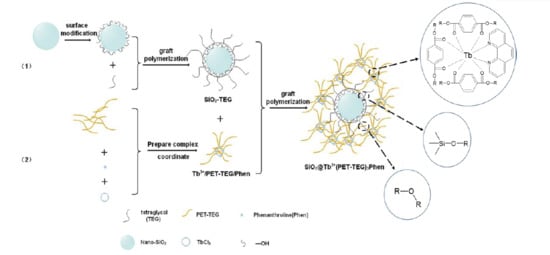
Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. The Preparation of Tb3+/PET–TEG/Phen

2.3. The Preparation of SiO2–TEG

2.4. The Preparation of SiO2@Tb3+(PET–TEG)3Phen
2.5. The Preparation of PET/SiO2@Tb3+(PET–TEG)3Phen
2.6. Characterization
3. Results and Discussion
3.1. Molecular Weight Determination of PET–TEG
3.2. Structure Characterization
3.3. Characterization of Fluorescence Properties
3.4. Morphological Characterization
3.5. Crystallization Behaviour of PET/SiO2@Tb3+(PET–TEG)3 Phen Hybrid
3.6. Effects on Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; Elmetwally, A. Ionic Liquid-Coordinated Ferrous Acetate Complex Immobilized on Bentonite as a Novel Separable Catalyst for PET Glycolysis. Ind. Eng. Chem. Res. 2015, 54, 12474–12481. [Google Scholar] [CrossRef]
- Bizer, C.; Lehmann, J.; Kobilarov, G.; Auer, S.; Becker, C.; Cyganiak, R.; Hellmann, S. DBpedia—A Crystallization Point for the Web of Data. Web Semant. Sci. Serv. Agents World Wide Web 2009, 7, 154–165. [Google Scholar] [CrossRef]
- Uzgiris, E.E.; Kornberg, R.D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen–antibody–complement complexes. Nature 1983, 301, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.C.; Hahm, W.G.; Im, S.S.; Oh, S.G. Poly(ethylene terephthalate)(PET) nanocomposites filled with fumed silicas by melt compounding. Macromol. Res. 2002, 10, 221–229. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Naveen, B.; Shiu, W.C.; Lu, S.W. An advanced preparation and characterization of the PET/MgAl-LDH nanocomposites. RSC Adv. 2014, 4, 25683–25691. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, X.; Liu, W.; Zheng, J.; Ruan, C.; Cui, P. Preparation and Properties of Poly(ethylene terephthalate)/Silica Nanocomposites. J. Macromol. Sci. Part B 2006, 45, 507–513. [Google Scholar] [CrossRef]
- Li, L.; Huang, R.; Zhang, L.; Hong, S. A new mechanism in the formation of PET extended-chain crystal. Polymer 2001, 42, 2085–2089. [Google Scholar]
- Hong, H.; Jiang, S.; An, L.; Feng, J. Influence of Shear on Crystallization Behavior of the β Phase in Isotactic Polypropylene with β-Nucleating Agent. Macromolecules 2004, 37, 2478–2483. [Google Scholar]
- Hu, Z.; Deibert, B.J.; Jing, L. ChemInform Abstract: Luminescent Metal-Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Liu, Y.-R.; Kong, R.-M.; Donovan, M.J.; Zhang, X.-B.; Tan, W.; Shen, G.-L.; Yu, R.-Q. Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label free nuclease enzymedetection. Biosens. Bioelectron. 2013, 42, 31–35. [Google Scholar] [CrossRef]
- Maillard, D.; Kumar, S.K.; Rungta, A.; Benicewicz, B.C.; Prud’homme, R.E. Polymer-Grafted-Nanoparticle Surfactants. Nano Lett. 2011, 11, 4569–4573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Biradar, A.V.; Duncan, C.T.; Asefa, T. Silica nanosphere-supported shaped Pd nanoparticles encapsulated with nanoporous silica shell: Efficient and recyclable nanocatalysts. J. Mater. Chem. 2010, 20, 7834–7841. [Google Scholar] [CrossRef]
- Zeng, C.; Lee, L.J. Poly(methyl methacrylate) and Polystyrene/Clay Nanocomposites Prepared by in-Situ Polymerization. Macromolecules 2001, 34, 4098–4103. [Google Scholar] [CrossRef]
- WHO. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Indian J. Tuberc. 2010, 57, 180–191. [Google Scholar]
- Knopp, D.; Dianping, T.; Reinhard, N. Review: Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 2009, 647, 14–30. [Google Scholar] [CrossRef]
- Santra, S.; Bagwe, R.P.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and Characterization of Fluorescent, Radio-Opaque, and Paramagnetic Silica Nanoparticles for Multimodal Bioimaging Applications. Adv. Mater. 2005, 17, 2165–2169. [Google Scholar] [CrossRef]
- Xu, H.; Goedel, W.A. Polymer−Silica Hybrid Monolayers as Precursors for Ultrathin Free-Standing Porous Membranes. Langmuir 2002, 18, 2363–2367. [Google Scholar] [CrossRef]
- Wu, J.H.; Li, X.S.; Zhao, Y.; Zhang, W.; Guo, L.; Feng, Y.Q. Application of liquid phase deposited titania nanoparticles on silica spheres to phosphopeptide enrichment and high performance liquid chromatography packings. J. Chromatogr. A 2011, 1218, 2944–2953. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef] [Green Version]
- Li, Q. Multifunctional Ionogels Incorporated with Lanthanide (Eu(3+), Tb(3+)) Complexes Covalently Modified Multi-Walled Carbon Nanotubes. Polymers 2018, 10, 1099. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yu, J.; Xi, X.; Sun, Y.; Shen, Y.; Yue, W.; Zhang, C.; Jiang, S. Preparation of Graphene/ITO Nanorod Metamaterial/U-Bent-Annealing Fiber Sensor and DNA Biomolecule Detection. Nanomaterials 2019, 9, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Sun, J.; Han, X.; Tursic-Wunder, A.; Toh, J.D.W.; Hong, W.; Gao, Y.-G.; Miao, Y. Polarisome scaffolder Spa2-mediated macromolecular condensation of Aip5 for actin polymerization. Nat. Commun. 2019, 10, 5078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahabadi, H.K.; Rudin, A. Effect of Solvent on Concentration Dependence of Hydrodynamic Volumes and GPC Elution Volumes. Polym. J. 1979, 11, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sanches, N.B.; Dias, M.L.; Pacheco, E. Comparative techniques for molecular weight evaluation of poly (ethylene terephthalate)(PET). Polym. Test. 2005, 24, 688–693. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Song, L.; Hong, N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Kim, K.; Shin, J.W.; Lee, Y.B.; Cho, M.Y.; Lee, S.H.; Park, D.H.; Jang, D.K.; Lee, C.J.; Joo, J. Poly(3-hexylthiophene)/Multiwalled Carbon Hybrid Coaxial Nanotubes: Nanoscale Rectification and Photovoltaic Characteristics. ACS Nano 2010, 4, 4197–4205. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Zheng, H.; Sun, W.; Jiao, J.; Wang, Y.; Huang, L.; Liu, J.; Wang, W.; Shen, W. Crystallization of Poly(ethylene terephthalate) via Silica Nanoparticles Tethered with Short Diblock PEG-PET Copolymers. Sci. Adv. Mater. 2016, 8, 1603–1611. [Google Scholar] [CrossRef]
- Mormino, M.G.; Fier, P.S.; Hartwig, J.F. Copper-mediated perfluoroalkylation of heteroaryl bromides with (phen)CuRF. Org. Lett. 2014, 16, 1744–1747. [Google Scholar] [CrossRef]
- Chung, K.; Tomljenovic-Hanic, S. Emission Properties of Fluorescent Nanoparticles Determined by Their Optical Environment. Nanomaterials 2015, 5, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-isothermal crystallization kinetic of poly (ethylene terephthalate)/fumed silica (PET/SiO2) prepared by in situ polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Sahoo, N.G.; Tan, Y.P.; Pan, Y.; Bao, H.; Li, L.; Chan, S.H.; Zhao, J. Poly(vinyl alcohol) Nanocomposites Filled with Poly(vinyl alcohol)-Grafted Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Batal, A.B.; Douglas, M.W.; Engram, A.E.; Parsons, C.M. Protein dispersibility index as an indicator of adequately processed soybean meal. Poult. Sci. 2000, 79, 1592–1596. [Google Scholar] [CrossRef]
- Shortreed, M.; Kopelman, R.; Kuhn, M.; Hoyland, B. Fluorescent Fiber-Optic Calcium Sensor for Physiological Measurements. Anal. Chem. 1996, 68, 1414–1418. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–15. [Google Scholar] [CrossRef]
- Shent, H.; Pugh, R.J.; Forssberg, E. A review of plastics waste recycling and the flotation of plastics. Resour. Conserv. Recycl. 1999, 25, 85–109. [Google Scholar] [CrossRef]







| Sample | Mn | Mw | Mw/Mn |
|---|---|---|---|
| LMPET | 11,500 | 29,095 | 2.531 |
| PET–TEG | 24,520 | 30,130 | 1.229 |
| Sample | Mass Fraction/% | ||||
|---|---|---|---|---|---|
| C1s | O1s | Si2p | Tb3d | N1s | |
| SiO2 | 8.65 | 62.57 | 28.79 | -- | -- |
| SiO2–TEG | 12.74 | 59.79 | 27.47 | -- | -- |
| SiO2@Tb3+(PET–TEG)3Phen | 52.65 | 36.63 | 9.61 | 0.79 | 0.33 |
| Elements | C | O | Si | Tb |
|---|---|---|---|---|
| Atomic wt % | 76.32 | 21.65 | 2.02 | 0.01 |
| Sample | W (%) | Tc (°C) | Tm (°C) | ∆Hc (J/g) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PET | 0 | 211.15 | 244.32 | 44.38 | 43.01 | 30.72 |
| SiO2–PET–TEG/PET | 2 | 224.01 | 261.86 | 45.88 | 45.27 | 33.00 |
| SiO2@Tb3+(PET–TEG)3/PET | 2 | 223.48 | 262.07 | 45.91 | 45.33 | 33.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Li, H.; Gong, X.; Liu, J.; Huang, L.; Wang, W.; Wang, Y.; Zhao, Z.; Belfiore, L.A.; et al. Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers 2020, 12, 568. https://doi.org/10.3390/polym12030568
Zhang Y, Wang Y, Li H, Gong X, Liu J, Huang L, Wang W, Wang Y, Zhao Z, Belfiore LA, et al. Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers. 2020; 12(3):568. https://doi.org/10.3390/polym12030568
Chicago/Turabian StyleZhang, Yanna, Yao Wang, Hao Li, Xuezhong Gong, Jixian Liu, Linjun Huang, Wei Wang, Yanxin Wang, Zhihuan Zhao, Laurence A. Belfiore, and et al. 2020. "Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET" Polymers 12, no. 3: 568. https://doi.org/10.3390/polym12030568
APA StyleZhang, Y., Wang, Y., Li, H., Gong, X., Liu, J., Huang, L., Wang, W., Wang, Y., Zhao, Z., Belfiore, L. A., & Tang, J. (2020). Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers, 12(3), 568. https://doi.org/10.3390/polym12030568