Simultaneous and Efficient Production of Furfural and Subsequent Glucose in MTHF/H2O Biphasic System via Parameter Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Furfural by the MTHF/H2O System
2.3. Enzymatic Hydrolysis
2.4. Analysis Methods
3. Results and Discussion
3.1. Furfural Yields by the MTHF/H2O System
3.2. Yields and Constituent Analysis of the Resulting Residues
3.3. Enzymatic Hydrolysis of the RM and Resulting Residues
3.4. Surface Morphology of the RM and Resulting Residues
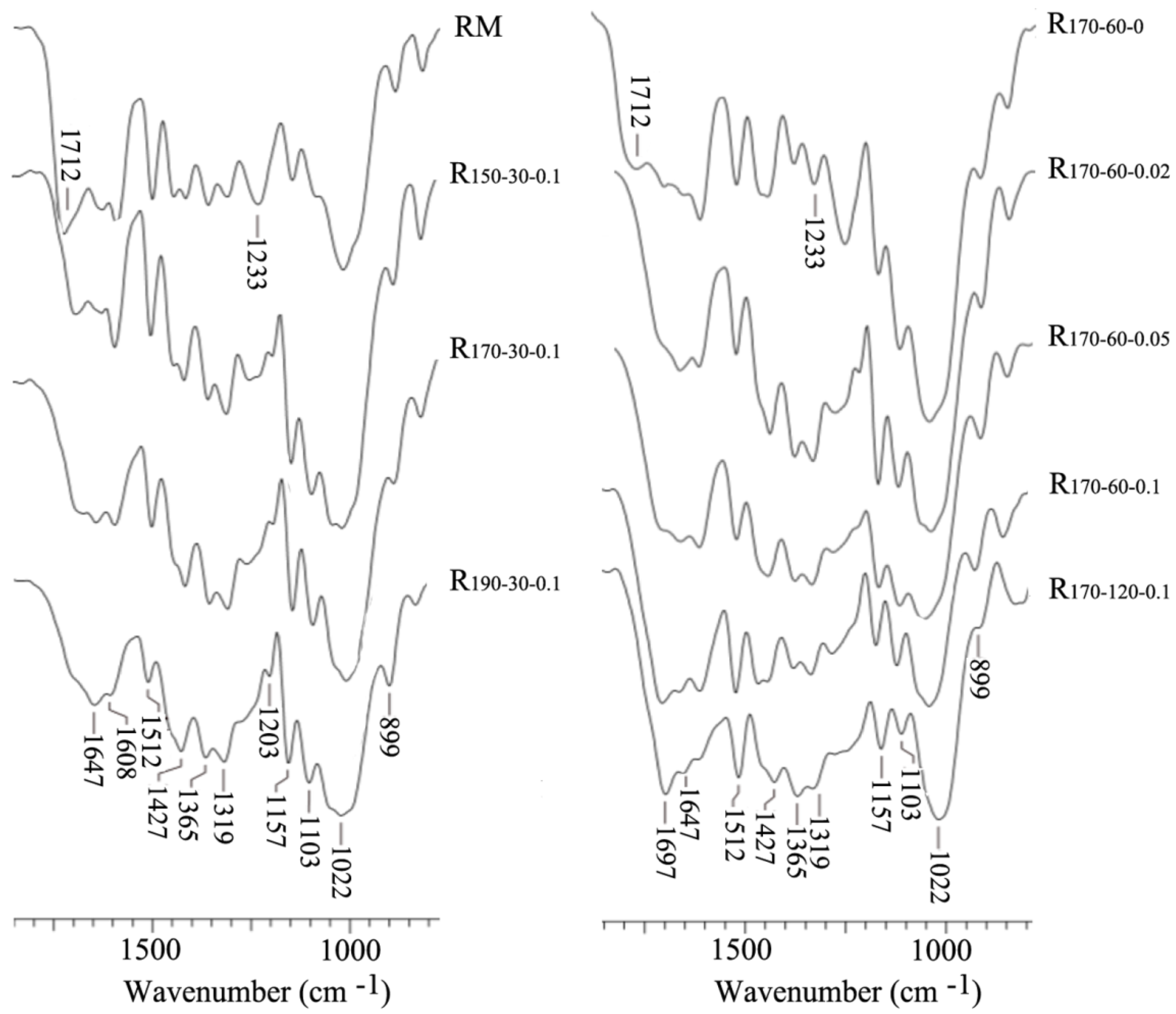
3.5. FT-IR of the RM and Resulting Residues
3.6. Crystallinity of Cellulose in the RM and Resulting Solid Residues
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yaghmur, A.; Aserin, A.; Garti, N. Furfural−cysteine model reaction in food grade nonionic oil/water microemulsions for selective flavor formation. J. Agric. Food Chem. 2002, 50, 2878–2883. [Google Scholar] [CrossRef]
- Sweygers, N.; Harrer, J.; Dewil, R.; Appels, L. A microwave-assisted process for the in-situ production of 5-hydroxymethylfurfural and furfural from lignocellulosic polysaccharides in a biphasic reaction system. J. Clean. Prod. 2018, 187, 1014–1024. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Zhong, L.; Peng, X.; Sun, R.; Fang, J.; Zheng, S. Conversion of xylose into furfural using lignosulfonic acid as catalyst in ionic liquid. J. Agric. Food Chem. 2014, 62, 7430–7435. [Google Scholar] [CrossRef] [PubMed]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. Innov. Sustain. Econ. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Zhang, T.W.; Li, W.Z.; Xu, Z.P.; Liu, Q.Y.; Ma, Q.Z.; Jameel, H.; Chang, H.M.; Ma, L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 2016, 209, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kashcheyeva, E.I.; Gismatulina, Y.A.; Budaeva, V.V. Pretreatments of non-woody cellulosic feedstocks for bacterial cellulose synthesis. Polymers 2019, 11, 1645. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Deng, A.J.; Ren, J.L.; Liu, C.F.; Lu, Q.; Zhong, L.J.; Peng, F.; Sun, R.C. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef]
- Loi, C.C.; Boo, H.C.; Mohammed, A.S.; Ariffin, A.A. A high performance liquid chromatography method for determination of furfural in crude palm oil. Food Chem. 2011, 128, 223–226. [Google Scholar] [CrossRef]
- Liu, J.; Gong, Z.; Yang, G.; Chen, L.; Huang, L.; Zhou, Y.; Luo, X. Novel kinetic models of xylan dissolution and degradation during ethanol based auto-catalyzed organosolv pretreatment of bamboo. Polymers 2018, 10, 1149. [Google Scholar] [CrossRef] [Green Version]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Riansa-ngawong, W.; Prasertsan, P. Optimization of furfural production from hemicellulose extracted from delignified palm pressed fiber using a two-stage process. Carbohyd. Res. 2011, 346, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green. Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Peleteiro, S.; Garrote, G.; Santos, V.; Parajó, J.C. Furan manufacture from softwood hemicelluloses by aqueous fractionation and further reaction in a catalyzed ionic liquid: A biorefinery approach. J. Clean. Prod. 2014, 76, 200–203. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Edit. 2014, 53, 11872–11875. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Glucose dehydration to 5-Hydroxymethylfurfural in a biphasic aystem over solid acid foams. ChemSusChem 2013, 6, 1697–1707. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Wettstein, S.G.; Dumesic, J.A. Conversion of hemicellulose to furfural and levulinic acid using biphasic reactors with alkylphenol solvents. ChemSusChem 2012, 5, 383–387. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Gallo, J.M.R.; Alonso, D.M.; Wettstein, S.G.; Lim, W.Y.; Dumesic, J.A. Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chem. Int. Edit. 2013, 52, 1270–1274. [Google Scholar] [CrossRef]
- Gupta, N.K.; Fukuoka, A.; Nakajima, K. Amorphous Nb2O5 as a selective and reusable catalyst for furfural production from xylose in biphasic water and toluene. ACS Catal. 2017, 7, 2430–2436. [Google Scholar] [CrossRef]
- Sun, S.L.; Cao, X.F.; Li, H.L.; Chen, X.; Tang, J.N.; Sun, S.N. Preparation of furfural from Eucalyptus by the MIBK/H2O pretreatment with biphasic system and enzymatic hydrolysis of the resulting solid fraction. Energy Convers. Manag. 2018, 173, 539–544. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of sugars and biomass to furans using heterogeneous catalysts in biphasic solvent systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, M.G.; Gupta, D.; Saha, B.; Patra, A.K.; Bhaumik, A.; Abu-Omar, M.M. Efficient solid acid catalyst containing Lewis and Brønsted acid sites for the production of furfurals. ChemSusChem 2014, 7, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Fang, Z.; Luo, J.; Su, T.C. Coproduction of furfural and easily hydrolyzable residue from sugar cane bagasse in the MTHF/aqueous biphasic system: Influence of acid species, NaCl addition, and MTHF. ACS. Sustain. Chem. Eng. 2016, 4, 5804–5813. [Google Scholar] [CrossRef]
- Lin, Q.X.; Li, H.L.; Wang, X.L.; Jian, L.F.; Ren, J.L.; Liu, C.F.; Sun, R.C. SO42−/Sn-MMT solid acid catalyst for xylose and xylan conversion into furfural in the biphasic system. Catalysts 2017, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Seemala, B.; Haritos, V.; Tanksale, A. Levulinic acid as a catalyst for the production of 5-hydroxymethylfurfural and furfural from lignocellulose biomass. ChemCatChem 2016, 8, 640–647. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Mao, J.D.; Holtman, K.M.; Franqui-Villanueva, D. Chemical structures of corn stover and its residue after dilute acid prehydrolysis and enzymatic hydrolysis: Insight into factors limiting enzymatic hydrolysis. J. Agric. Food Chem. 2010, 58, 11680–11687. [Google Scholar] [CrossRef]
- Sun, S.L.; Huang, Y.; Sun, R.C.; Tu, M.B. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Rong, C.G.; Ding, X.F.; Zhu, Y.C.; Li, Y.; Wang, L.L.; Qu, Y.N.; Ma, X.Y.; Wang, Z.C. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts. Carbohyd. Res. 2012, 350, 77–80. [Google Scholar] [CrossRef]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef]
- Teramoto, Y.; Nishio, Y. Biodegradable cellulose diacetate-graft-poly(l-lactide)s: Enzymatic hydrolysis behavior and surface morphological characterization. Biomacromolecules 2004, 5, 407–414. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yu, J.; Zhang, X.; Tan, T. The effects of four different pretreatments on enzymatic hydrolysis of sweet sorghum bagasse. Bioresour. Technol. 2011, 102, 4585–4589. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, I.A.; Lignos, G.D.; Bakker, R.R.; Koukios, E.G. Effect of low severity dilute-acid pretreatment of barley straw and decreased enzyme loading hydrolysis on the production of fermentable substrates and the release of inhibitory compounds. J. Clean. Prod. 2012, 32, 45–51. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Venkatesh, B.; Dale, B.E. Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnol. Bioeng. 2007, 96, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Bian, J.; Li, M.F.; Xu, H.; Xiao, B.; Sun, R.C. Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour. Technol. 2014, 159, 41–47. [Google Scholar] [CrossRef]
- Li, M.; Tu, M.; Cao, D.; Bass, P.; Adhikari, S. Distinct roles of residual xylan and lignin in limiting enzymatic hydrolysis of organosolv pretreated loblolly pine and sweetgum. J. Agric. Food Chem. 2013, 61, 646–654. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Structural elucidation of sorghum lignins from an integrated biorefinery process based on hydrothermal and alkaline treatments. J. Agric. Food Chem. 2014, 62, 8120–8128. [Google Scholar] [CrossRef]
- Huang, C.X.; Dong, H.L.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.T.; Lee, Y.H.; Beardmore, D.H. Mechanism of the enzymatic hydrolysis of cellulose: Effects of major structural features of cellulose on enzymatic hydrolysis. Biotechnol. Bioeng. 1980, 22, 177–199. [Google Scholar] [CrossRef]
- Banerjee, R.; Chintagunta, A.D.; Ray, S. A cleaner and eco-friendly bioprocess for enhancing reducing sugar production from pineapple leaf waste. J. Clean. Prod. 2017, 149, 387–395. [Google Scholar] [CrossRef]
- Sun, S.L.; Sun, S.N.; Wen, J.L.; Zhang, X.M.; Peng, F.; Sun, R.C. Assessment of integrated process based on hydrothermal and alkaline treatments for enzymatic saccharification of sweet sorghum stems. Bioresour. Technol. 2015, 175, 473–479. [Google Scholar] [CrossRef]
- Liu, J.; Sidhu, S.S.; Wang, M.L.; Tonnis, B.; Habteselassie, M.; Mao, J.; Huang, Q. Evaluation of various fungal pretreatment of switchgrass for enhanced saccharification and simultaneous enzyme production. J. Clean. Prod. 2015, 104, 480–488. [Google Scholar] [CrossRef]
- Koo, B.W.; Min, B.C.; Gwak, K.S.; Lee, S.M.; Choi, J.W.; Yeo, H.; Choi, I.G. Structural changes in lignin during organosolv pretreatment of Liriodendron tulipifera and the effect on enzymatic hydrolysis. Biomass. Bioenerg. 2012, 42, 24–32. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Progr. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Varanasi, P.; Li, C.; Liu, H.; Melnichenko, Y.B.; Simmons, B.A.; Kent, M.S.; Singh, S. Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromolecules 2011, 12, 933–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J.N. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energ. Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]




| Entry | Cellulose a | Hemicelluloses | KL | Yields |
|---|---|---|---|---|
| R170-60-0 | 54.7 | 7.6 | 21.6 | 59.0 |
| R170-60-0.02 | 62.8 | ND b | 23.0 | 57.9 |
| R170-60-0.05 | 64.3 | ND | 27.0 | 50.1 |
| R170-60-0.1 | 51.1 | ND | 28.6 | 48.9 |
| R150-30-0.1 | 65.4 | ND | 23.2 | 50.0 |
| R170-30-0.1 | 67.1 | ND | 24.0 | 49.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Cao, X.; Li, H.; Zhu, Y.; Li, Y.; Jiang, W.; Wang, Y.; Sun, S. Simultaneous and Efficient Production of Furfural and Subsequent Glucose in MTHF/H2O Biphasic System via Parameter Regulation. Polymers 2020, 12, 557. https://doi.org/10.3390/polym12030557
Sun S, Cao X, Li H, Zhu Y, Li Y, Jiang W, Wang Y, Sun S. Simultaneous and Efficient Production of Furfural and Subsequent Glucose in MTHF/H2O Biphasic System via Parameter Regulation. Polymers. 2020; 12(3):557. https://doi.org/10.3390/polym12030557
Chicago/Turabian StyleSun, Shaolong, Xuefei Cao, Huiling Li, Yingbo Zhu, Yijing Li, Wei Jiang, Yang Wang, and Shaoni Sun. 2020. "Simultaneous and Efficient Production of Furfural and Subsequent Glucose in MTHF/H2O Biphasic System via Parameter Regulation" Polymers 12, no. 3: 557. https://doi.org/10.3390/polym12030557






