Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Gels Obtaining
2.3. Morphological and Structural Characterization
2.4. Swelling and Sorption Experiments
3. Results and Discussion
3.1. Gels Structure and Morphology
3.2. Gels Swelling and Adsorption Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.D.; Liu, X.H.; Yuan, H.Y.; Liu, H.; Liu, C.T.; Li, T.X.; Yan, C.; Yan, X.R.; Shen, C.Y.; Guo, Z.H. Non-covalently functionalized graphene strengthened poly(vinyl alcohol). Mater. Des. 2018, 139, 372–379. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.J.; Zhang, X.N.; Song, Y.H.; Zhao, Y.P.; Chen, L.; Su, F.M.; Li, L.B.; Wu, Z.L.; Zheng, Q. Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Wang, X.; Su, M.; Liu, C.; Shen, C.; Liu, X. Poly (vinyl alcohol)/Graphene Nanocomposite Hydrogel Scaffolds for Control of Cell Adhesion. J. Renew. Mater. 2020, 8, 89–99. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Ghanaatian, E.; Entezam, M. Mechanical properties and drug release rate of poly(vinyl alcohol)/poly(ethylene glycol)/clay nanocomposite hydrogels: Correlation with structure and physical properties. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Liu, L.S.; Kost, J.; Yan, F.; Spiro, R.C. Hydrogels from Biopolymer Hybrid for Biomedical, Food, and Functional Food Applications. Polymers (Basel) 2012, 4, 997–1011. [Google Scholar] [CrossRef]
- El Halah, A.; Lopez-Carrasquero, F.; Contreras, J. Applications of hydrogels in the adsorption of metallic ions. Cienc. Ing. 2018, 39, 57–70. [Google Scholar]
- Guenther, M.; Gerlach, G.; Sorber, J.; Suchaneck, G.; Arndt, K.F.; Richter, A. pH sensors based on polyelectrolytic hydrogels. Smart Struct. Mater. 2005, 5759, 540–548. [Google Scholar] [CrossRef]
- Duquette, D.; Dumont, M.J. Comparative studies of chemical crosslinking reactions and applications of bio-based hydrogels. Polym. Bull. 2019, 76, 2683–2710. [Google Scholar] [CrossRef]
- Crescenzi, V.; Paradossi, G.; Desideri, P.; Dentini, M.; Cavalieri, F.; Amici, E.; Lisi, R. New hydrogels based on carbohydrate and on carbohydrate-synthetic polymer networks. Polym. Gels. Netw. 1997, 5, 225–239. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite Cryogel with Polyelectrolyte Complexes for Growth Factor Delivery. Pharmaceutics 2019, 11, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagri, L.P.; Saini, R.K.; Bajpai, A.K.; Choubey, R. Silver hydroxyapatite reinforced poly(vinyl alcohol)-starch cryogel nanocomposites and study of biodegradation, compressive strength and antibacterial activity. Polym. Eng. Sci. 2019, 59, 254–263. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Lotfipour, F.; Alami-Milani, M.; Salatin, S.; Hadavi, A.; Jelyehgari, M. Freeze-thaw-induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: The experimental design and optimization. Res. Pharm. Sci. 2019, 14, 175–189. [Google Scholar] [CrossRef]
- Santos, C.; Silva, C.J.; Buttel, Z.; Guimaraes, R.; Pereira, S.B.; Tamagnini, P.; Zille, A. Preparation and characterization of polysaccharides/PVA blend nanofibrous membranes by electrospinning method. Carbohyd. Polym. 2014, 99, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohyd. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Daheriya, P. Kappa-Carrageenan/PVA Filmswith Antibacterial Properties: Part 1. Optimization of Preparation Conditions and Preliminary Drug Release Studies. J. Macromol. Sci. A 2014, 51, 286–295. [Google Scholar] [CrossRef]
- El-Fawal, G.F.; Yassin, A.M.; El-Deeb, N.M. The Novelty in Fabrication of Poly Vinyl Alcohol/kappa-Carrageenan Hydrogel with Lactobacillus bulgaricus Extract as Anti-inflammatory Wound Dressing Agent. AAPS PharmSciTech 2017, 18, 1605–1616. [Google Scholar] [CrossRef]
- Soares, S.F.; Simoes, T.R.; Trindade, T.; Daniel-da-Silva, A.L. Highly Efficient Removal of Dye from Water Using Magnetic Carrageenan/Silica Hybrid Nano-adsorbents. Water Air Soil Poll. 2017, 228. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Khan, R.U. Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. 2019, 9, 12282–12290. [Google Scholar] [CrossRef] [Green Version]
- Sukhlaaied, W.; Riyajan, S.A. Synthesis and properties of carrageenan grafted copolymer with poly(vinyl alcohol). Carbohyd. Polym. 2013, 98, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Dafader, N.C.; Manir, M.S.; Alam, M.F.; Swapna, S.P.; Akter, T.; Huq, D. Effect Of Kappa-Carrageenan On The Properties Of Poly(Vinyl Alcohol) Hydrogel Prepared By The Application Of Gamma Radiation. SOP Trans. Appl. Chem. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Ye, L.; Cui, M.; Yang, B.G.; Li, J.J.; Sun, H.; Yao, F.L. Physically crosslinked poly(vinyl alcohol)-carrageenan composite hydrogels: Pore structure stability and cell adhesive ability. RSC Adv. 2015, 5, 78180–78191. [Google Scholar] [CrossRef]
- Hosseinzadeh, H. Freezing-Thawing Assisted Synthesis of Novel Carrageenan Nanomagnetic Beads for Controlled Release of Antitumor Drugs. Cell. Chem. Technol. 2017, 51, 529–537. [Google Scholar]
- Chopra, P.; Nayak, D.; Nanda, A.; Ashe, S.; Rauta, P.R.; Nayak, B. Fabrication of poly(vinyl alcohol)-Carrageenan scaffolds for cryopreservation: Effect of composition on cell viability. Carbohyd. Polym. 2016, 147, 509–516. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Patachia, S.; Croitoru, C. Increasing the adsorption capacity and selectivity of poly(vinyl alcohol) hydrogels by an alternative imprinting technique. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Roata, I.C.; Croitoru, C.; Pascu, A.; Stanciu, E.M. Characterization of Physically Crosslinked Ionic Liquid-lignocellulose Hydrogels. Bioresources 2018, 13, 6110–6121. [Google Scholar]
- Varca, G.H.C.; Ferraz, C.C.; Mathor, M.B.; Lopes, P.S.; Rogero, S.O.; Rogero, J.O. Encapsulation and nano-encapsulation of papain active sites to enhance radiolytic stability and decrease toxicity. In Nanoscale Radiation Engineering of Advanced Materials for Potential Biomedical Applications; IAEA RADIATION TECHNOLOGY REPORTS: Vienna, Austria, 2015; pp. 31–46. [Google Scholar]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Jain, E.; Kumar, A. Designing Supermacroporous Cryogels Based on Polyacrylonitrile and a Polyacrylamide-Chitosan Semi-interpenetrating Network. J. Biomat. Sci. Polym. Ed. 2009, 20, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Braet, F.; deZanger, R.; Wisse, E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: A study on hepatic endothelial cells. J. Microsc. Oxf. 1997, 186, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.P.; Heng, L.Y. Complexation between Carrageenan and Methylene Blue for Sensor Design. In Proceedings of the 2013 Ukm Fst Postgraduate Colloquium, Selangor, Malaysian, 3–4 July 2013; Volume 1571, pp. 717–724. [Google Scholar] [CrossRef]
- Pehlivan, E.; Arslan, G. Removal of metal ions using lignite in aqueous solution—Low cost biosorbents. Fuel Process. Technol. 2007, 88, 99–106. [Google Scholar] [CrossRef]
- Caccavo, D.; Lamberti, G.; Cafaro, M.M.; Barba, A.A.; Kazlauske, J.; Larsson, A. Mathematical modelling of the drug release from an ensemble of coated pellets. Br. J. Pharmacol. 2017, 174, 1797–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.L.; Pan, Y.M.; Shen, C.Y.; Liu, C.T.; Liu, X.H. Facile Thermally Impacted Water-Induced Phase Separation Approach for the Fabrication of Skin-Free Thermoplastic Polyurethane Foam and Its Recyclable Counterpart for Oil-Water Separation. Macromol. Rapid Comm. 2018, 39. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yang, H.G.; Chen, Z.H.; Chen, N.; Pang, X.C.; Zhang, L.; Minari, T.; Liu, X.Y.; Liu, H.Z.; Chen, J.Z. Recyclable Oil-Absorption Foams via Secondary Phase Separation. ACS Sustain. Chem. Eng. 2018, 6, 13834–13843. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Laupretre, F. X-ray diffraction analysis of poly(vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Daud, W.R.W. Synthesis and characterization of modified kappa-carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Kantoglu, O.; Caykara, T.; Guven, O. Preparation and characterization of polysaccaride interpolymer complexes: I-PVA/iota-carrageenan. J. Appl. Polym. Sci. 2013, 127, 500–507. [Google Scholar] [CrossRef]
- Xu, W.G.; Asai, S.; Sumita, M. Spectroscopic study of ethylene vinyl alcohol copolymer and poly(vinyl alcohol). Sen’i Gakkaishi 1997, 53, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.M.N.; Moreira, A.P.D.; Carvalho, C.W.P.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Mendes, M.F.; Oliveira, R.N. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C Bio. S 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zinoun, M.; Cosson, J.; Deslandes, E. Physicochemical Characterization of Carrageenan from Gigartina-Teedii (Rooth) Lamouroux (Gigartinales, Rhodophyta). J. Appl. Phycol. 1993, 5, 23–28. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Ribeiro-Claro, P.J.A.; van de Velde, F. VIBRATIONAL SPECTROSCOPY (FTIR-ATR AND FT-RAMAN) A Rapid and Useful Tool for Phycocolloid Analysis. In Proceedings of the Biodevices 2009: Proceedings of the International Conference on Biomedical Electronics and Devices, Porto, Portugal, 14–17 January 2009; pp. 131–136. [Google Scholar]
- Kenawy, E.; Kamoun, E.A.; Eldin, M.S.M.; El-Meligy, M.A. Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arab. J. Chem. 2014, 7, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of glutaraldehyde on thermal and mechanical properties of starch and polyvinyl alcohol blends. Des. Monomers Polym. 2019, 22, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, G.C.; Sederholm, C.H. Correlation of Infrared Stretching Frequencies and Hydrogen Bond Distances in Crystals. J. Chem. Phys. 1956, 24, 639–641. [Google Scholar] [CrossRef]
- Zaltariov, M.F.; Filip, D.; Varganici, C.D.; Macocinschi, D. Atr-Ftir and Thermal Behavior Studies of New Hydrogel Formulations Based on Hydroxypropyl Methylcellulose/Poly(Acrilic Acid) Polymeric Blends. Cell. Chem. Technol. 2018, 52, 619–631. [Google Scholar]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.H.; Liu, H.; Wang, Y.M.; Guo, Z.H.; Liu, C.T. Multi-walled carbon nanotube in a miscible PEO/PMMA blend: Thermal and rheological behavior. Polym. Test. 2019, 75, 367–372. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Garcia-Carvajal, Z.Y.; Jimenez-Palomar, I.; Jimenez-Avalos, J.A.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Gomez, I.; Otazo, E.M.; Hernandez, H.; Rubio, E.; Varela, J.; Ramirez, M.; Barajas, I.; Gordillo, A.J. Thermal degradation study of PVA derivative with pendant phenylthionecarbamate groups by DSC/TGA and GC/MS. Polym. Degrad. Stabil. 2015, 112, 132–136. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Ettelaie, R.; Rafe, A. Kinetic study of kappa-carrageenan degradation and its impact on mechanical and structural properties of chitosan/kappa-carrageenan film. Carbohyd. Polym. 2016, 142, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Fathi, E.; Atyabi, N.; Imani, M.; Alinejad, Z. Physically crosslinked polyvinyl alcohol-dextran blend xerogels: Morphology and thermal behavior. Carbohyd. Polym. 2011, 84, 145–152. [Google Scholar] [CrossRef]
- Salleh, M.S.N.; Nor, N.N.M.; Mohd, N.; Draman, S.F.S. Water Resistance and Thermal Properties of Polyvinyl Alcohol-Starch Fiber Blend Film. In Proceedings of the 6th International Advances in Applied Physics and Materials Science Congress & Exhibition (Apmas 2016), Melville, NY, USA, 1–3 June 2017; Volume 1809. [Google Scholar] [CrossRef] [Green Version]
- van de Velde, F.; Antipova, A.S.; Rollema, H.S.; Burova, T.V.; Grinberg, N.V.; Pereira, L.; Gilsenan, P.M.; Tromp, R.H.; Rudolph, B.; Grinberg, V.Y. The structure of kappa/iota-hybrid carrageenans II. Coil-helix transition as a function of chain composition. Carbohyd. Res. 2005, 340, 1113–1129. [Google Scholar] [CrossRef] [Green Version]
- Cha, W.I.; Hyon, S.H.; Ikada, Y. Microstructure of Poly(Vinyl Alcohol) Hydrogels Investigated with Differential Scanning Calorimetry. Makromol. Chem. 1993, 194, 2433–2441. [Google Scholar] [CrossRef]
- El-Zaher, N.A.; Osiris, W.G. Thermal and structural properties of poly(vinyl alcohol) doped with hydroxypropyl cellulose. J. Appl. Polym. Sci. 2005, 96, 1914–1923. [Google Scholar] [CrossRef]
- Stavropoulou, A.; Papadokostaki, K.G.; Sanopoulou, M. Thermal properties of poly(vinyl alcohol)-solute blends studied by TMDSC. J. Appl. Polym. Sci. 2004, 93, 1151–1156. [Google Scholar] [CrossRef]
- Braudo, E.E.; Muratalieva, I.R.; Plaschina, I.G.; Tolstoguzov, V.B.; Markovich, I.S. Studies on the Mechanisms of Gelation of Kappa-Carrageenan and Agarose. Colloid. Polym. Sci. 1991, 269, 1148–1156. [Google Scholar] [CrossRef]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocolloid. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Wang, T.; Chai, C.X. Swelling of pH-sensitive chitosan-poly(vinyl alcohol) hydrogels. J. Appl. Polym. Sci. 2006, 102, 4665–4671. [Google Scholar] [CrossRef]
- Xiao, C.M.; Yang, M.L. Controlled preparation of physical cross-linked starch-g-PVA hydrogel. Carbohyd. Polym. 2006, 64, 37–40. [Google Scholar] [CrossRef]
- Imtiaz, N.; Niazi, M.B.K.; Fasim, F.; Khan, B.A.; Bano, S.A.; Shah, G.M.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an Original Transparent PVA/Gelatin Hydrogel: In Vitro Antimicrobial Activity against Skin Pathogens. Int. J. Polym. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Zangi, R.; Engberts, J.B.F.N. Physisorption of hydroxide ions from aqueous solution to a hydrophobic surface. J. Am. Chem. Soc. 2005, 127, 2272–2276. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. Czech. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Malana, M.A.; Zohra, R. The release behavior and kinetic evaluation of tramadol HCl from chemically cross linked Ter polymeric hydrogels. Daru 2013, 21. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Vashishtha, M. Development of novel hydrogels by modification of sterculia gum through radiation cross-linking polymerization for use in drug delivery. Nucl. Instrum. Meth. B 2008, 266, 2009–2020. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Zainuddin Whittaker, A.K. Water diffusion into radiation crosslinked PVA-PVP network hydrogels. Radiat. Phys. Chem. 2011, 80, 213–218. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels (Basel) 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. N. Y. 2017. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wu, F.C.; Liu, B.L.; Wu, K.T.; Tseng, R.L. A new linear form analysis of Redlich-Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Wang, J.; Wang, Y.M.; Liu, Q.; Zhong, Y.J.; Wang, Y.; Li, L.; Lincoln, S.F.; Guo, X.H. Preparation of a poly(acrylic acid) based hydrogel with fast adsorption rate and high adsorption capacity for the removal of cationic dyes. RSC Adv. 2019, 9, 21075–21085. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, H. Synthesis of carrageenan/multi-walled carbon nanotube hybrid hydrogel nanocomposite for adsorption of crystal violet from aqueous solution. Pol. J. Chem. Technol. 2015, 17, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.R.; Bazmizeynabad, F.; Seyyedi, B. kappa-Carrageenan beads as new adsorbent to remove crystal violet dye from water: Adsorption kinetics and isotherm. Desalin. Water Treat. 2015, 53, 2529–2539. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Anirudh, T.R.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Effic. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Boujraf, S.; Taouda, H.; Zerrouq, F. Assessment of adsorption kinetics for removal potential of Crystal Violet dye from aqueous solutions using Moroccan pyrophyllite. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 23, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef] [PubMed]
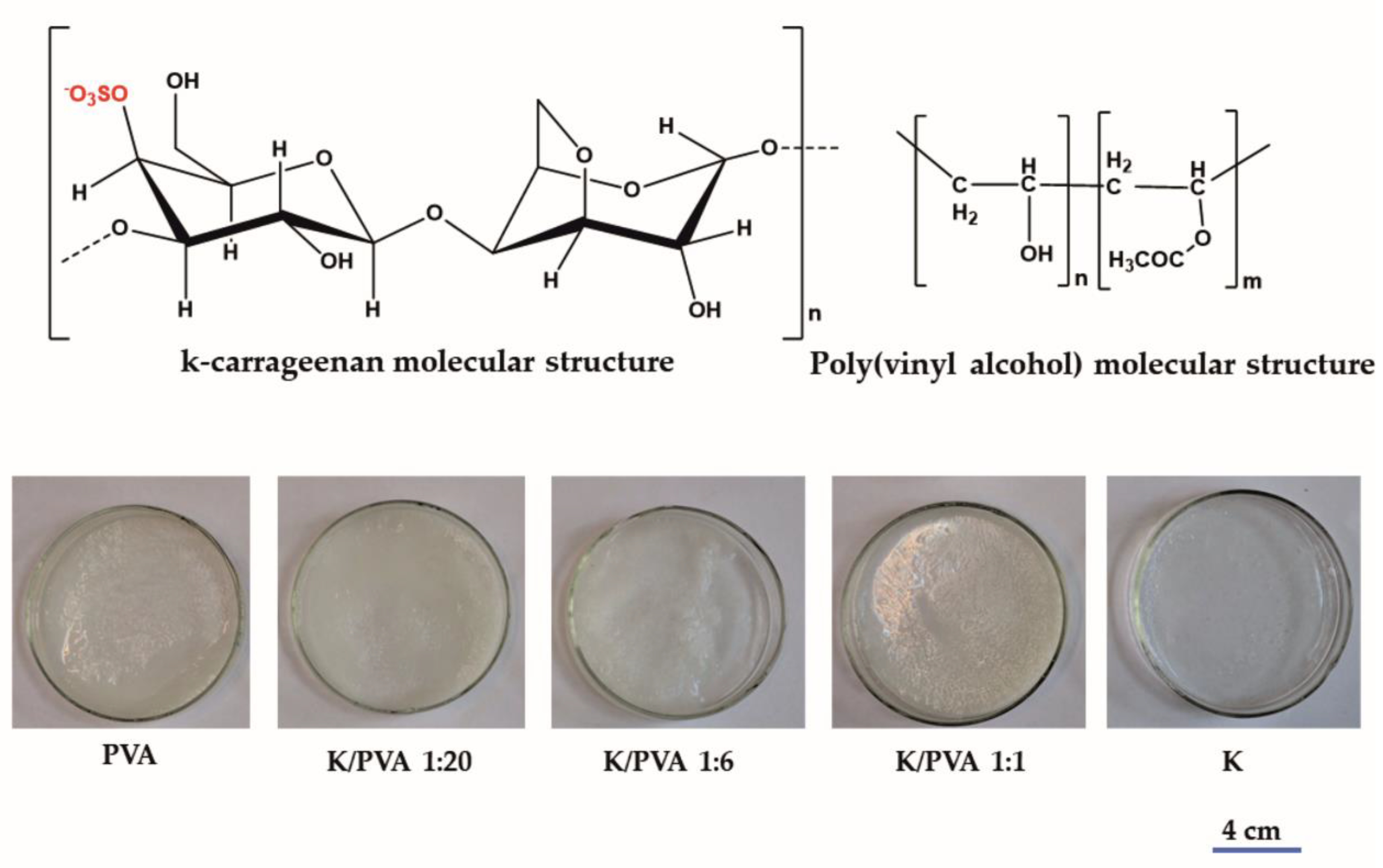
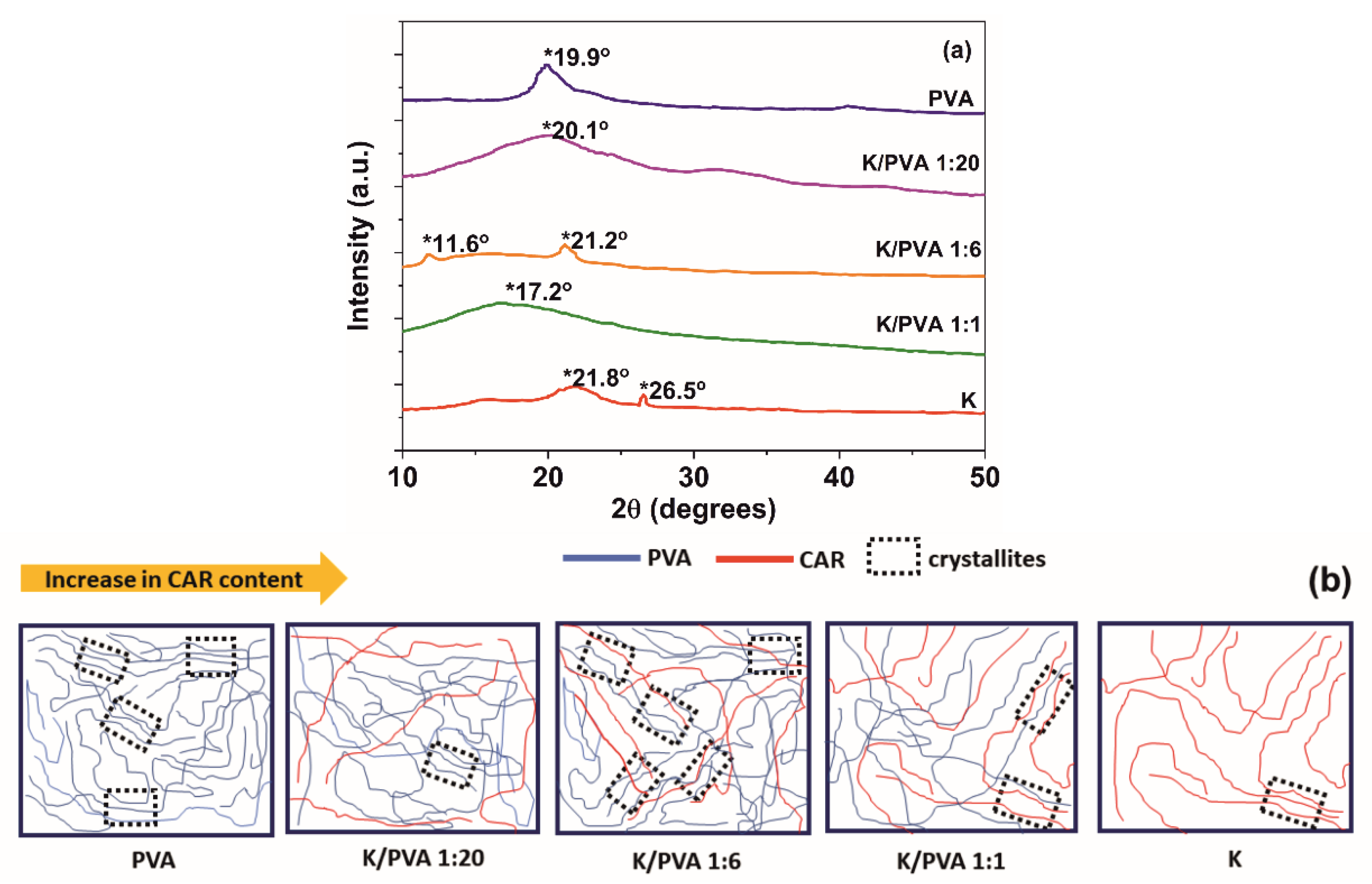
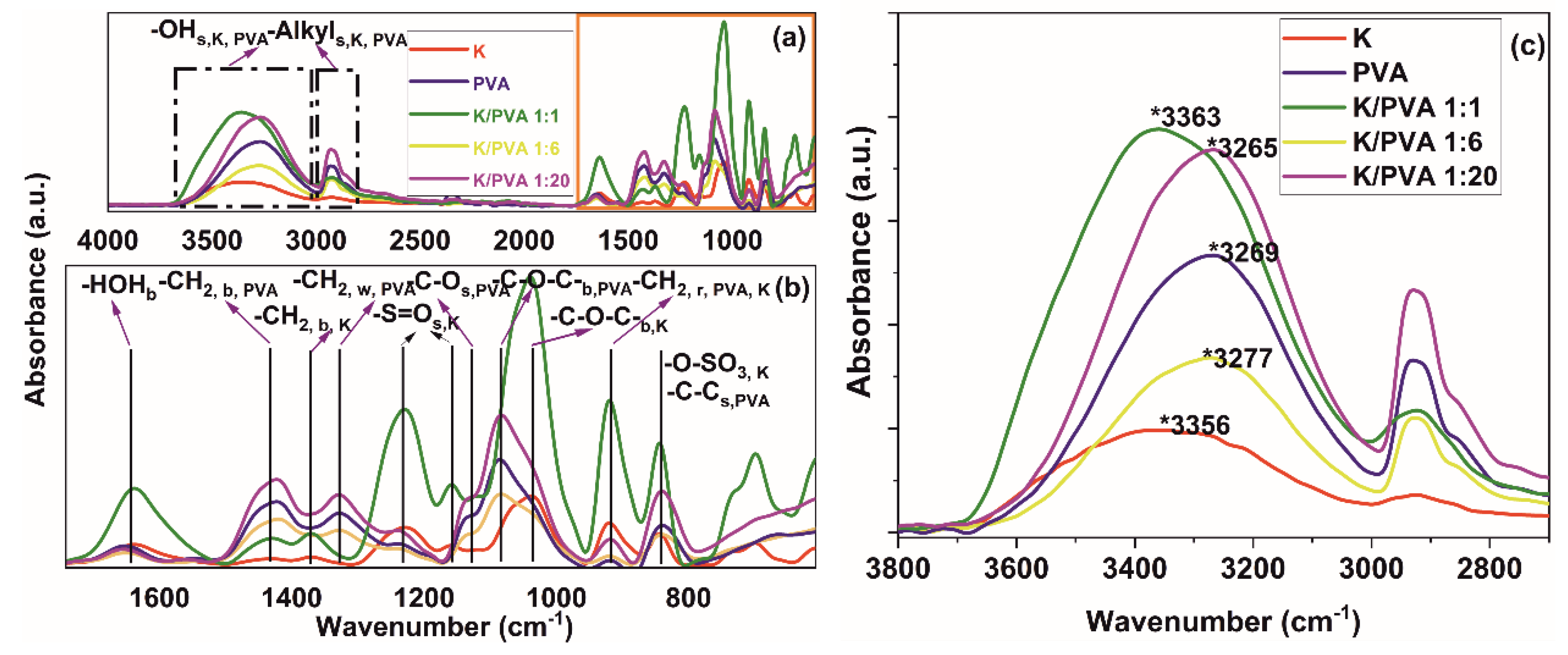
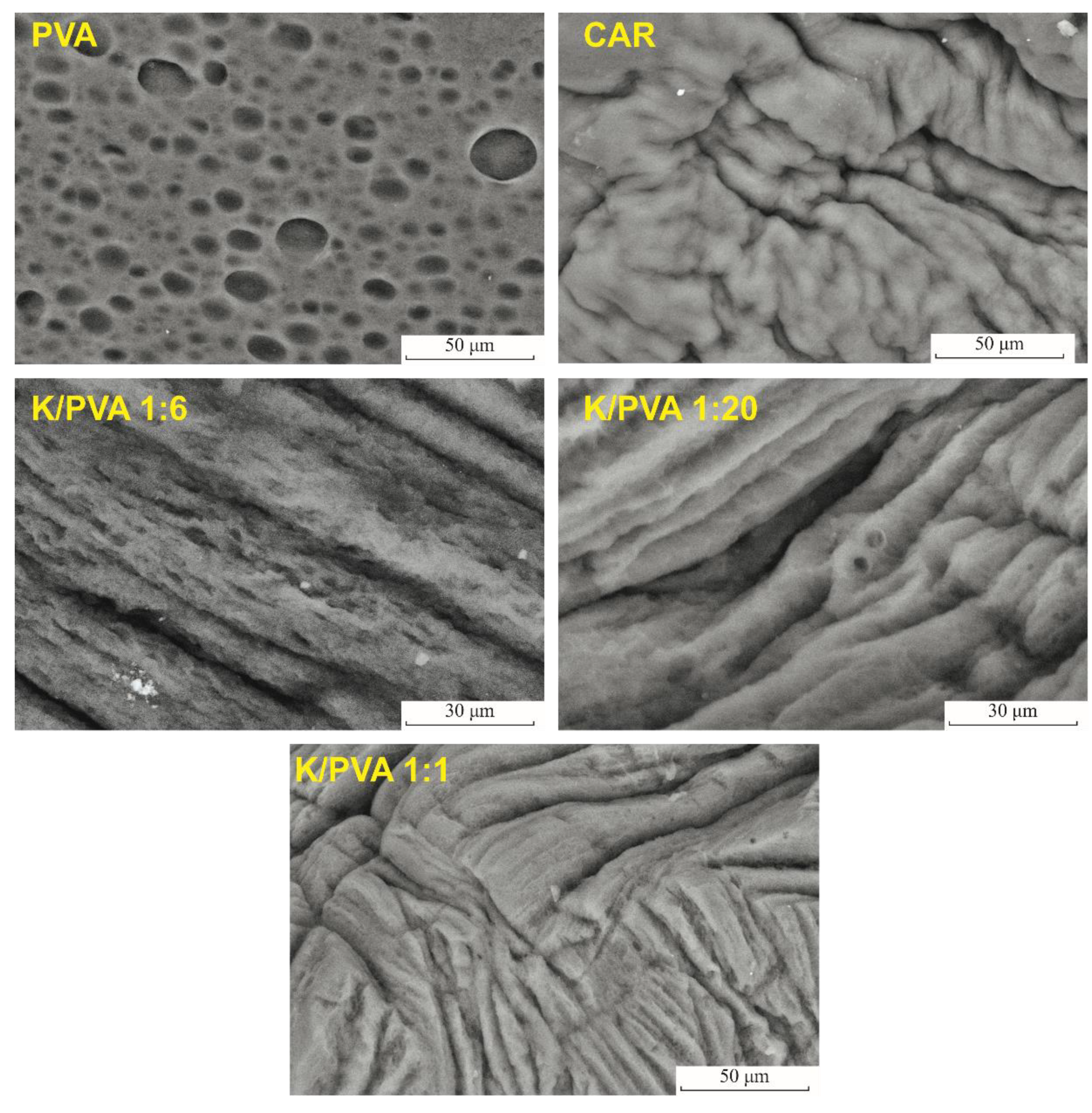

| Sample Code | PVA | CAR | wcar | Gels SC* (%) | Shore 00 Hardness * | Thickness * δ (mm) | G (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Vol. (mL) | Amount (g) | Vol. (mL) | Amount (g) | ||||||
| PVA | 10 | 1 | - | - | 0 | 9.85 | 68 | 2.85 | 82.24 |
| K/PVA 1:20 | 7.5 | 0.75 | 2.5 | 0.037 | 0.047 | 7.59 | 64 | 2.94 | 76.15 |
| K/PVA 1:6 | 4.8 | 0.48 | 5.2 | 0.078 | 0.139 | 5.98 | 69 | 3.02 | 83.07 |
| K/PVA 1:1 | 1.3 | 0.13 | 8.7 | 0.13 | 0.5 | 2.62 | 45 | 3.11 | 64.80 |
| K | - | - | 10 | 0.15 | 1 | 1.54 | 12 | 3.16 | 0.75 |
| Sample Code | FTIR | XRD | Pore volume Ratio Pr (%) | |||
|---|---|---|---|---|---|---|
| CrI | R (Å) | EH (kcal) | CrXRD (%) | DXRD (nm) | ||
| PVA | 0.143 | 2.926 | 6.523 | 4.2 | 5.79 | 8.63 |
| K/PVA 1:20 | 0.119 | 2.927 | 6.386 | 4.0 | 5.05 | 6.42 |
| K/PVA 1:6 | 0.188 | 2.920 | 6.592 | 4.8 | 4.81 | 5.53 |
| K/PVA 1:1 | 0.024 | 2.924 | 4.914 | 2.3 | 6.02 | 3.48 |
| K | - | 2.929 | 5.034 | 1.1 | - | 2.31 |
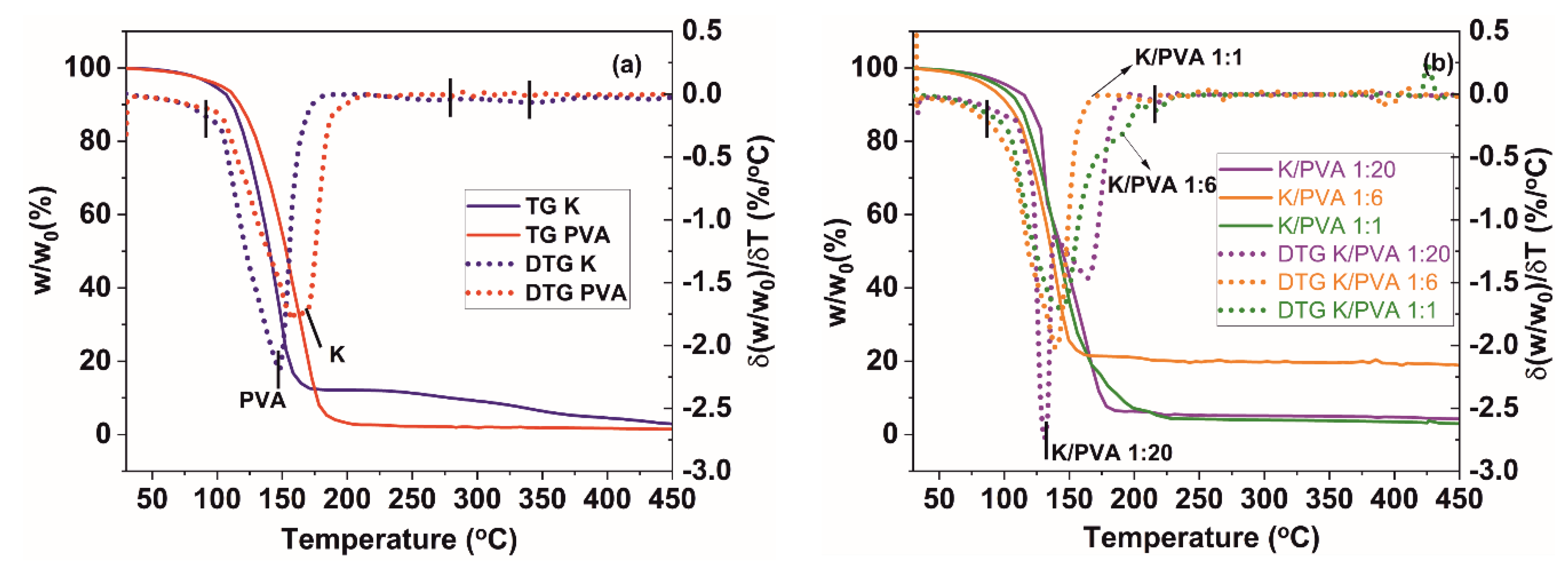
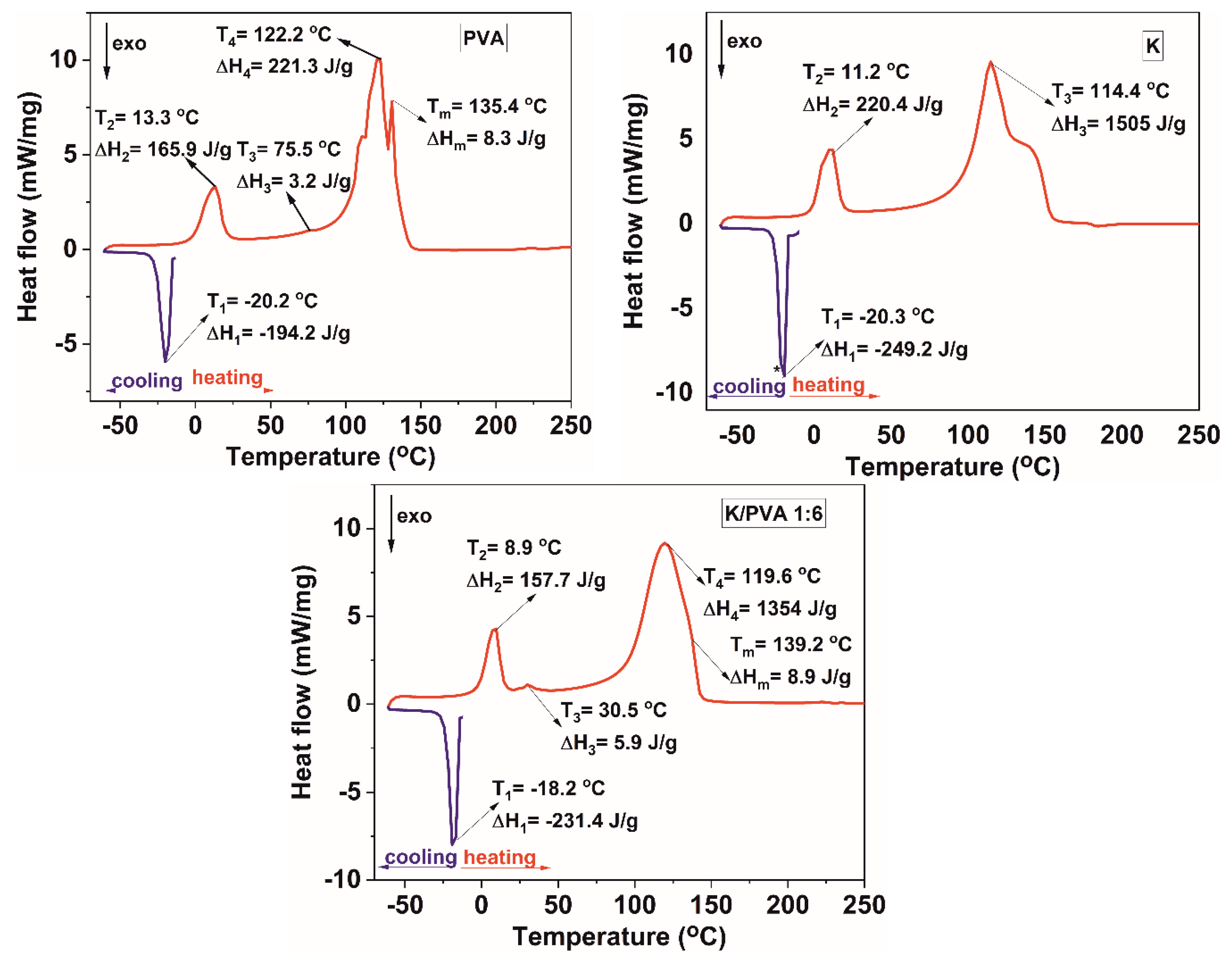
| Sample Code | TGA/DTG Results | DTA Results | DSC Results | ||||
|---|---|---|---|---|---|---|---|
| Twater (°C) | Tdegrad (°C) | ∆mdegrad (%) | ∆mresidue (%) | TDTA (°C) | Tg (°C) | CrDSC (%) | |
| PVA | 100.2 | 146.5 273.5 340.3 | 95.5 | 4.0 | 170.7 | 75.5 | 5.5 |
| K/PVA 1:20 | 109.2 | 133.6 167.1 225.5 | 91.4 | 4.6 | 132.7 169.7 | 60.5 | 5.2 |
| K/PVA 1:6 | 113.1 | 149.1 185.9 214.7 | 37.8 | 19.4 | 145.8 | 30.5 | 5.9 |
| K/PVA 1:1 | 104.0 | 145.7 206.7 268.8 | 90.7 | 3.5 | 145.5 | 29.5 | 3.2 |
| K | 106.8 | 176.8 288.1 333.6 | 91.9 | 1.2 | 150.4 | - | - |
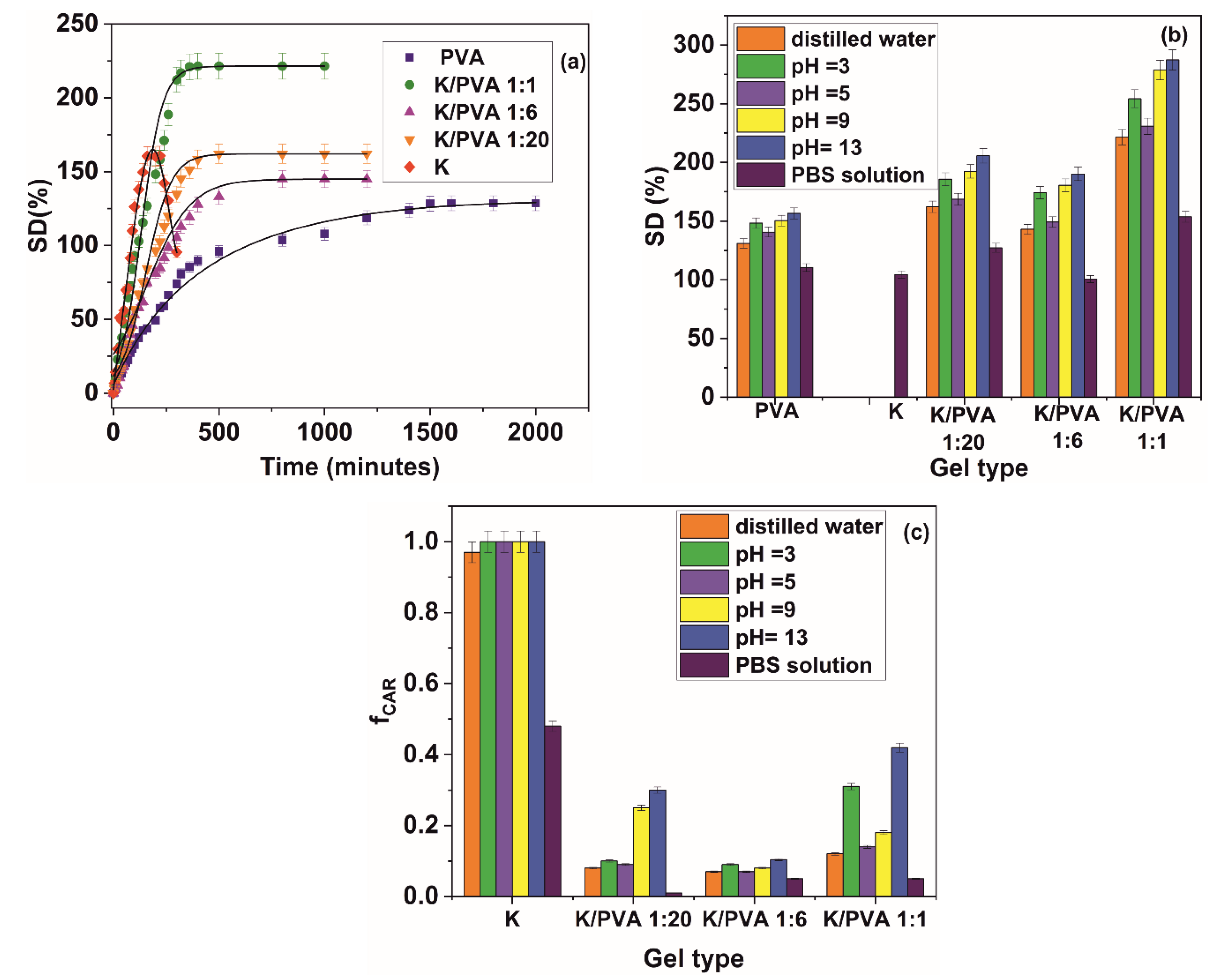
| Material | Parameter | Swelling Medium | |||||
|---|---|---|---|---|---|---|---|
| Distilled Water | pH = 3 | pH = 5 | pH = 9 | pH = 13 | PBS | ||
| PVA | ν (cm/min × 103) | 1.82 | 1.89 | 1.84 | 1.86 | 2.11 | 1.67 |
| De (mm2/min × 105) | 1.82 (0.992) | 1.94 (0.984) | 1.90 (0.990) | 1.84 (0.994) | 2.01 (0.982) | 1.43 (0.992) | |
| Dl (mm2/min × 102) | 6.72(0.977) | 8.92 (0.971) | 8.87 (0.989) | 8.79 (0.977) | 8.94 (0.975) | 5.11 (0.976) | |
| k (min−0.5 × 103) | 4.10 (0.964) | 4.89 (0.960) | 5.16 (0.976) | 4.63 (0.983) | 4.75 (0.974) | 3.46 (0.988) | |
| n | 0.73 (0.964) | 0.85 (0.960) | 0.82 (0.976) | 0.84 (0.983) | 0.91 (0.974) | 0.68 (0.988) | |
| K/PVA 1:20 | ν (cm/min × 103) | 1.89 | 1.52 | 0.93 | 0.95 | 1.61 | 0.72 |
| De (mm2/min × 105) | 6.31 (0.968) | 6.58 (0.972) | 6.40 (0.992) | 6.61 (0.988) | 6.72 (0.988) | 5.62 (0.995) | |
| Dl (mm2/min × 101) | 2.30 (0.979) | 2.65 (0.989) | 2.40 (0.995) | 2.69 (0.985) | 2.73 (0.992) | 1.95 (0.984) | |
| k (min−0.5 × 103) | 1.96 (0.967) | 1.99 (0.973) | 1.97 (0.989) | 2.01 (0.9) | 4.75 (0.974) | 3.46 (0.988) | |
| n | 0.99 (0.967) | 0.85 (0.960) | 0.82 (0.976) | 0.84 (0.983) | 0.91 (0.974) | 0.68 (0.988) | |
| K/PVA 1:6 | ν (cm/min × 103) | 1.65 | 1.78 | 1.69 | 1.71 | 1.82 | 1.38 |
| De (mm2/min × 105) | 6.66 (0.986) | 7.80 (0.989) | 6.75 (0.992) | 6.72 (0.994) | 7.84 (0.992) | 5.42 (0.991) | |
| Dl (mm2/min × 101) | 2.97 (0.988) | 3.18 (0.978) | 3.08 (0.977) | 3.11 (0.976) | 3.24 (0.972) | 1.47 (0.973) | |
| k (min−0.5 × 103) | 4.0 (0.980) | 4.92 (0.991) | 4.27 (0.993) | 4.20 (0.988) | 5.02 (0.987) | 3.20 (0.988) | |
| n | 0.93 (0.980) | 0.96 (0.991) | 0.94 (0.993) | 0.96 (0.988) | 1.00 (0.987) | 0.71 (0.988) | |
| K/PVA 1:1 | ν (cm/min × 103) | 3.66 | 3.90 | 3.69 | 3.73 | 3.98 | 1.89 |
| De (mm2/min × 104) | 8.24 (0.993) | 8.97 (0.998) | 8.28 (0.997) | 8.27 (0.991) | 8.89 (0.992) | 5.43 (0.992) | |
| Dl (mm2/min × 101) | 3.95(0.970) | 4.72 (0.975) | 4.12 (0.970) | 4.06 (0.978) | 4.80 (0.980) | 2.99 (0.989) | |
| k (min−0.5 × 103) | 5.23 (0.995) | 5.39 (0.994) | 5.28 (0.989) | 5.23 (0.995) | 5.23 (0.995) | 5.23 (0.988) | |
| n | 0.95 (0.995) | 0.97 (0.994) | 0.96 (0.989) | 0.95 (0.995) | 0.95 (0.995) | 0.62 (0.988) | |
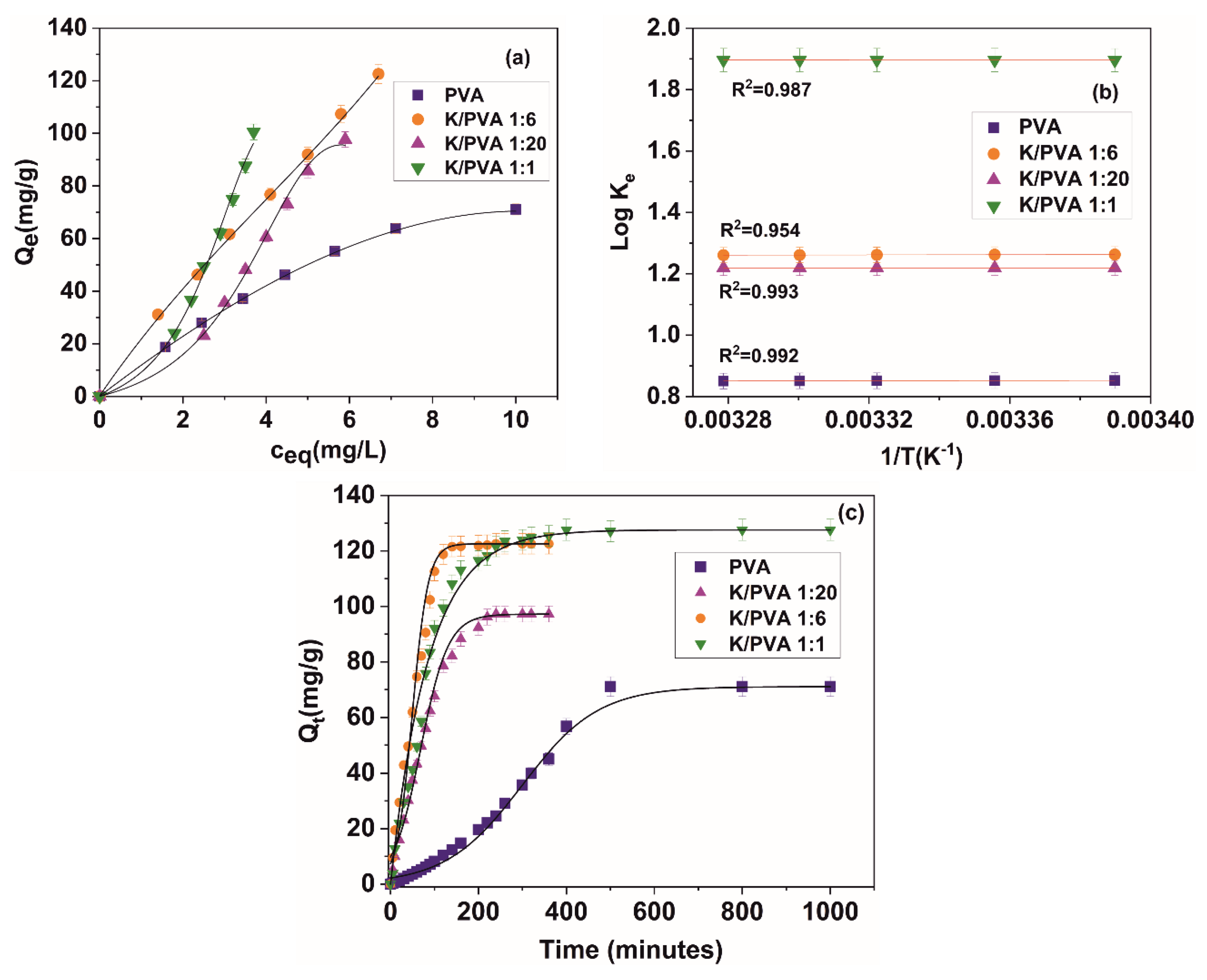
| Isotherm Model | Isotherm Equation (Linearized Form) | Equation Parameters | |||
|---|---|---|---|---|---|
| PVA | K/PVA 1:20 | K/PVA 1:6 | K/PVA 1:1 | ||
| Langmuir | KL = 0.092 Qm = 153.84 RL: 0.11 ÷ 0.35 R2 = 0.953 | KL = 0.103 Qm = 76.92 RL: 0.11 ÷ 0.32 R2 = 0.731 | KL = 0.035 Qm = 625.11 RL: 0.26 ÷ 0.58 R2 = 0.789 | KL = 0.176 Qm = 55.55 RL: 0.06 ÷ 0.22 R2 = 0.909 | |
| Freundlich | KF = 14.270 1/nF = 0.77 R2 = 0.979 | KF = 5.371 1/nF = 1.70 R2 = 0.976 | KF = 22.510 1/nF = 0.88 R2 = 0.998 | KF = 7.882 1/nF = 1.94 R2 = 0.997 | |
| Radke–Prausnitz | KRP = 0.146 Qm = 79.42 m = 0.65 R2 = 0.970 | KRP = 0.763 Qm = 92.90 m = 1.50 R2 = 0.967 | KRP = 0.111 Qm = 123.97 m = 0.90 R2 = 0.998 | KRP = 0.940 Qm = 112.59 m = 2.00 R2 = 0.995 | |
| Redlich–Peterson | KR = 8.372 g = 0.74 αR = 0.11 Qm = 76.10 R2 = 0.979 | KR = 1.372 g = 0.79 αR = 0.013 Qm = 99.91 R2 = 0.979 | KR = 1.619 g = 0.81 αR = 0.013 Qm = 124.59 R2 = 0.998 | KR = 1.631 g = 0.86 αR = 0.014 Qm = 116.54 R2 = 0.909 | |
| Dubinin–Radushkevich | Qm = 63.10 β: = 9 10−7 E = 745 R2 = 0.914 | Qm = 142.25 β: = 3 10−6 E = 408 R2 = 0.995 | Qm = 130.08 β: = 8 10−7 E = 791 R2 = 0.865 | Qm = 112.44 β: = 2 10−6 E = 500 R2 = 0.992 | |
| Flory–Huggins | KFH = 2.2 10−5 n = 2.85 ∆G° = −26.48 R2 = 0.774 | KFH = 5.378 n = 2.27 ∆G° = 4.16 R2 = 0.951 | KFH = 1.8 10−10 n = 1.90 ∆G° = −55.44 R2 = 0.879 | KFH = 6.529 n = 2.05 ∆G° = 4.65 R2 = 0.996 | |
| − | Thermodynamic parameters according to Equations (5) and (6) and Figure 9b for c0 = 80 mg/L (at 25 °C) | ∆H° = −6.7 ∆S° = 15.6 ∆G° = −11.3 | ∆H = 10.5 ∆S° = 23.4 ∆G° = 11.1 | ∆H° = −14.5 ∆S° = 22.0 ∆G° = −21.0 | ∆H° = 19.7 ∆S° = 36.5 ∆G° = 20.7 |
| Kinetic Model | Model Equation (Linearized Form) | Equation Parameters | |||
|---|---|---|---|---|---|
| PVA | K/PVA 1:20 | K/PVA 1:6 | K/PVA 1:1 | ||
| Pseudo-first-order | k1 = 1.8 × 10−3 R2 = 0.920 | k1 = 6.3 × 10−3 R2 = 0.899 | k1 = 0.018 R2 = 0.839 | k1 = 9.1 × 10−3 R2 = 0.849 | |
| Pseudo-second-order | k2 = 0.4 × 10−5 R2 = 0.956 | k2 = 1.1 × 10−4 R2 = 0.961 | k2 = 7.5 × 10−4 R2 = 0.974 | k2 = 9.4 × 10−3 R2 = 0.908 | |
| Intraparticle diffusion | (20% of the first portion of the kinetic data) | kd = 0.144 C = 3.9 × 10−8 R2 = 1 | kd = 2.314 C = 3.1 × 10−12 R2 = 1 | kd = 2.354 C = 0.254 R2 = 1 | kd = 1.739 C = 3.6 × 10−16 R2 = 1 |
| Liquid film diffusion | kfd = 2.1 × 10−3 R2 = 0.891 | kfd = 1.5 × 10−2 R2 = 0.901 | kfd = 2.6 × 10−2 R2 = 0.924 | kfd = 1.2 × 10−2 R2 = 0.973 | |
| Elovich | α = 2.1 × 10−17 β = 2.1 × 10−3 R2 = 0.634 | α = 1.2 × 10−8 β = 5.8 × 10−2 R2 = 0.325 | α = 6 × 10−7 β = 0.199 R2 = 0.251 | α = 9.1 × 10−4 β = 2.9 × 10−2 R2 = 0.929 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, C.; Pop, M.A.; Bedo, T.; Cosnita, M.; Roata, I.C.; Hulka, I. Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers 2020, 12, 560. https://doi.org/10.3390/polym12030560
Croitoru C, Pop MA, Bedo T, Cosnita M, Roata IC, Hulka I. Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers. 2020; 12(3):560. https://doi.org/10.3390/polym12030560
Chicago/Turabian StyleCroitoru, Catalin, Mihai Alin Pop, Tibor Bedo, Mihaela Cosnita, Ionut Claudiu Roata, and Iosif Hulka. 2020. "Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications" Polymers 12, no. 3: 560. https://doi.org/10.3390/polym12030560
APA StyleCroitoru, C., Pop, M. A., Bedo, T., Cosnita, M., Roata, I. C., & Hulka, I. (2020). Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers, 12(3), 560. https://doi.org/10.3390/polym12030560








