Salt-Free Dyeing of Modified Cotton through Graft Polymerization with Highly Enhanced Dye Fixation and Good Strength Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
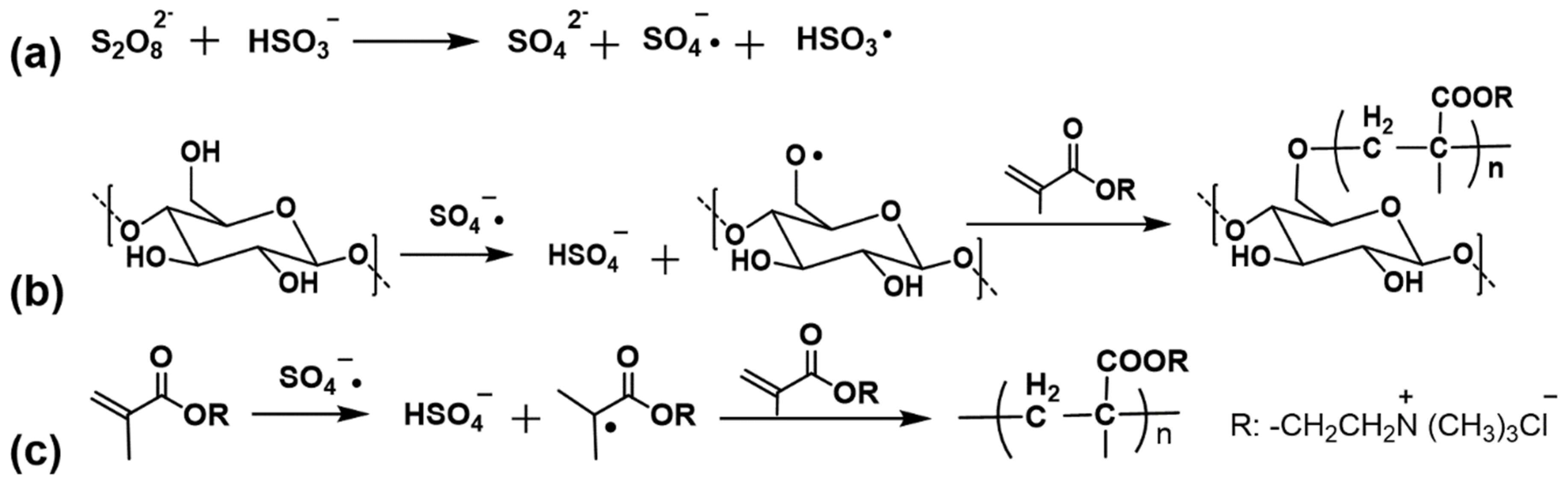
2.2. Graft Polymerization of DMC onto Cotton
2.3. Dyeing of Modified and Unmodified Cotton Fabrics
2.4. Degree of Substitution (DS) Measurement
2.5. Characterization
2.6. Color Measurement
2.7. Color Fastness
2.8. Strength Testing
3. Results and Discussion
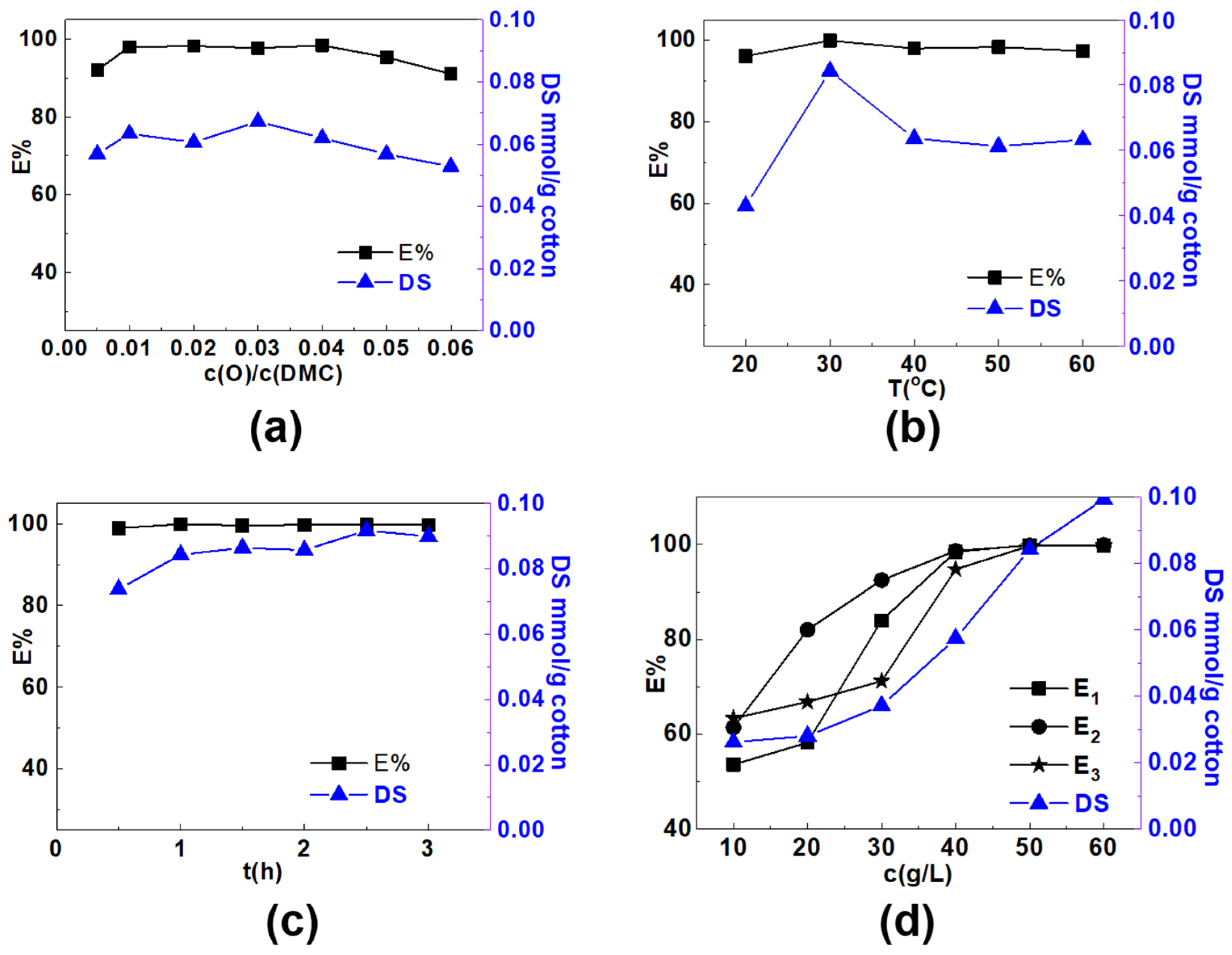
3.1. Optimization of Preparation Conditions of the Modified Cotton Fibers
3.2. Characterization of Cationic Fibers
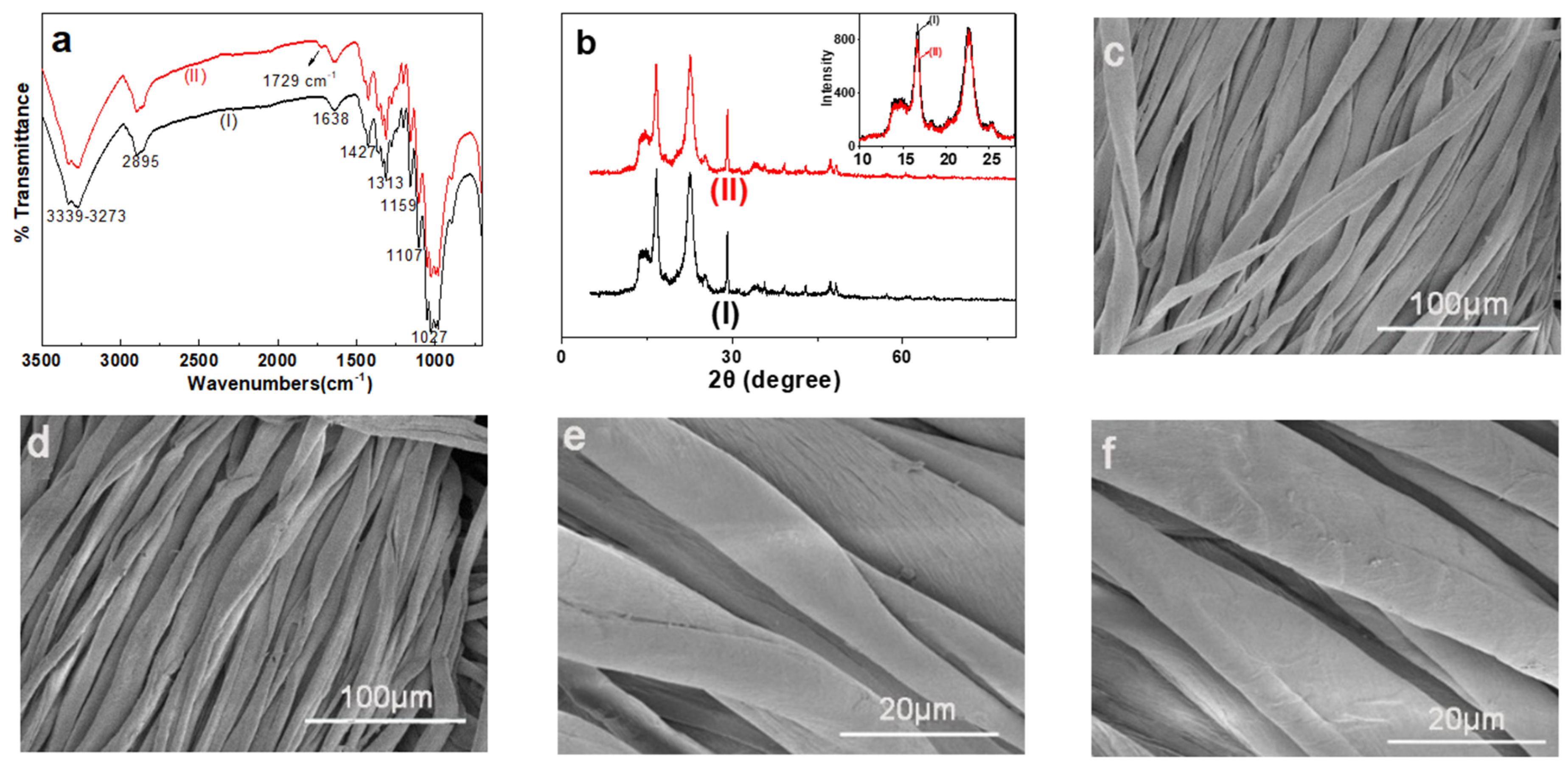
3.2.1. FTIR Analysis of the Modified Cotton
3.2.2. XRD Analysis
3.2.3. SEM Analysis
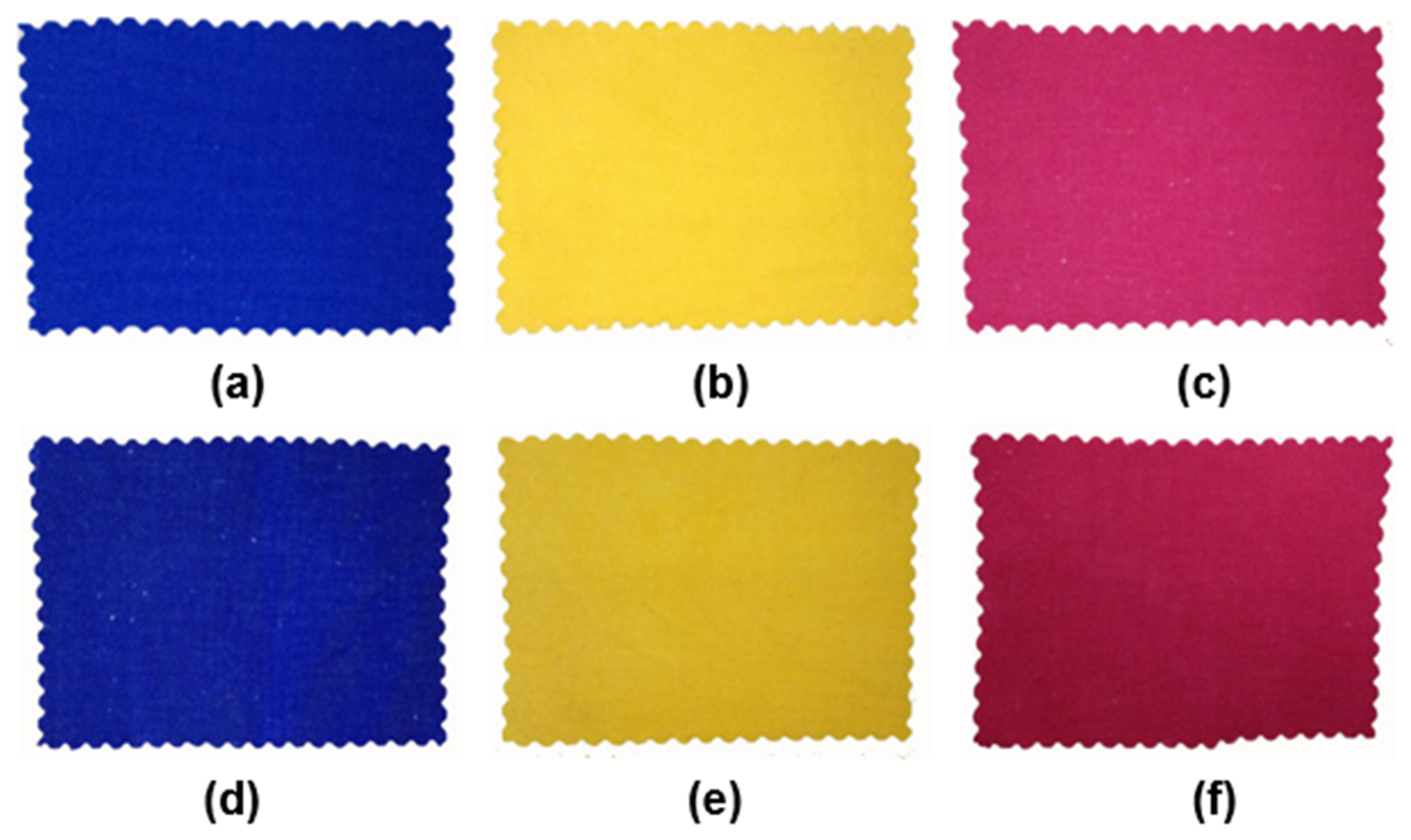
3.2.4. Colorimetric Properties
3.2.5. Application Performance
3.2.6. Strength Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.Q.; Liu, F.; Chen, X.S.; Ma, X.J.; Zeng, H.Q.; Yang, Z.M. Transcriptome profiling of early developing cotton fiber by deep-sequencing reveals significantly differential expression of genes in a fuzzless/lintless mutant. Genomics 2010, 96, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muresan, E.I.; Balan, G.; Popescu, V. Durable hydrophobic treatment of cotton fabrics with glycidyl stearate. Ind. Eng. Chem. Res. 2013, 52, 6270–6276. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.; Hossain, M.A.; Zakaria, M.; Islam, M.N.; Uddin, M.Z. Chitosan coated cotton fiber: Physical and antimicrobial properties for apparel use. J. Polym. Environ. 2016, 25, 334–342. [Google Scholar] [CrossRef]
- Samanta, A.K.; Sharmistha, C.; Guha, R.T. Dyeing of jute with reactive dyes: Optimisation of the process variables and assessment of colourfastness characteristics. J. Inst. Eng. (India) Ser. E 2012, 93, 15–24. [Google Scholar] [CrossRef]
- Rizk, H.F.; Ibrahim, S.A. Synthesis, fastness properties, color assessment and antimicrobial activity of some azo reactive dyes having pyrazole moiety. Dyes Pigment 2015, 112, 86–92. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Chung, T.S.; Weber, M.; Maletzko, C. Low-pressure nanofiltration hollow fiber membranes for effective fractionation of dyes and inorganic salts in textile wastewater. Environ. Sci. Technol. 2018, 52, 3676–3684. [Google Scholar] [CrossRef]
- Acharya, S.; Abidi, N.; Rajbhandari, R.; Meulewaeter, F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 2014, 21, 4693–4706. [Google Scholar] [CrossRef]
- Hamaky, Y.H.; Tawfeek, S.; Ibrahim, D.F.; Maamoun, D.; Gaber, S. Printing cotton fabrics with reactive dyes of high reactivity from an acidic printing paste. Color. Technol. 2010, 123, 365–373. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, G.; Song, X.Y. An antimicrobial cationic reactive dye: Synthesis and applications on cellulosic fibers. J. Appl. Polym. Sci. 2010, 108, 1917–1923. [Google Scholar] [CrossRef]
- Fu, S.; Hinks, D.; Hauser, P.; Ankeny, M. High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 2013, 20, 3101–3110. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhuang, L.H.; Sun, J.; Zheng, C.L. Salt-free dyeing of ramie fabric with an amino-terminated hyperbranched polymer. Cellulose 2014, 21, 3725–3736. [Google Scholar] [CrossRef]
- Siddiqua, U.H.; Ali, S.; Iqbal, M.; Hussain, T. Relationship between structures and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq. 2017, 241, 839–844. [Google Scholar] [CrossRef]
- Li, R.; Gu, F. Dyeing properties of PECH-amine cationized cotton with acid dyes. J. Appl. Polym. Sci. 2010, 100, 3302–3306. [Google Scholar]
- Boonroeng, S.; Srikulkit, K.; Xin, J.H.; Liang, H. Preparation of a novel cationic curcumin and its properties evaluation on cotton fabric. Fiber. Polym. 2015, 16, 2426–2431. [Google Scholar] [CrossRef]
- Ma, W.; Meng, M.; Yan, S.; Zhang, S. Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation. Chin. J. Chem. Eng. 2016, 24, 175–179. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Zhang, A.; Sun, R. Synergistic effects of graft polymerization and polymer blending on the flexibility of xylan-based films. Carbohydr. Polym. 2017, 181, 1128–1135. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release npk fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. A 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, C.; Zhang, A.; Ren, J. Synthesis of thermoplastic xylan-lactide copolymer with amidine-mediated organocatalyst in ionic liquid. Sci. Rep. 2017, 7, 551. [Google Scholar] [CrossRef] [Green Version]
- Sekine, A.; Seko, N.; Tamada, M.; Suzuki, Y. Biodegradable metal adsorbent synthesized by graft polymerization onto nonwoven cotton fabric. Radiat. Phys. Chem. 2010, 79, 16–21. [Google Scholar] [CrossRef]
- Ou, K.; Wu, X.; Wang, B.; Meng, C.; Dong, X.; He, J. Controlled in situ graft polymerization of dmaema onto cotton surface via si-arget atrp for low-adherent wound dressings. Cellulose 2017, 24, 5211–5224. [Google Scholar] [CrossRef]
- Wang, C.X.; Ren, Y.; Lv, J.C.; Zhou, Q.Q.; Ma, Z.P.; Qi, Z.M. In situ synthesis of silver nanoparticles on the cotton fabrics modified by plasma induced vapor phase graft polymerization of acrylic acid for durable multifunction. Appl. Surf. Sci. 2017, 396, 1840–1848. [Google Scholar] [CrossRef]
- Xia, D.; Bao, H.; Ou, K.; Yao, J.; Wei, Z.; He, J. Polymer-grafted modification of cotton fabrics by si-arget atrp. Fiber. Polym. 2015, 16, 1478–1486. [Google Scholar]
- Princia, E.; Pedemonte, E.; Gentile, G.; Cocca, M.; Martuscelli, E. Synthesis and mechanical characterisation of cellulose based textiles grafted with acrylic monomers. Eur. Polym. J. 2006, 42, 51–60. [Google Scholar] [CrossRef]
- Princi, E.; Vicini, S.; Pedemonte, E.; Arrighi, V.; Mcewen, I.J. New polymeric materials for paper and textiles conservation. II. Grafting polymerization of ethyl acrylate/methyl methacrylate copolymers onto linen and cotton. J. Appl. Polym. Sci. 2010, 103, 90–99. [Google Scholar] [CrossRef]
- Ma, W.; Wang, T.; Li, H.; Zhang, S. Cotton fabric modification through ceric (IV) ion-initiated graft copolymerisation of 2-methacryloyloxyethyltrimethyl ammonium chloride to enhance the fixation of reactive dyes. Cellulose 2015, 22, 4035–4047. [Google Scholar] [CrossRef]
- Xue, J.Q.; Wang, Y.J.; Du, Y.W.; Xue, Y.F.; Wen, D.D. Synthesis and characterization of modified chitosan by graft polymerization. Adv. Mater. Res. 2011, 239, 2843–2846. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2015, 59, 1729–1738. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mokhtar, S.M. Chemically induced graft copolymerization of itaconic acid onto cellulose fibers. Polym. Test 2002, 21, 337–343. [Google Scholar] [CrossRef]
- Suo, A.; Qian, J.; Yu, Y.; Zhang, W. Synthesis and properties of carboxymethyl cellulose-graft-poly(acrylic acid-co-acrylamide) as a novel cellulose-based superabsorbent. J. Appl. Polym. Sci. 2010, 103, 1382–1388. [Google Scholar] [CrossRef]
- Xiu, H.J.; Han, Q.; Wang, L.J.; Zhang, R. Effect of different initiator systems on graft copolymerization of cellulose fiber with caprolactam. Adv. Mater. Res. 2011, 391, 1306–1310. [Google Scholar] [CrossRef]
- Storsberg, J.; Ritter, H. Cyclodextrins in polymer synthesis: Free radical polymerization of cyclodextrin host-guest complexes of methyl methacrylate or styrene from homogenous aqueous solution. Macromol. Rapid Commun. 2015, 21, 236–241. [Google Scholar] [CrossRef]
- Mostafa, T.B.; Naguib, H.F.; Sabaa, M.W.; Mokhtar, S.M. Graft copolymerization of itaconic acid onto chitin and its properties. Polym. Int. 2010, 54, 221–225. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Antibacterial and acid and cationic dyeable bamboo cellulose (rayon) fabric on grafting. Carbohydr. Polym. 2012, 88, 1281–1287. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Graft copolymerization of acrylamide onto bamboo rayon and fiber dyeing with acid dyes. Iran. Polym. J. 2012, 21, 43–49. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.Y.; Spanswick, J. Synthesis of hydroxy-telechelic poly(methyl acrylate) and polystyrene by atom transfer radical coupling. Macromolecules 2004, 37, 106–111. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R. Chemically induced graft copolymerization of 4-vinyl pyridine onto carboxymethyl chitosan. Polym. Bull. 2011, 67, 693–707. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A.; Rana, R.K. Ascorbic acid/H2O2-initiated graft copolymerization of methyl methacrylate onto abelmoschus esculentus fiber: A kinetic approach. Int. J. Polym. Anal. Charact. 2013, 18, 1–14. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Khaliel, A.B.; Mohamed, E.H. Enhancing water resistance of paper by graft copolymerization reaction. J. Appl. Polym. Sci. 2013, 127, 4446–4455. [Google Scholar] [CrossRef]
| Cotton | K/S | L* | a* | b* | Whiteness | Yellowness |
|---|---|---|---|---|---|---|
| Original | 0.1 | 94.2 | −0.8 | 0.8 | 81.0 | 1.4 |
| Modified | 0.1 | 94.1 | −0.7 | 0.7 | 81.5 | 1.1 |
| Dyes | No. | E% | R% | F% | K/S | Wash Fastness | Rub Fastness | Light Fastness | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staining | Change | Dry | Wet | ||||||||
| Cotton | Wool | ||||||||||
| C. I. Reactive Blue 19 | 1 | 99.9 | 96.9 | 96.8 | 11.5 | 4–5 | 3 | 4 | 4–5 | 4 | 7 |
| 2 | 81.1 | 82.8 | 67.2 | 9.7 | 4–5 | 3 | 4 | 4–5 | 4 | 6–7 | |
| C. I. Reactive Yellow 145 | 1 | 99.8 | 98.9 | 98.7 | 4.2 | 4–5 | 4–5 | 4–5 | 4–5 | 4 | 5–6 |
| 2 | 96.7 | 78.4 | 75.8 | 3.7 | 4–5 | 4 | 4–5 | 4–5 | 4 | 4–5 | |
| C. I. Reactive Red 195 | 1 | 99.7 | 97.6 | 97.3 | 11.3 | 4–5 | 4 | 4–5 | 4 | 3 | 3–4 |
| 2 | 91.0 | 87.0 | 79.2 | 8.5 | 4–5 | 4 | 4 | 4 | 3–4 | 2–3 | |
| Cotton | Tensile Strength/N | Tearing Strength/N | ||
|---|---|---|---|---|
| Warp | Weft | Warp | Weft | |
| Original (N0) | 536.2 | 496.0 | 11.2 | 10.6 |
| Modified (N1) | 523.5 | 482.4 | 11.0 | 10.6 |
| Dec% | −2.4 | −2.7 | −1.8 | 0 |
| Cotton | Tensile Strength /N | Tearing Strength /N | |||
|---|---|---|---|---|---|
| Warp | Weft | Warp | Weft | ||
| Undyed and original | 536.2 | 496.0 | 11.2 | 10.6 | |
| Dyed with C. I. Reactive Blue 19 | 1 | 518.7 | 481.3 | 11.1 | 10.2 |
| 2 | 528.7 | 472.2 | 10.6 | 10.1 | |
| Dyed with C. I. Reactive Yellow 145 | 1 | 513.4 | 489.9 | 10.9 | 10.9 |
| 2 | 535.8 | 482.0 | 10.8 | 10.2 | |
| Dyed with C. I. Reactive Red 195 | 1 | 519.0 | 489.3 | 10.8 | 10.6 |
| 2 | 514.9 | 473.2 | 10.7 | 10.2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Du, S.; Yan, S.; Yu, X.; Zhang, Z.; Zhang, S. Salt-Free Dyeing of Modified Cotton through Graft Polymerization with Highly Enhanced Dye Fixation and Good Strength Properties. Polymers 2020, 12, 462. https://doi.org/10.3390/polym12020462
Ma W, Du S, Yan S, Yu X, Zhang Z, Zhang S. Salt-Free Dyeing of Modified Cotton through Graft Polymerization with Highly Enhanced Dye Fixation and Good Strength Properties. Polymers. 2020; 12(2):462. https://doi.org/10.3390/polym12020462
Chicago/Turabian StyleMa, Wei, Sen Du, Shumin Yan, Xiaolin Yu, Zhongjian Zhang, and Shufen Zhang. 2020. "Salt-Free Dyeing of Modified Cotton through Graft Polymerization with Highly Enhanced Dye Fixation and Good Strength Properties" Polymers 12, no. 2: 462. https://doi.org/10.3390/polym12020462





