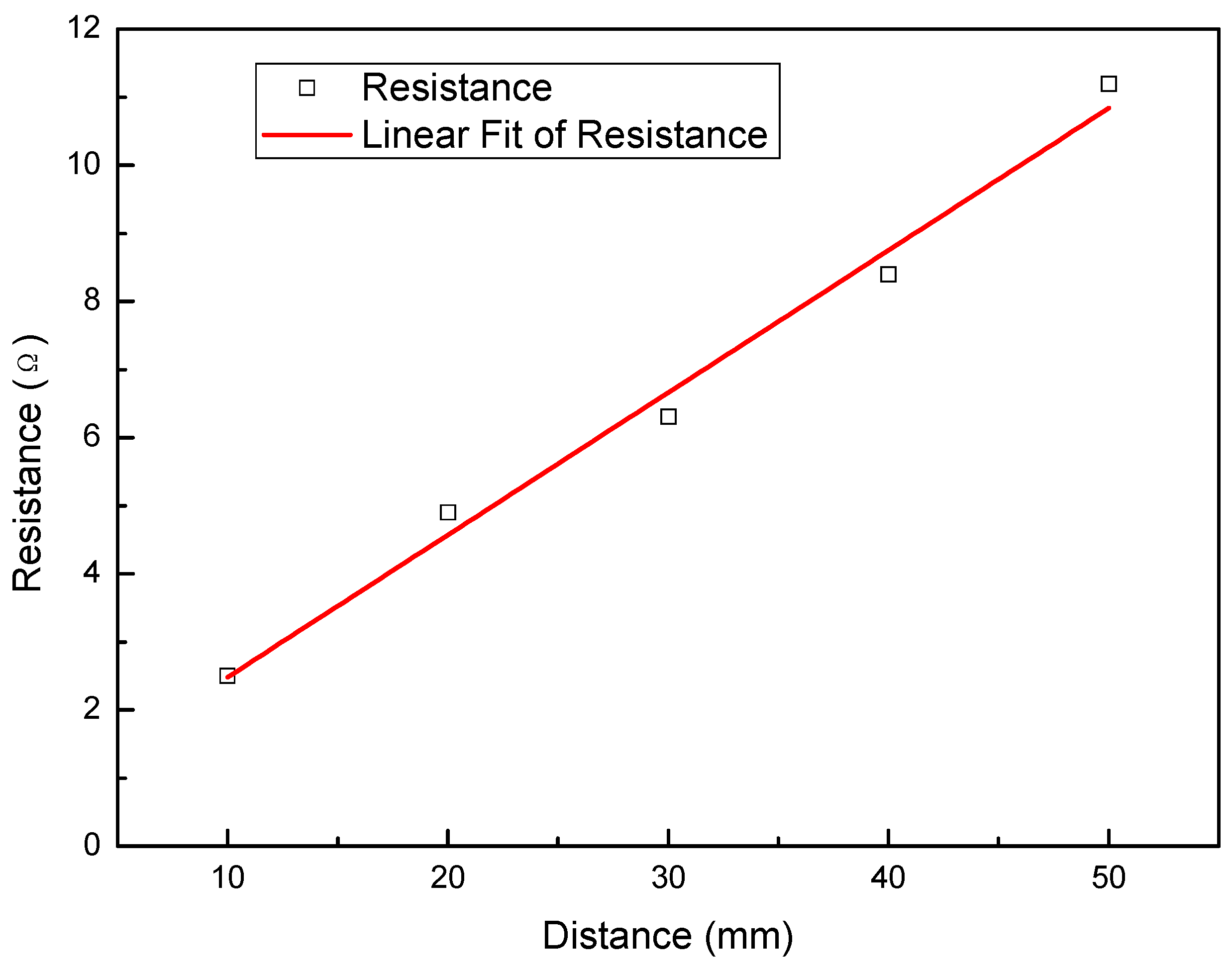
3.4. Surface Resistance
3.5. Effect of IPMC Length on Actuation
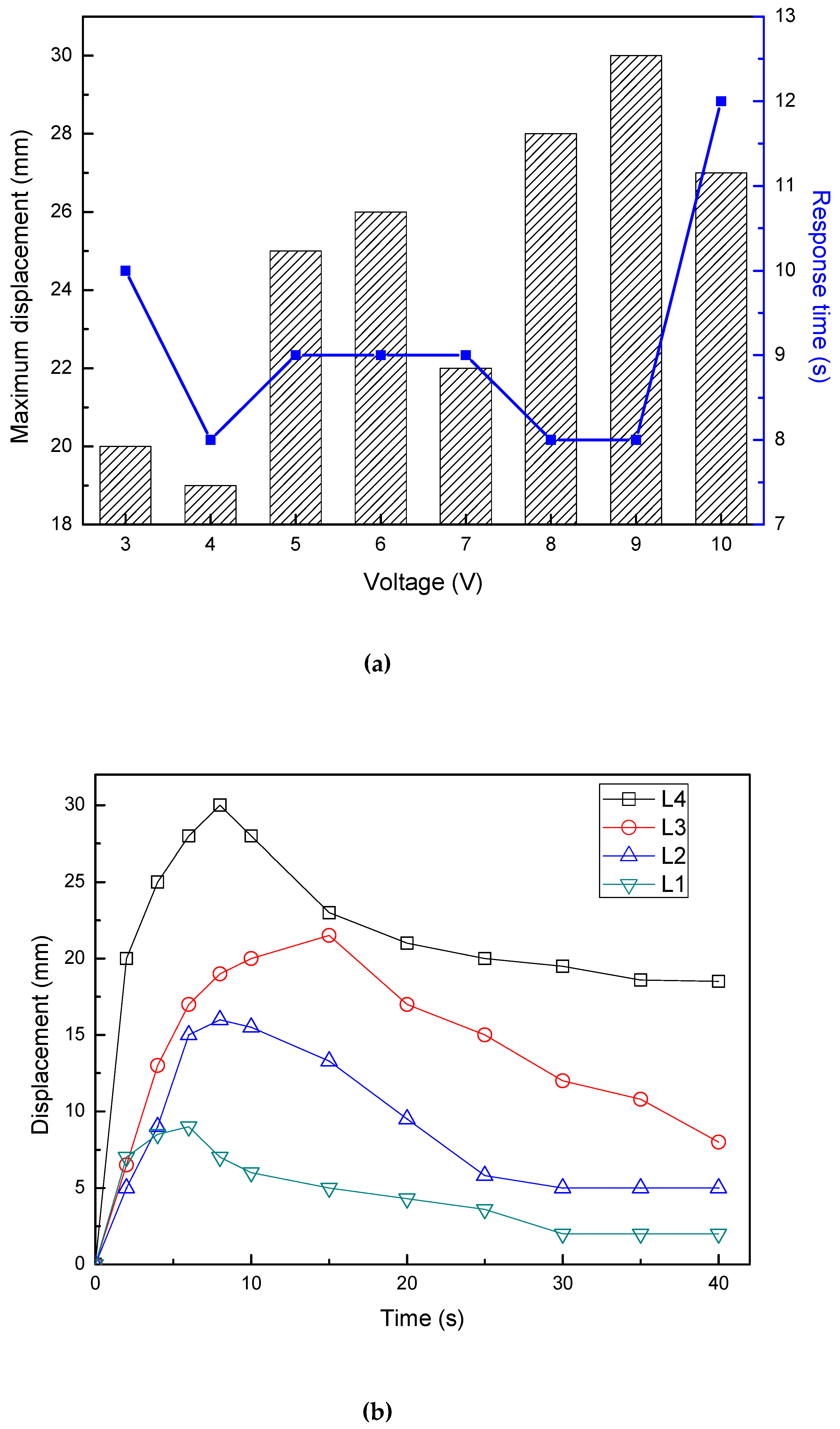
3.5.1. Effect of IPMC Length on Displacement
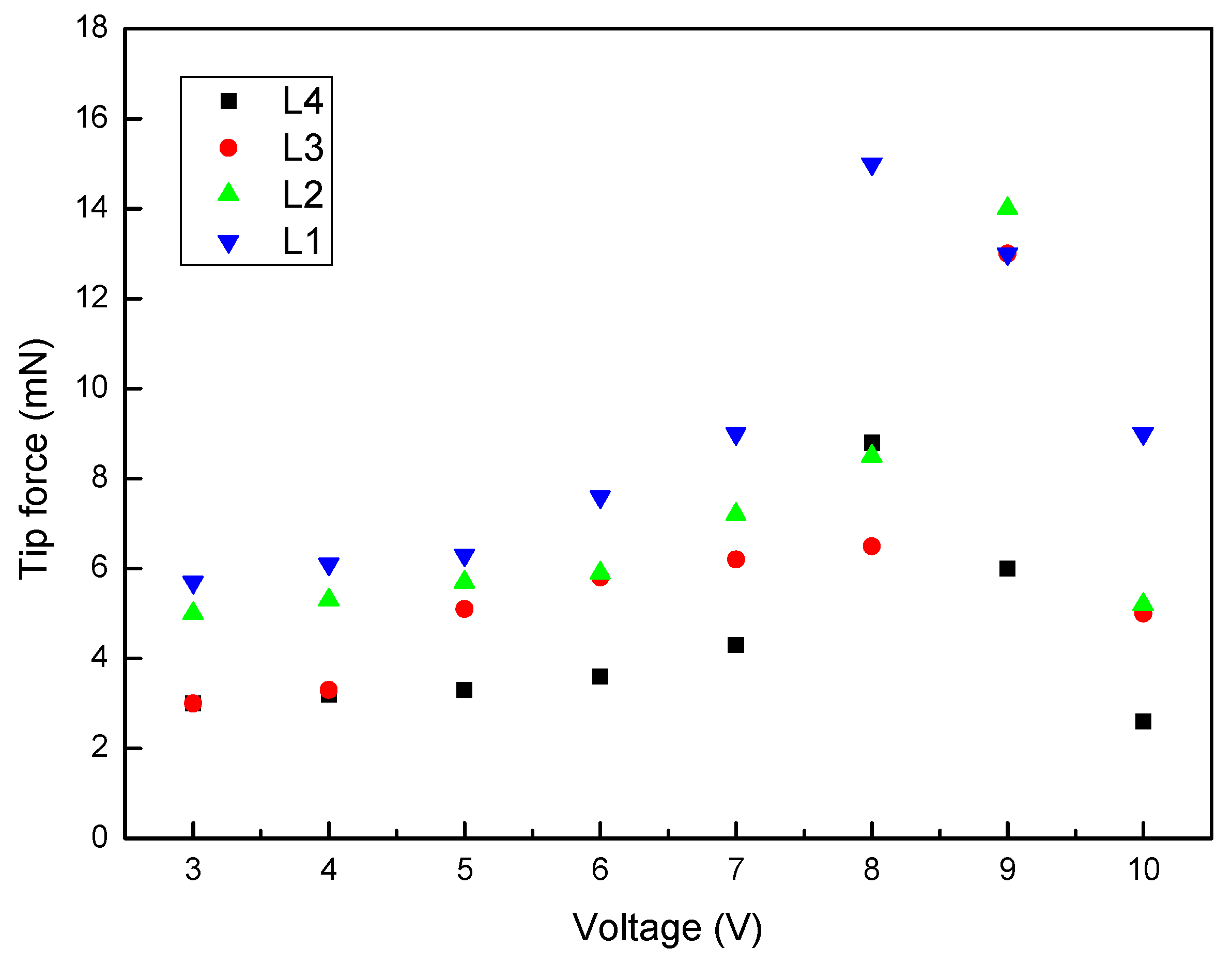
3.5.2. Effect of IPMC Length on Tip Force
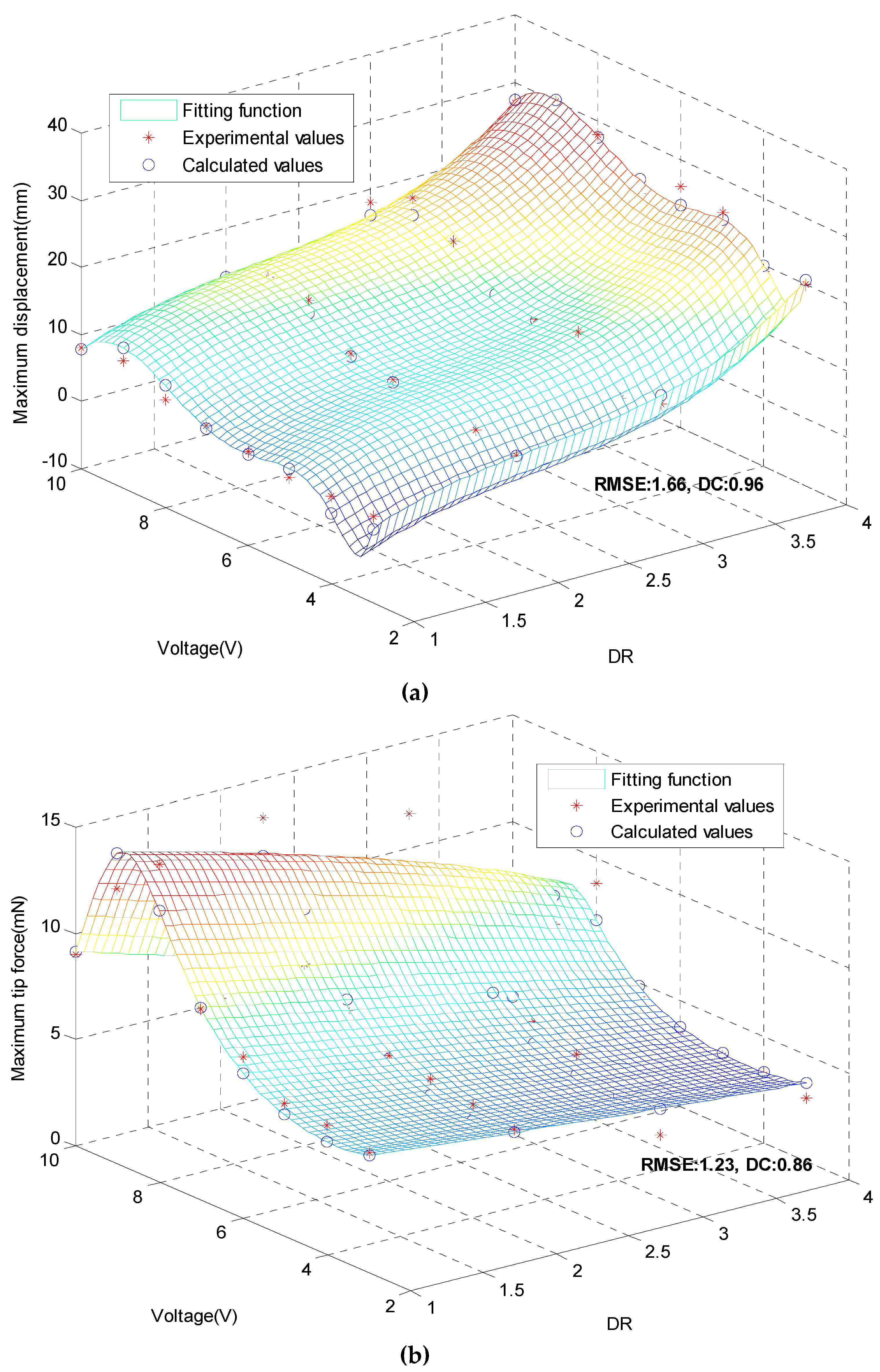
3.6. Displacements Model and Tip Forces Model.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhandari, B.; Lee, G.Y.; Ahn, S.H. A review on IPMC material as actuators and sensors: Fabrications, characteristics and applications. Int. J. Precis. Eng. Manuf. 2012, 13, 141–163. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, Y.J.; Zhu, Z.C.; Wang, J.H.; He, Q.S.; Luo, M.Z. The Effects of Dimensions on the Deformation Sensing Performance of Ionic Polymer-Metal Composites. Sensors 2019, 9, 2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, C.; Pugal, D.; Oh, I.K.; Kim, K.J.; Asaka, K. Recent advances in ionic polymer–metal composite actuators and their modeling and applications. Prog. Polym. Sci. 2013, 38, 1037–1066. [Google Scholar] [CrossRef]
- Shen, Q.; Trabia, S.; Stalbaum, T.; Palmre, V.; Kim, K.; Oh, I.l.-K. A multiple-shape memory polymer-metal composite actuator capable of programmable control, creating complex 3D motion of bending, twisting, and oscillation. Sci. Rep. 2016, 6, 24462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, C.; Ru, J.; Zhu, Z.; Luo, B.; Chen, H. A three-electrode structured ionic polymer carbon-composite actuator with improved electromechanical performance. Smart Mater. Struct. 2018, 27, 085017. [Google Scholar] [CrossRef]
- Chang, L.; Yu, L.; Li, C.; Niu, Q.; Hu, Y.; Lu, P.; Zhu, Z.; Wu, Y. Ionic polymer with single-layered electrodes: A novel strategy for ionic actuator design. Smart Mater. Struct. 2018, 27, 105046. [Google Scholar] [CrossRef]
- Wang, J.; McDaid, A.; Sharma, R.; Aw, K. A compact ionic polymer metal composite (IPMC) system with inductive sensor for closed loop feedback. Actuators 2015, 4, 114–126. [Google Scholar] [CrossRef]
- Cheong, H.R.; Nguyen, N.T.; Khaw, M.K.; Teoh, B.Y.; Chee, P.S. Wirelessly activated device with an integrated ionic polymer metal composite (IPMC) cantilever valve for targeted drug delivery. Lab Chip 2018, 18, 3207–3215. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Guo, S. Development and evaluation of flytapinspired micorobot. Microsyst. Technol. 2015, 22, 1949–1958. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Y.; Hu, X.; Sun, X.; Chang, L.; Lu, P. An easily fabricated high performance ionic polymer based sensor network. Appl. Phys. Lett. 2016, 109, 073504. [Google Scholar] [CrossRef]
- Feng, G.H.; Liu, K.M. Fabrication and characterization of a micromachined swirl-shaped ionic polymer metal composite actuator with electrodes exhibiting asymmetric resistance. Sensors 2014, 14, 8380–8397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeli, E.; Ganjian, M.; Rastegar, H.; Kolahdouz, M.; Kolahdouz, Z.; Zhang, G.Q. Humidity sensor based on the ionic polymer metal composite. Sensor Actuators B Chem. 2017, 247, 498–504. [Google Scholar] [CrossRef]
- Wu, G.; Hu, Y.; Liu, Y.; Zhao, J.; Chen, X.; Whoehling, V.; Plesse, C.; Nguyen, G.T.M.; Vidal, F.; Chen, W. Graphitic carbon nitride nanosheet electrode-based high-performance ionic actuator. Nat. Commun. 2015, 6, 7258. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.F.; Yang, Q.; Niu, Q.Z.; Wang, Y.J.; Liu, Y.F.; Lu, P.; He, Q.S.; Wu, Y.C.; Hu, Y. High-performance ionic polymer–metal composite actuators fabricated with microneedle roughening. Smart Mater. Struct. 2019, 28, 015007. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Chu, H.D.; Lim, S.H.; Yim, T.E.; Kim, Y.J.; Kim, T.H. Preparation and characterization of imidazolium-PEO-based Ionene/PVDF(HFP)/LiTFSI as a novel Gel polymer electrolyte. Macromol. Res. 2015, 23, 38–44. [Google Scholar] [CrossRef]
- Song, D.S.; Han, D.G.; Rhee, K.; Kim, D.M.; Jho, J.Y. Fabrication and Characterization of an Ionic Polymer-Metal Composite Bending Sensor. Macromol. Res. 2017, 25, 1205–1211. [Google Scholar] [CrossRef]
- Li, S.F.; Yip, J. Characterization and Actuation of Ionic Polymer Metal Composites with Various Thicknesses and Lengths. Polymer 2019, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.Q.; Qian, W.Z.; Estevez, L.; Liu, H.L.; Liu, Y.X.; Jiang, T.; Wang, K.S.; Guo, W.L.; Giannelis, E.P. Enhanced actuation in functionalized carbon nanotube–Nafion composites. Sensor Actuators B Chem. 2011, 156, 187–193. [Google Scholar] [CrossRef]
- Oh, C.; Kim, S.; Kim, H.; Park, G.; Kim, J.; Ryu, J. Panpan Li, a Sunghwan Lee, b Kwangsoo Noa and Seungbum HongEffects of membrane thickness on the performance of ionic polymer–metal composite actuators. RSC Adv. 2019, 9, 14621. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, H.; Wang, Y.; Zhu, Z.; Li, D. Effect of Dehydration on the Mechanical and Physicochemical Properties of Gold- and Palladium -Ionomeric Polymer-Metal Composite (IPMC) Actuators. Electrochim. Acta 2014, 129, 450–458. [Google Scholar] [CrossRef]
- Palmre, V.; Kim, S.J.; Pugal, D.; Kim, K. Improving electromechanical output of IPMC by high surface area Pd-Pt electrodes and tailored ionomer membrane thickness. Int. J. Smart Nano Mater. 2014, 5, 99–113. [Google Scholar] [CrossRef]
- Zu, L.; Li, Y.; Lian, H.; Hu, Y.; Chang, W.; Liu, B.; Liu, Y.; Ao, X.; Li, Q.; Cui, X. The Enhancement Effect of Mesoporous Graphene on Actuation of Nafion—Based IPMC. Macromol. Mater. Eng. 2016, 301, 1076–1083. [Google Scholar] [CrossRef]
- Ru, J.; Wang, Y.; Chang, L.; Chen, H.; Li, D. Preparation and characterization of water-soluble carbon nanotube reinforced Nafion membranes and so-based ionic polymer metal composite actuators. Smart Mater. Struct. 2016, 25, 095006. [Google Scholar] [CrossRef]
- Uchida, M.; Taya, M. Solid polymer electrolyte actuator using electrode reaction. Polymer 2001, 42, 9281–9285. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. The effect of surface-electrode resistance on the performance of ionic polymer-metal composite (IPMC) artificial muscles. Smart Mater. Struct. 2000, 9, 543–551. [Google Scholar] [CrossRef]
- Johanson, U.; Maeorg, U.; Sammelselg, V.; Brandell, D.; Punning, A.; Kruusmaa, M.; Aabloo, A. Electrode reactions in Cu-Pt coated ionic polymer actuators. Sensor Actuators B Chem. 2008, 131, 340–346. [Google Scholar] [CrossRef]
- Takagi, K.; Tomita, N.; Asaka, K. A simple method for obtaining large deformation of IPMC actuators utilizing copper tape. Adv. Robot. 2014, 28, 513–521. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Zhang, X.; Tian, A. Prediction of Actuation property of Cu Ionic Polymer-Metal Composites Based on Back Propagation Neural Network. ACS Omega. ACS Omega 2020, in press. [Google Scholar]
- Yang, L.; Zhang, D.; Zhang, X.; Tian, A.; Hui, X.; Yang, J. Fabrication of Cu/Nafion-Based Ionic Polymer Metal Composites by electroless plating method. Integr. Ferroelectr. 2020, in press. [Google Scholar]
| No. | Thickness (μm) | Width (mm) | Length (mm) | Clamped Part (mm) | Free Part (mm) |
|---|---|---|---|---|---|
| L1 | 187 | 10 | 20 | 10 | 10 |
| L2 | 187 | 10 | 30 | 10 | 20 |
| L3 | 187 | 10 | 40 | 10 | 30 |
| L4 | 187 | 10 | 50 | 10 | 40 |
| No. | DR | Voltage/V | Displacement | Tip Force | ||||
|---|---|---|---|---|---|---|---|---|
| Experimental Value/mm | Calculated Value/mm | Error Rate/% | Experimental Value/mN | Calculated Value/mN | Error Rate/% | |||
| 1 | 4 | 6.5 | 21.50 | 23.20 | 7.91 | 3.80 | 4.21 | 10.79 |
| 2 | 4 | 9.5 | 28.50 | 29.90 | 4.91 | 4.80 | 4.34 | 9.58 |
| 3 | 3 | 7.3 | 14.00 | 13.69 | 2.21 | 6.40 | 7.02 | 9.69 |
| 4 | 2 | 7.5 | 12.00 | 11.15 | 7.08 | 8.60 | 9.25 | 7.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, D.; Zhang, X.; Tian, A. Fabrication and Actuation of Cu-Ionic Polymer Metal Composite. Polymers 2020, 12, 460. https://doi.org/10.3390/polym12020460
Yang L, Zhang D, Zhang X, Tian A. Fabrication and Actuation of Cu-Ionic Polymer Metal Composite. Polymers. 2020; 12(2):460. https://doi.org/10.3390/polym12020460
Chicago/Turabian StyleYang, Liang, Dongsheng Zhang, Xining Zhang, and Aifen Tian. 2020. "Fabrication and Actuation of Cu-Ionic Polymer Metal Composite" Polymers 12, no. 2: 460. https://doi.org/10.3390/polym12020460






