Short- and Long-Term Epoxy Modification of Bitumen: Modification Kinetics, Rheological Properties, and Microstructure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Modified Bitumen Processing
2.3. Samples Testing
3. Results and Discussion
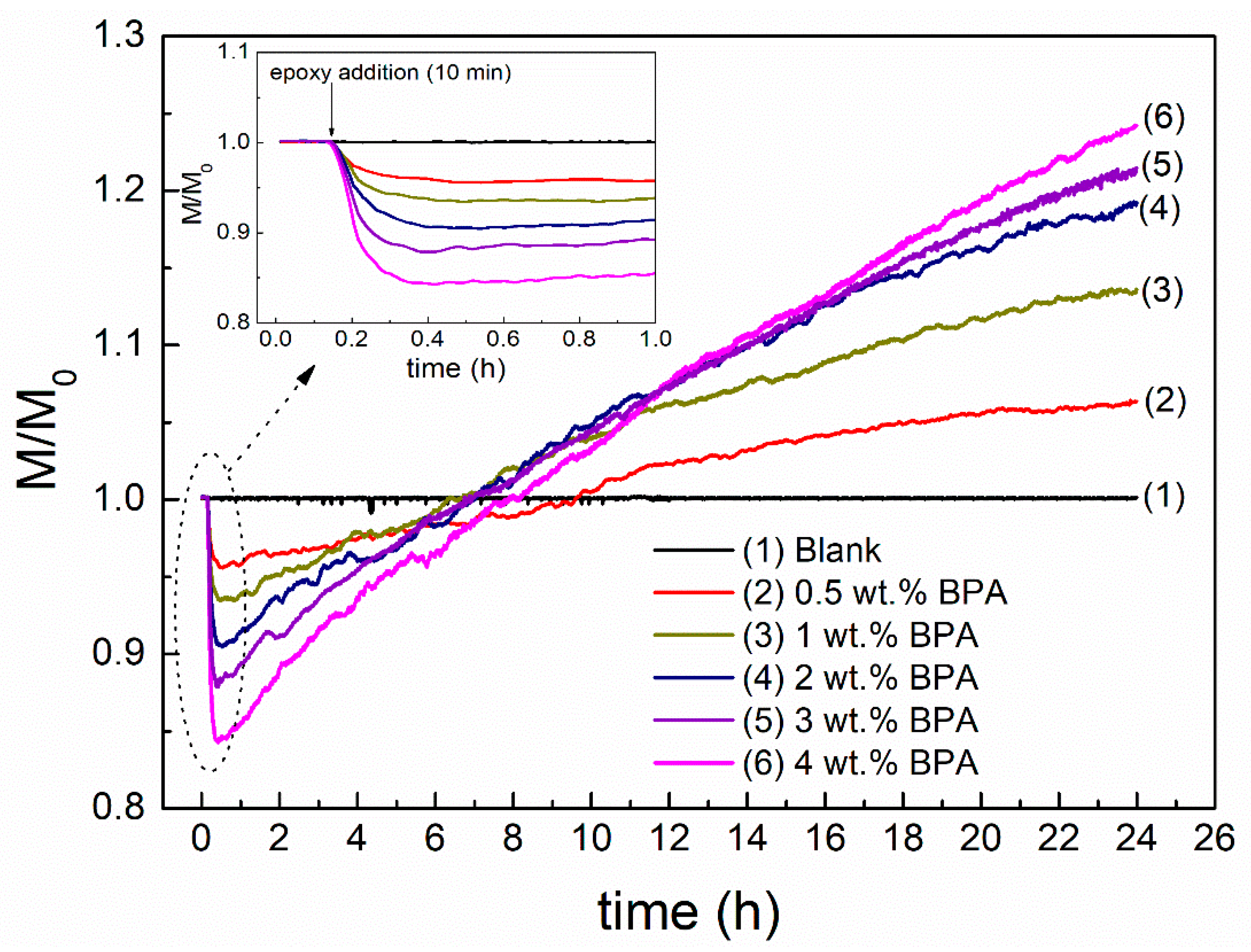
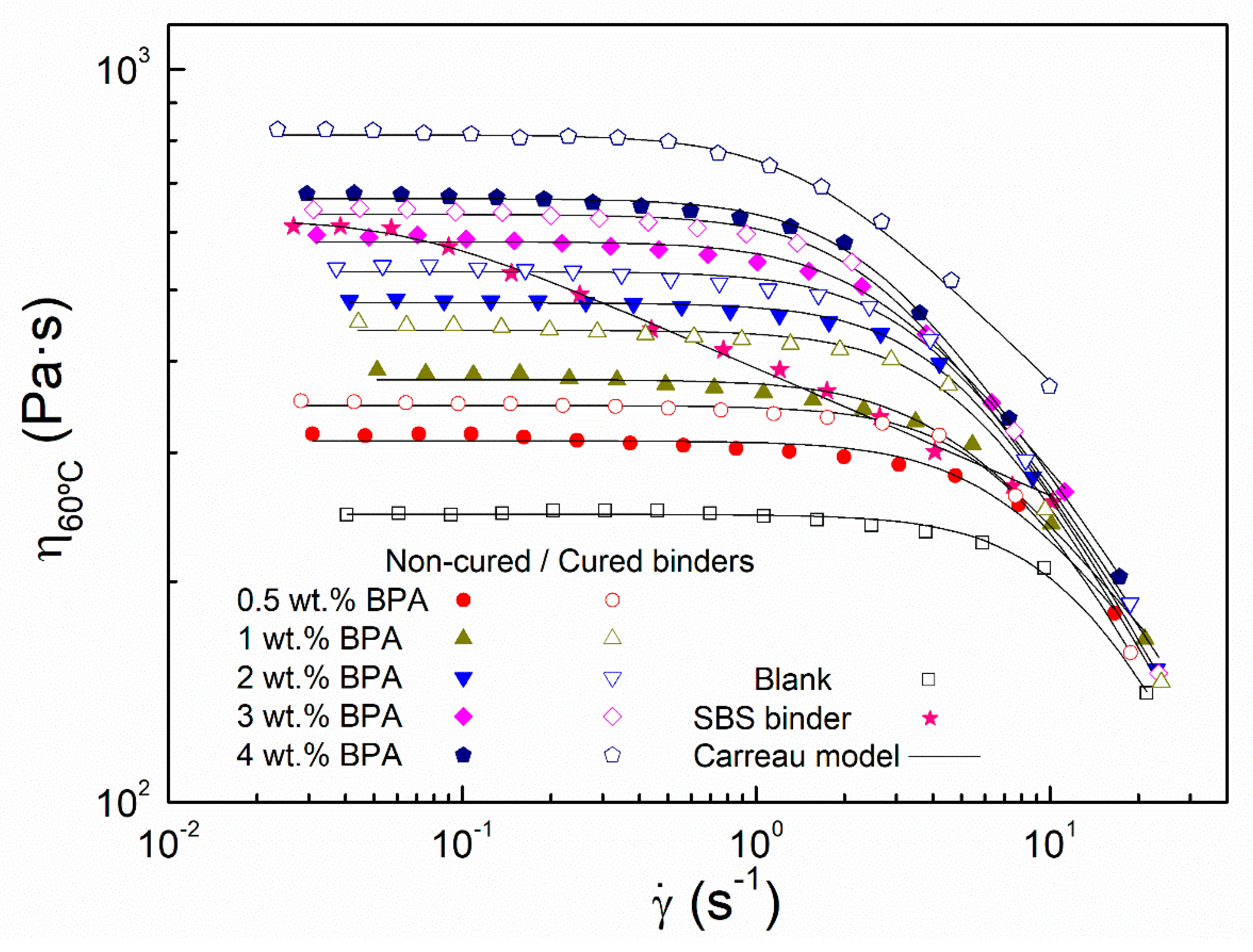
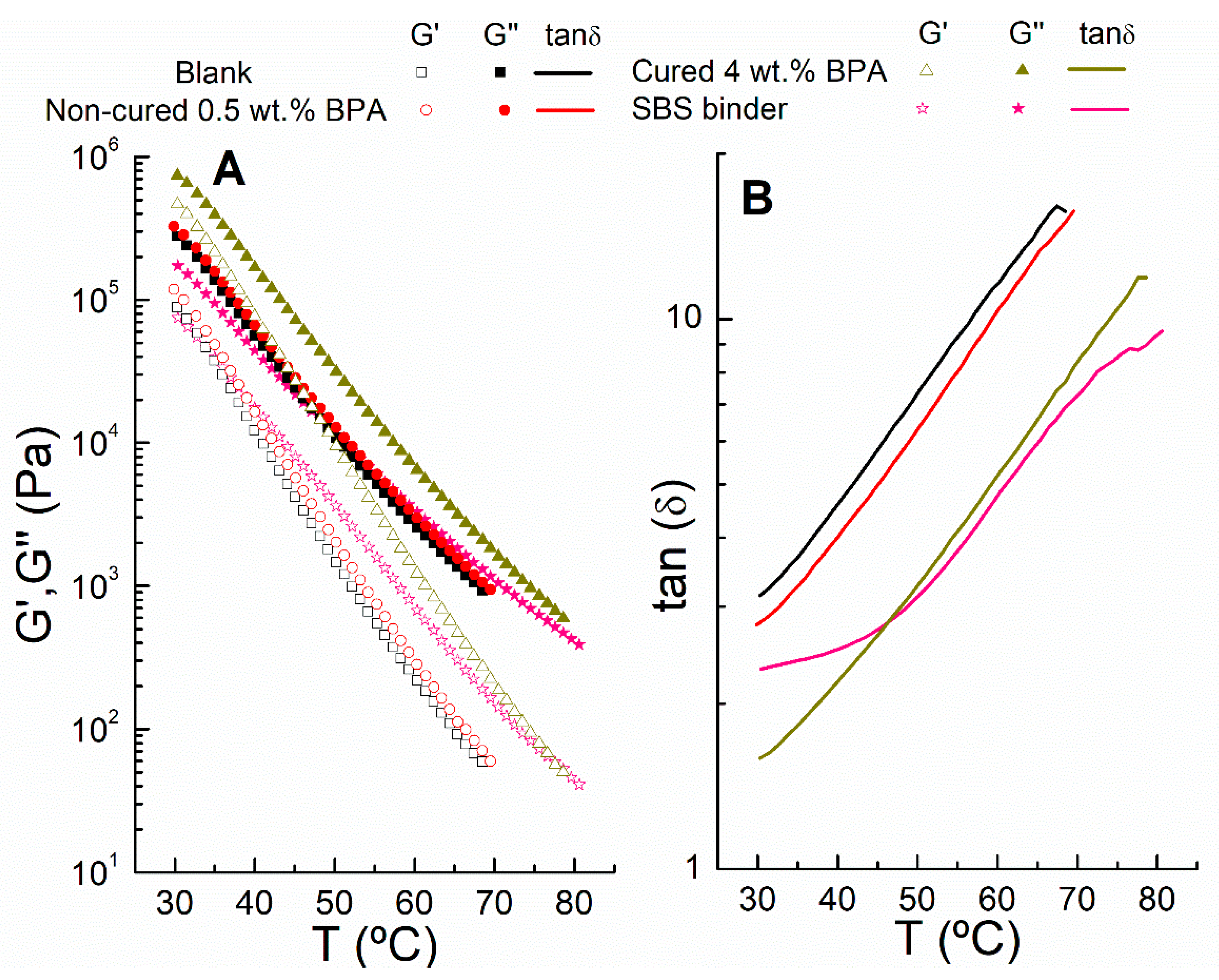
3.1. Short- and Long-Term Rheological Modification
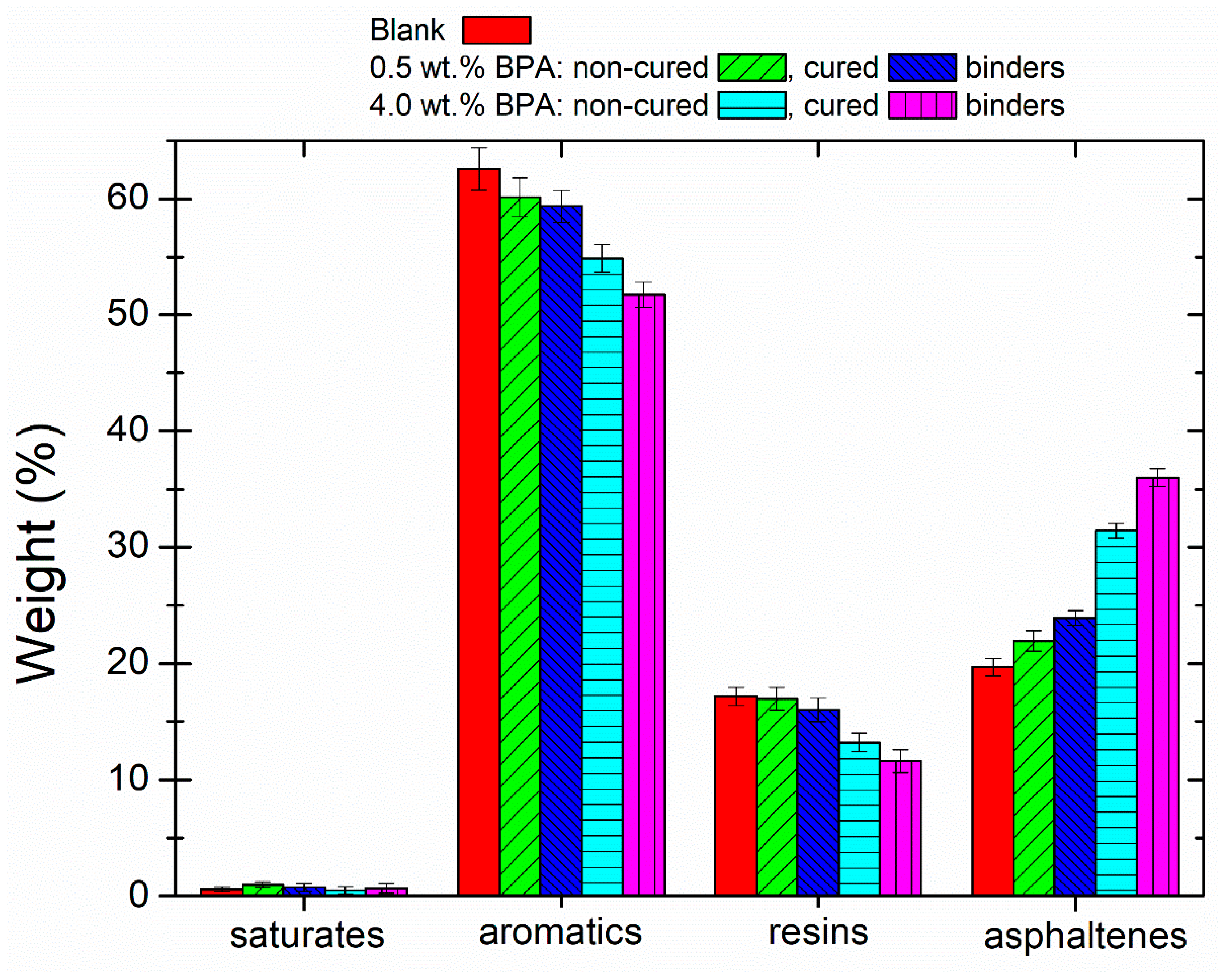
3.2. Physicochemical Characteristics and Microstructure of Epoxy-Modified Bitumens
4. Concluding Remarks
- Rheokinetic curves and rheological tests have shown epoxy binders present an enhanced behavior after 24 h mixing at 180 °C (particularly, at high epoxy concentration). Moreover, this improvement is able to continue under ambient curing for at least one month. As a result, the cured 4 wt % epoxy binder presents viscosity values at 60 °C, much higher than those obtained for the SBS binder.
- Temperature sweep tests in oscillatory shear reveal that, above 50 °C, this epoxy binder displays elastic properties similar to the reference rubbery binder.
- The improvement in the thermo-rheological properties occurred during either the mixing stage or the ambient curing. In this regards, FTIR and SARAs tests confirm a chemical modification through esterification/etherification reactions between the oxirane groups of BPA and the carbonyl groups available in aromatics and resins.
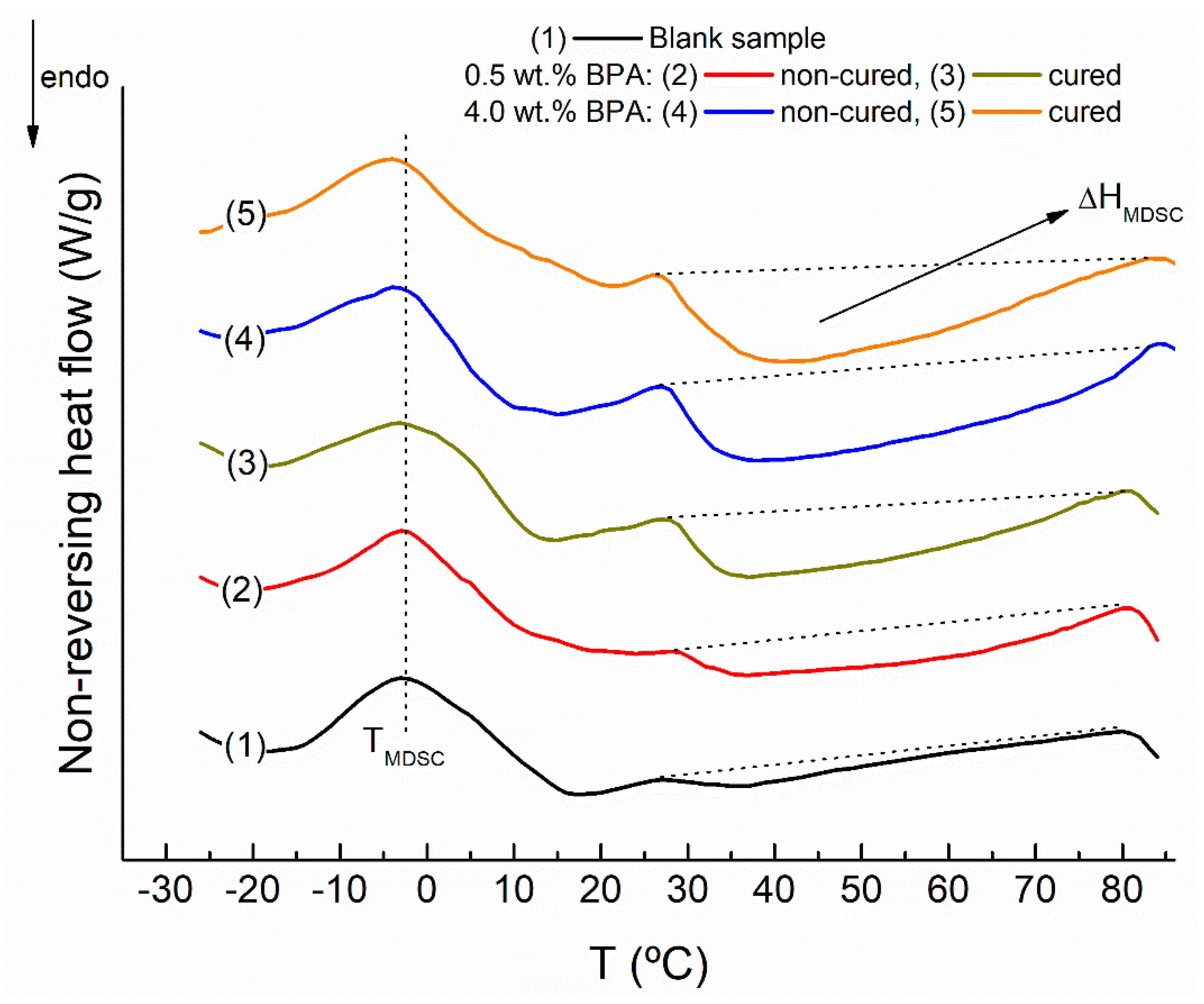
- New bitumen compounds are quantified in the asphaltenic fraction. Likewise, these high molecular weight compounds are more structured and ordered, as confirmed by the increase in the area corresponding to the fourth endothermic event in the nonreversing heat flow curves.
- Interestingly, in addition to the improved rheological response observed at 60 °C, all epoxy binders exhibit viscosity values, at 135 °C, lower than 3 Pa·s. According to AASHTO MP320, the compaction of their resultant asphalt mixes would be carried out successfully.
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Borzędowska-Labuda, K.; Wojtkiewicz, A.; Janik, H. Developments of methods improving storage stability of bitumen modified with ground tire rubber: A review. Fuel Process. Technol. 2017, 159, 272–279. [Google Scholar] [CrossRef]
- González, V.; Martínez-Boza, F.J.; Gallegos, C.; Pérez-Lepe, A.; Páez, A. A study into the processing of bitumen modified with tire crumb rubber and polymeric additives. Fuel Process. Technol. 2019, 95, 137–143. [Google Scholar] [CrossRef]
- Senise, S.; Carrera, V.; Cuadri, A.A.; Navarro, F.J.; Partal, P. Hybrid Rubberised Bitumen from Reactive and Non-Reactive Ethylene Copolymers. Polymers 2019, 11, 1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasileiro, L.; Moreno-Navarro, F.; Tauste-Martínez, R.; Matos, J.; Rubio-Gámez, M.C. Reclaimed polymers as asphalt binder modifiers for more sustainable roads: A review. Sustainability 2019, 11, 646. [Google Scholar] [CrossRef] [Green Version]
- Cuciniello, G.; Leandri, P.; Filippi, S.; Lo Presti, D.; Losa, M.; Airey, G. Effect of ageing on the morphology and creep and recovery of polymer-modified bitumens. Mater. Struct. 2018, 51, 136. [Google Scholar] [CrossRef] [Green Version]
- Brasileiro, L.L.; Moreno-Navarro, F.; Tauste Martínez, R.; del Sol-Sánchez, M.; Elias Matos, J.M.; Rubio-Gámez, M.C. Study of the feasibility of producing modified asphalt bitumens using flakes made from recycled polymers. Constr. Build. Mater. 2018, 208, 269–282. [Google Scholar] [CrossRef]
- Airey, G.D. Styrene butadiene styrene polymer modification of road bitumens. J. Mater. Sci. 2004, 39, 951–959. [Google Scholar] [CrossRef]
- Fawcett, A.H.; McNally, T. Blends of bitumen with polymers having a styrene component. Polym. Eng. Sci. 2001, 41, 1251–1264. [Google Scholar] [CrossRef]
- Lu, X.; Isacsson, U. Effect of ageing on bitumen chemistry and rheology. Constr. Build. Mater. 2002, 16, 15–22. [Google Scholar] [CrossRef]
- Apostolidis, P.; Liu, X.; Erkens, S.; Scarpas, A. Evaluation of epoxy modification in bitumen. Constr. Build. Mater. 2019, 208, 361–368. [Google Scholar] [CrossRef]
- Masson, F.J. Brief review of the chemistry of polyphosphoric acid (PPA) and bitumen. Energy Fuels 2008, 22, 2637–2640. [Google Scholar] [CrossRef] [Green Version]
- Martín-Alfonso, M.J.; Partal, P.; Navarro, F.J.; García-Morales, M.; Bordado, J.C.M.; Diogo, A.C. Effect of processing temperature on the bitumen/MDI-PEG reactivity. Fuel Process. Technol. 2009, 90, 525–530. [Google Scholar] [CrossRef]
- Baldino, N.; Angelico, R.; Caputo, P.; Gabriele, D.; Oliviero Rossi, C. Effect of high water salinity on the adhesion properties of model bitumen modified with a smart additive. Constr. Build. Mater. 2019, 225, 642–648. [Google Scholar] [CrossRef]
- Cuadri, A.A.; García-Morales, M.; Navarro, F.J.; Partal, P. Isocyanate-functionalized castor oil as a novel bitumen modifier. Chem. Eng. Sci. 2013, 97, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Roman, C.; Navarro, F.J.; Garcia-Morales, M.; McNally, T. Physico-chemistry control of the linear viscoelastic behaviour of bitumen/montmorillonite/MDI ternary composites: Effect of the modification sequence. Fuel Process. Technol. 2016, 143, 195–203. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Carrera, V.; Izquierdo, M.A.; García-Morales, M.; Navarro, F.J. Bitumen modifiers for reduced temperature asphalts: A comparative analysis between three polymeric and non-polymeric additives. Constr. Build. Mater. 2014, 51, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Chichun, H.; Jianying, Z.; Zhen, L.; Manfred Partl, N.; Rui, L. Laboratory evaluation of waterborne epoxy bitumen emulsion for pavement preventative maintenance application. Constr. Build. Mater. 2019, 197, 220–227. [Google Scholar]
- Jianying, Y.; Peiliang, C.; Shaopeng, W. Laboratory investigation of the properties of asphalt modified with epoxy resin. J. Appl. Polym. Sci. 2009, 113, 3557–3563. [Google Scholar]
- Bagshaw, S.A.; Herrington, P.R.; Wu, J.P. Preliminary examination of chipseals prepared with epoxy-modified bitumen. Constr. Build. Mater. 2015, 88, 232–240. [Google Scholar] [CrossRef]
- Xiao, Y.; van de Ven, M.F.C.; Molenaar, A.A.A.; Su, Z.; Zandvoort, F. Characteristics of two-component epoxy modified bitumen. Mater. Struct. 2011, 44, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Peiliang, C.; Jianying, Y.; Shuanfa, C. Effects of epoxy resin contents on the rheological properties of epoxy-asphalt blends. J. Appl. Polym. Sci. 2010, 118, 3678–3684. [Google Scholar] [CrossRef]
- Xu, X.; Yu, J.; Xue, L.; Zhang, C.; He, B.; Wu, M. Structure and performance evaluation on aged SBS modified bitumen with bi- or tri-epoxy reactive rejuvenating system. Constr. Build. Mater. 2017, 151, 479–486. [Google Scholar] [CrossRef]
- Xu, S.; Yu, J.; Hu, C.; Qin, D.; Xue, L. Laboratory evaluation of rejuvenation effect of reactive rejuvenator on aged SBS modified bitumen. Mater. Struct. 2017, 50, 233. [Google Scholar] [CrossRef]
- Ecker, A. The application of Iatroscan-technique for analysis of bitumen. Pet. Coal 2001, 43, 51–53. [Google Scholar]
- Carrera, V.; García-Morales, M.; Navarro, F.J.; Partal, P.; Gallegos, C. Bitumen chemical foaming for asphalt paving applications. Ind. Eng. Chem. Res. 2010, 49, 8538–8543. [Google Scholar] [CrossRef]
- Morea, F.; Agnusdei, J.O.; Zerbino, R. Comparison of methods for measuring zero shear viscosity in asphalts. Mater. Struct. 2010, 43, 499–507. [Google Scholar] [CrossRef]
- Biro, S.; Gandhi, T.; Amirkhanian, S. Determination of zero shear viscosity of warm asphalt binders. Constr. Build. Mater. 2009, 23, 2080–2086. [Google Scholar] [CrossRef]
- Yuliestyan, A.; Cuadri, A.A.; García-Morales, M.; Partal, P. Influence of polymer melting point and Melt Flow Index on the performance of ethylene-vinyl-acetate modified bitumen for reduced-temperature application. Mater. Des. 2016, 96, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Martin-Alfonso, J.E.; Cuadri, A.A.; Torres, J.; Hidalgo, M.E.; Partal, P. Use of plastic wastes from greenhouse in asphalt mixes manufactured by dry process. Road Mater. Pavement 2019, 20, S265–S281. [Google Scholar] [CrossRef]
- Handle, F.; Füssl, J.; Neudl, S.; Grossegger, D.; Hofko, B.; Eberhardsteiner, L.; Hospodka, M.; Blab, R.; Grothe, H. The bitumen microstructure: A fluorescent approach. Mater. Struct. 2016, 49, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Navarro, F.J.; Jasso, M.; Zanzotto, L. Physicochemical softening of a bituminous binder by a reactive surfactant (dodecenyl succinic anhydride, DSA). Constr. Build. Mater. 2019, 222, 766–775. [Google Scholar] [CrossRef]
- Ortega, F.J.; Navarro, F.J.; García-Morales, M.; McNally, T. Effect of shear processing on the linear viscoelastic behaviour and microstructure of bitumen/montmorillonite/MDI ternary composites. J. Ind. Eng. Chem. 2017, 48, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Carrera, V.; Partal, P.; García-Morales, M.; Gallegos, C.; Pérez-Lepe, A. Effect of processing on the rheological properties of poly-urethane/urea bituminous products. Fuel Process. Technol. 2010, 91, 1139–1145. [Google Scholar] [CrossRef]
- Carrera, V.; Partal, P.; García-Morales, M.; Gallegos, C.; Páez, A. Influence of bitumen colloidal nature on the design of isocyanate-based bituminous products with enhanced rheological properties. Ind. Eng. Chem. Res. 2009, 48, 8464–8470. [Google Scholar] [CrossRef]
- Cuadri, A.A.; García-Morales, M.; Navarro, F.J.; Partal, P. Processing of bitumens modified by a bio-oil-derived polyurethane. Fuel 2014, 118, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Masson, F.J.; Polomark, G.M. Bitumen microstructure by modulated differential scanning calorimetry. Thermochim. Acta 2001, 374, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Masson, F.J.; Polomark, G.M.; Collins, P. Time-dependent microstructure of bitumen and its fractions by modulated Differential Scanning Calorimetry. Energy Fuels 2002, 16, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F.J.; Partal, P.; García-Morales, M.; Martín-Alfonso, M.J.; Martinez-Boza, F.; Gallegos, C.; Bordado, J.M.C.; Diogo, A.C. Bitumen modification with reactive and non-reactive (virgin and recycled) polymers: A comparative analysis. J. Ind. Eng. Chem. 2009, 15, 458–464. [Google Scholar] [CrossRef]



| (Pa·s) | λ (s) | s | ||
|---|---|---|---|---|
| Blank | 247 ± 7 | 0.08 ± 0.02 | 0.42 ± 0.01 | |
| SBS | 625 ± 9 | 14.89 ± 0.02 | 0.09 ± 0.01 | |
| Noncured binders | 0.5 wt % BPA | 311 ± 6 | 0.15 ± 0.02 | 0.27 ± 0.01 |
| 1 wt % BPA | 377 ± 6 | 0.24 ± 0.02 | 0.24 ± 0.01 | |
| 2 wt % BPA | 480 ± 7 | 0.21 ± 0.02 | 0.36 ± 0.01 | |
| 3 wt % BPA | 582 ± 9 | 0.40 ± 0.02 | 0.25 ± 0.01 | |
| 4 wt % BPA | 667 ± 10 | 0.43 ± 0.02 | 0.29 ± 0.01 | |
| Cured binders | 0.5 wt % BPA | 347 ± 6 | 0.14 ± 0.02 | 0.38 ± 0.01 |
| 1 wt % BPA | 441 ± 7 | 0.19 ± 0.02 | 0.37 ± 0.01 | |
| 2 wt % BPA | 530 ± 7 | 0.27 ± 0.02 | 0.32 ± 0.01 | |
| 3 wt % BPA | 634 ± 9 | 0.40 ± 0.02 | 0.31 ± 0.01 | |
| 4 wt % BPA | 815 ± 11 | 0.71 ± 0.02 | 0.28 ± 0.01 |
| ΔHMDSC (J/g) | TMDSC (°C) | |
|---|---|---|
| Blank | 0.34 ± 0.05 | −2.5 ± 0.5 |
| noncured 0.5 wt % BPA | 0.95 ± 0.06 | −3.2 ± 0.5 |
| cured 0.5 wt % BPA | 1.27 ± 0.06 | −3.2 ± 0.6 |
| noncured 4.0 wt % BPA | 2.09 ± 0.07 | −3.6 ± 0.5 |
| cured 4.0 wt % BPA | 2.41 ± 0.07 | −4.7 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadri, A.A.; Delgado-Sánchez, C.; Navarro, F.J.; Partal, P. Short- and Long-Term Epoxy Modification of Bitumen: Modification Kinetics, Rheological Properties, and Microstructure. Polymers 2020, 12, 508. https://doi.org/10.3390/polym12030508
Cuadri AA, Delgado-Sánchez C, Navarro FJ, Partal P. Short- and Long-Term Epoxy Modification of Bitumen: Modification Kinetics, Rheological Properties, and Microstructure. Polymers. 2020; 12(3):508. https://doi.org/10.3390/polym12030508
Chicago/Turabian StyleCuadri, Antonio A., Clara Delgado-Sánchez, Francisco Javier Navarro, and Pedro Partal. 2020. "Short- and Long-Term Epoxy Modification of Bitumen: Modification Kinetics, Rheological Properties, and Microstructure" Polymers 12, no. 3: 508. https://doi.org/10.3390/polym12030508






