Abstract
The free radical copolymerization of electron-acceptor and electron-donor vinyl monomers represents a particular case of sequence-controlled polymerization. The reactions of maleic anhydride (MA) or related compounds (acceptor comonomers) with α-olefins (donor comonomers) result in the formation of the alternating copolymers that have clear prospects for petrochemical and biomedical applications. However, in contrast to the well-established polymerization of acrylate monomers, these processes have not been studied theoretically using the density functional theory (DFT) calculations. In our research, we performed a comprehensive theoretical analysis of the free radical copolymerization of MA and closely related maleimide with different structural types of olefins at mpw1pw91/6-311g(d) level of the DFT. The results of our calculations clearly indicated the preference of the alternating reaction mode for the copolymerization of MA with α-olefins, isobutylene and prospective unsaturated monomers, as well as methylenealkanes. The DFT modeling of the thermally induced Alder-ene reaction between MA and olefins allowed to exclude this reaction from the scope of possible side processes at moderately high temperatures. Comparative analysis of MA and N-methylmaleimide (MMI) reactivity shown that the use of MMI instead of MA makes no sense in terms of the reaction rate and selectivity.
1. Introduction
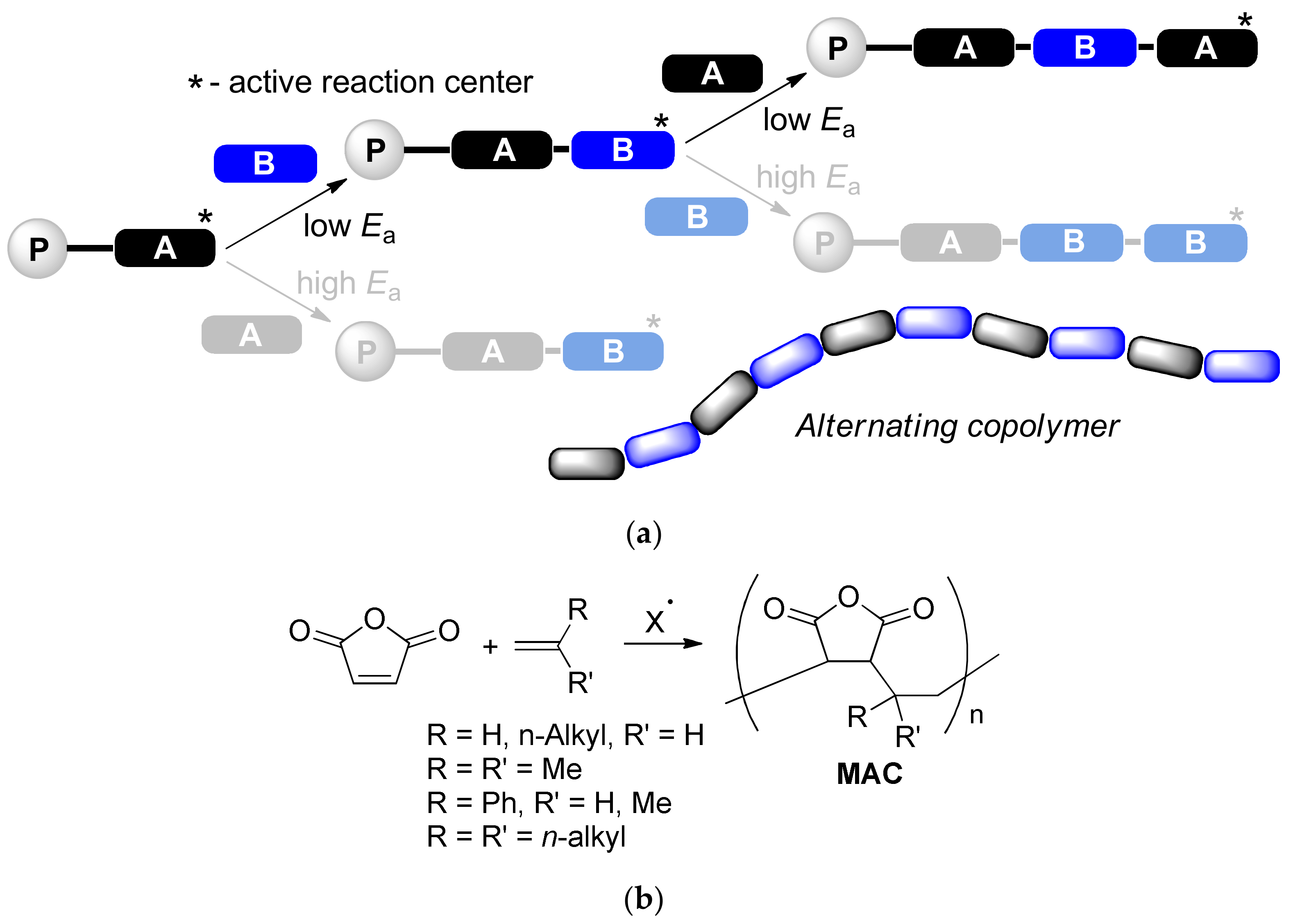
Sequence-controlled polymerization (SCP, Scheme 1a) represents an efficient approach to macromolecules with a determined structure and desired characteristics [1]. Free radical alternating copolymerization of donor and acceptor vinyl monomers is a special case of SCP, which attracts the attention of researchers owing to its experimental simplicity and wide selection of the monomers. Different derivatives of the unsaturated acids such as acrylates, acrylamides, maleic anhydride (MA) or maleimide can be used as an acceptor comonomers; donor comonomers are represented by vinyl ethers and olefins including substituted styrenes. Maleic anhydride copolymers (MAC) with donor monomers are highly attractive due to low cost, structural variability and high reactivity of succinic anhydride subunits (Scheme 1b). To date, MAC with ethylene [2,3], isobutylene [4,5,6] and styrenes [7,8] are synthesized and structurally characterized, alternating microstructure of these copolymers was confirmed by spectral methods [3,9,10]. MA copolymers with linear α-olefins are usually attributed to alternating copolymers [1,11,12,13,14,15,16] (Scheme 1), however these macromolecules are regarded as statistic in the few publications [17,18]. The wide spectra of the functional derivatives of MAC are considered as prospective membranes and films for power cells [19,20,21], pigments [22,23,24], polymers for biomedical applications [16,25,26,27,28,29,30,31,32], and highly efficient depressor additives and flow improvers for fuels and oils [15,18,33,34,35,36,37,38,39,40,41,42,43,44,45].
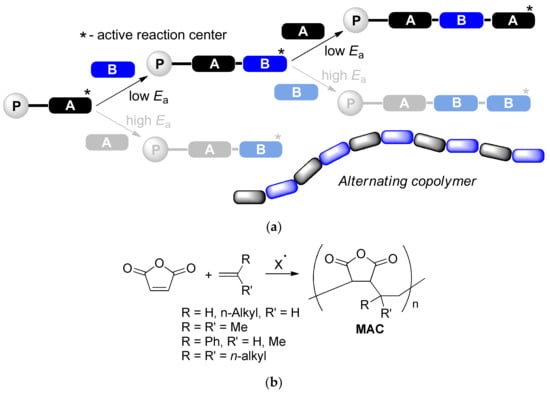
Scheme 1.
(a) Sequence-controlled polymerization and formation of the alternating copolymers; (b) Maleic anhydride copolymers (MAC) with olefins.
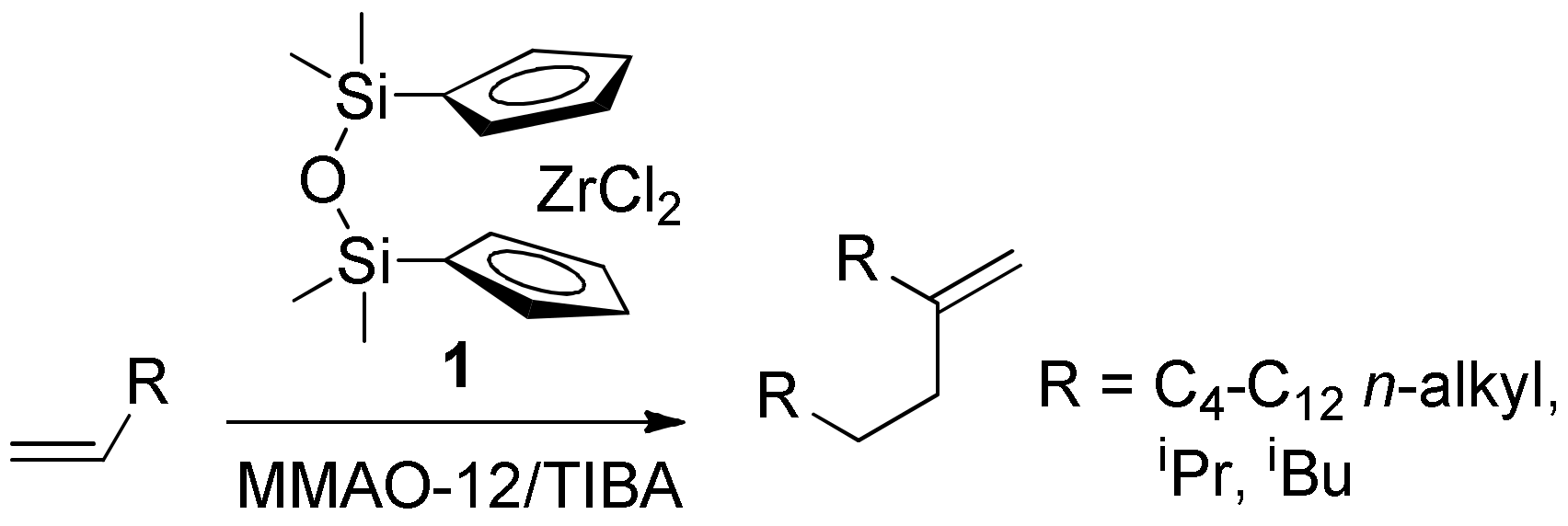
Recently we performed copolymerization of MA with methylenealkanes RC(=CH2)CH2CH2R that are close structural analogs of isobutylene [46]. The ratio of comonomers in the products of copolymerization was ~1:1 (by 1H NMR and elemental analysis). The high synthetic availability of the methylenealkanes that can be obtained by zirconocene-catalyzed dimerization of α-olefins (Scheme 2) [47,48,49] makes it possible to consider methylenealkane/MA copolymers as low-cost materials that contain amphiphilic subunits and branched alkyl substituents with regulated length. The perceived potential of the practical application of these materials required a better understanding of the reaction mechanism and limitations.
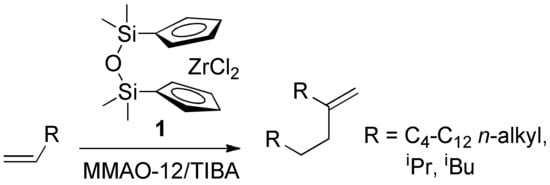
Scheme 2.
Selective dimerization of α-olefins.
The reasons of the formation of alternate polymers by MA and olefins are still unclear in detail. The methods of quantum chemistry based on the density functional theory were successfully used for the analysis of different radical polymerization processes [50,51,52], including homo- and copolymerization of acrylates [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71], acrylamides [70,72,73], and acrylonitrile [74]. However, as far as we know, DFT-based methods have not been extensively used for visualization and comprehensive analysis of the free radical alternating copolymerization of donor and cyclic acceptor monomers, except the publications of Matsumoto et al., devoted to the copolymerization of N-methylmaleimide (MMI) with olefins [75], and MA with 2,4-dimethylpenta-1,3-diene [76].
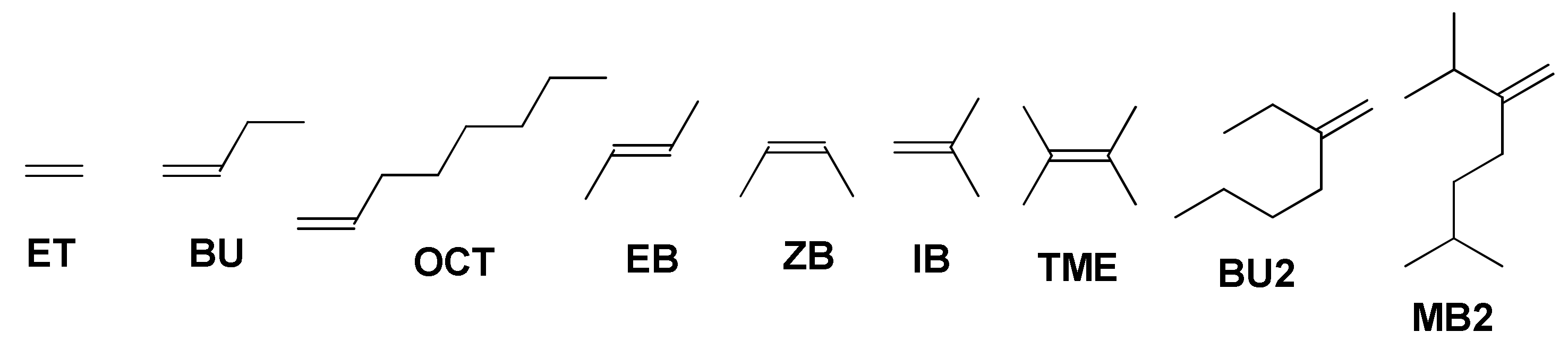
In our study, we present the results of the DFT modeling of copolymerization of MA and closely related MMI with a number of olefins, namely, ethylene (ET), but-1-ene (BU), oct-1-ene (OCT) (E)-but-2-ene (EB), (Z)-but-2-ene (ZB), 2-methylprop-1-ene (isobutylene IB), 2,3-dimethylbut-2-ene (tetramethylethylene TME), 3-methyleneheptane (vinylidene dimer of but-1-ene BU2) and 2,6-dimethyl-3-methyleneheptane (vinylidene dimer of 3-methylbut-1-ene MB2) presented in Scheme 3. The results of our calculations allowed to describe the reaction mechanism in some detail, and predicted the limitations of the reaction by the structure of the olefin comonomer. The following material illustrates some fundamental principles of free radical polymerization mechanism, and therefore may be of interest for the chemists who work in the fields of both polymer science and higher education.
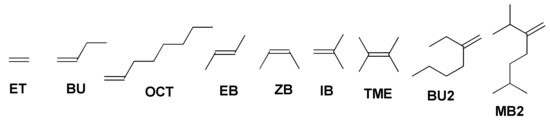
Scheme 3.
Molecular structures of the olefins studied in the reactions with MA.
2. Materials and Methods
2.1. DFT Calculations
The initial cartesian coordinates of the stationary points had been found by PRIRODA program (version 4.0, M.V. Lomonosov Moscow University, Moscow, Russia) [77] using the 3ζ basis. The final optimization and determination of the thermodynamic parameters for stationary points and transition states were carried out using Gaussian 09 program [78] for gas phase at 298.15 K, the root mean square (RMS) force criterion was 3 × 10−4. The mpw1pw91 functional [79] and 6-311g(d) basis set [80] were used in the optimizations. Transition states were found by energy scanning with sequential changing of key geometric parameters with a step of 0.01 Å followed by Berny optimization, and confirmed by intrinsic reaction coordinate (IRC) simulations.
For comparison of the free activation energies for Alder-ene reaction and alternating copolymerization of MA and BU2 we optimized the structures of the starting compounds, intermediates and transition states at 80 °C.
Cartesian coordinates, molecular structure plots, and key energy parameters of the optimized structures are presented in the Supporting Information.
2.2. General Experimentsl Remarks
TIBA (1 M solution in hexane, Merck, NJ, USA), MMAO-12 (1.52 M solution in toluene, Merck, NJ, USA), azobisisobutyronitrile (AIBN, Merck, NJ, USA), benzoyl peroxide (Merck, NJ, USA), mesitylene (Merck, NJ, USA) and CDCl3 (99.8% 2H, Cambridge Isotope Laboratories, Inc., MS, USA) were used as purchased. Toluene (Merck, NJ, USA) was refluxed over sodium and stored under argon atmosphere. Maleic anhydride (MA, Merck, NJ, USA) was recrystallized from toluene before use. Hex-1-ene (Merck, NJ, USA) was stored over Na wire and distilled under argon. The 1H NMR spectra were recorded on a Bruker AVANCE 400 spectrometer (400 MHz, Bruker, MS, USA) at 20 °C. The chemical shifts are reported in ppm relative to the solvent residual peaks.
Zirconium complex 1 was prepared in accordance with published procedure [81]. Dimerization of hex-1-ene was conducted in liquid olefin media in the presence of 1, activated by TIBA and MMAO-12 using the protocol described previously [47,49], 5-methyleneundecane was separated by the vacuum distillation, b. p. 80 °C at 7 Torr.
2.3. The Alder-Ene Reaction of MA with 5-Methyleneundecane
5-Methyleneundecane (4.03 g, 24 mmol) and MA (2.45 g, 25 mmol) were placed in round-bottom flask, prefilled with argon. Mesitylene (10 mL) was added, and the mixture was heated (130 °C) with stirring within 8 h. The formation of the product of Alder-ene reaction was controlled by GC. The product was separated by distillation (B. p. 133–138 °C/0.1 Torr). The yield was 5.37 g (84%). The control experiments were performed with the same reagent and solvent loading at 80 °C. After 4 h of stirring, the sample of the reaction mixture was analyzed by 1H NMR spectroscopy (see Supplementary Figure S1). The products of Alder-ene reaction were not detected.
2.4. Copolymerization of MA and 5-Methyleneundecane
Briefly, 5-methyleneundecane (4.03 g, 24 mmol), MA (2.35 g, 24 mmol), and toluene (10 mL) were placed in a round-bottom flask, prefilled with argon (note that freshly recrystallized MA should be used, the presence of the traces of the maleic acid leads to the isomerization of 5-methyleneundecane). The mixture was heated to 80 °C with stirring, and 0.24 mL of 1M solution of AIBN in toluene (0.24 mmol) was added. After 4 h of stirring, the mixture was cooled, the solvent was removed under reduced pressure. The residue was washed by methanol, and dried in vacuo. The yield of the product was 5.0 g (78%), white powder. For C16H26O3 calculated, %: C 72.14, H 9.84, O 18.02; found, %: C 72.21, H 10.03, O 17.76. For NMR spectra, see Supplementary Figures S2–S4.
In parallel, we performed the controlled experiments on homopolymerization of MA and 5-methyleneundecane, initiated at 80 °C by AIBN. After 2 h of heating, no sign of the reaction appeared. In the presence of benzoyl peroxide at 100 °C, we detected the formation of the traces of MA homopolymer, while 5-methyleneundecane was inert.
3. Results
3.1. MA–Olefin Association
The charge-transfer interaction between donor and acceptor monomers, called “templating”, was proposed as a factor that facilitates the alternating polymerization [1,8]. To confirm or refute this assumption for MA–olefin copolymerization [82], we analyzed the interactions between MA and olefins presented in Scheme 3. The complexes formed are substantially different by geometry as interatomic distances between MA and olefin molecules exceeded the sum of the Van-der-Vaals radii (Figure 1). Even in gas phase, the values of the calculated free energies and free enthalpies of the formation of these complexes (ΔGf and ΔHf, see Table 1) suggest that such an association can hardly be expected to carry much weight for the interaction of MA with olefins.
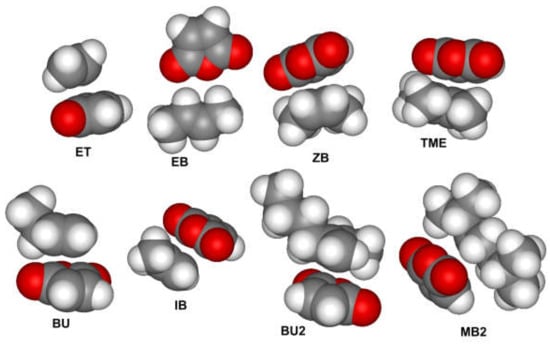
Figure 1.
Optimized geometries of the adducts of MA and olefins.

Table 1.
Calculated free energies and free enthalpies of the formation of MA-olefin complexes.
3.2. The Alder-Ene Reaction between MA and Methylenealkanes
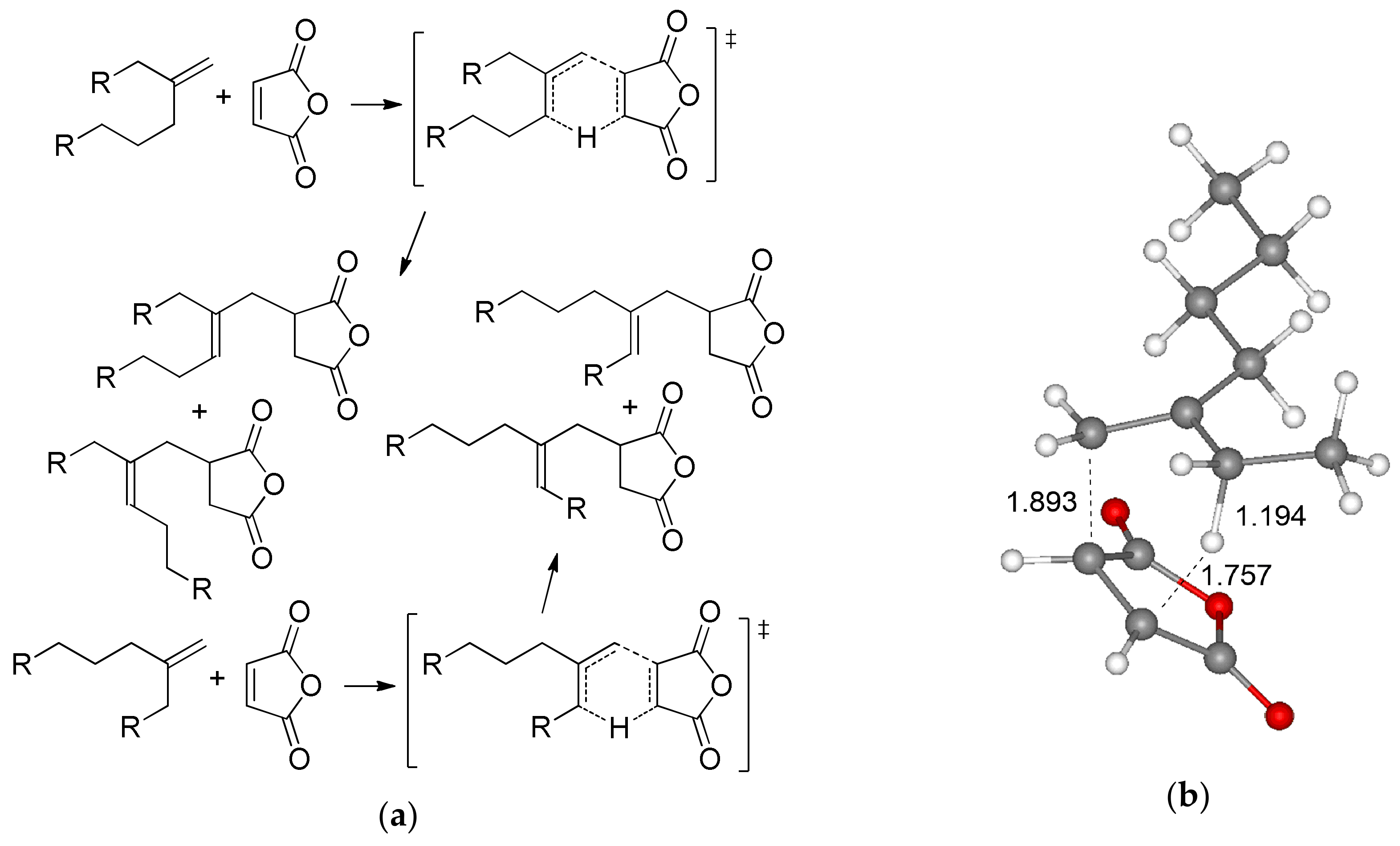
Before the detailed analysis of radical copolymerization of MA and olefins, the possible side process, namely, thermally induced Alder-ene reaction with a formation of isomeric alkenyl-substituted succinic anhydrides (Figure 2a) should have been taken into account. We optimized four possible transition states (TS) of the ene reaction between MA and BU2, found the TS with minimal free energy (Figure 2b), and calculated the differences between the free energies and free enthalpies of this transition state and MA–BU2 complex. The value of ΔG≠ (33.9 kcal/mol, respectively) suggested that this reaction may proceed only at elevated temperatures.
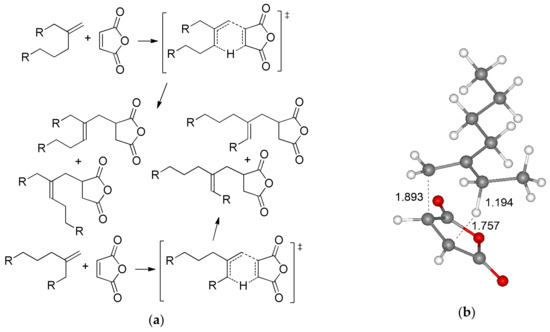
Figure 2.
(a) The Alder-ene reaction between MA and methylenealkanes; (b) Optimized geometry of the transition state of the Alder-ene reaction between MA and 3-methyleneheptane (BU2).
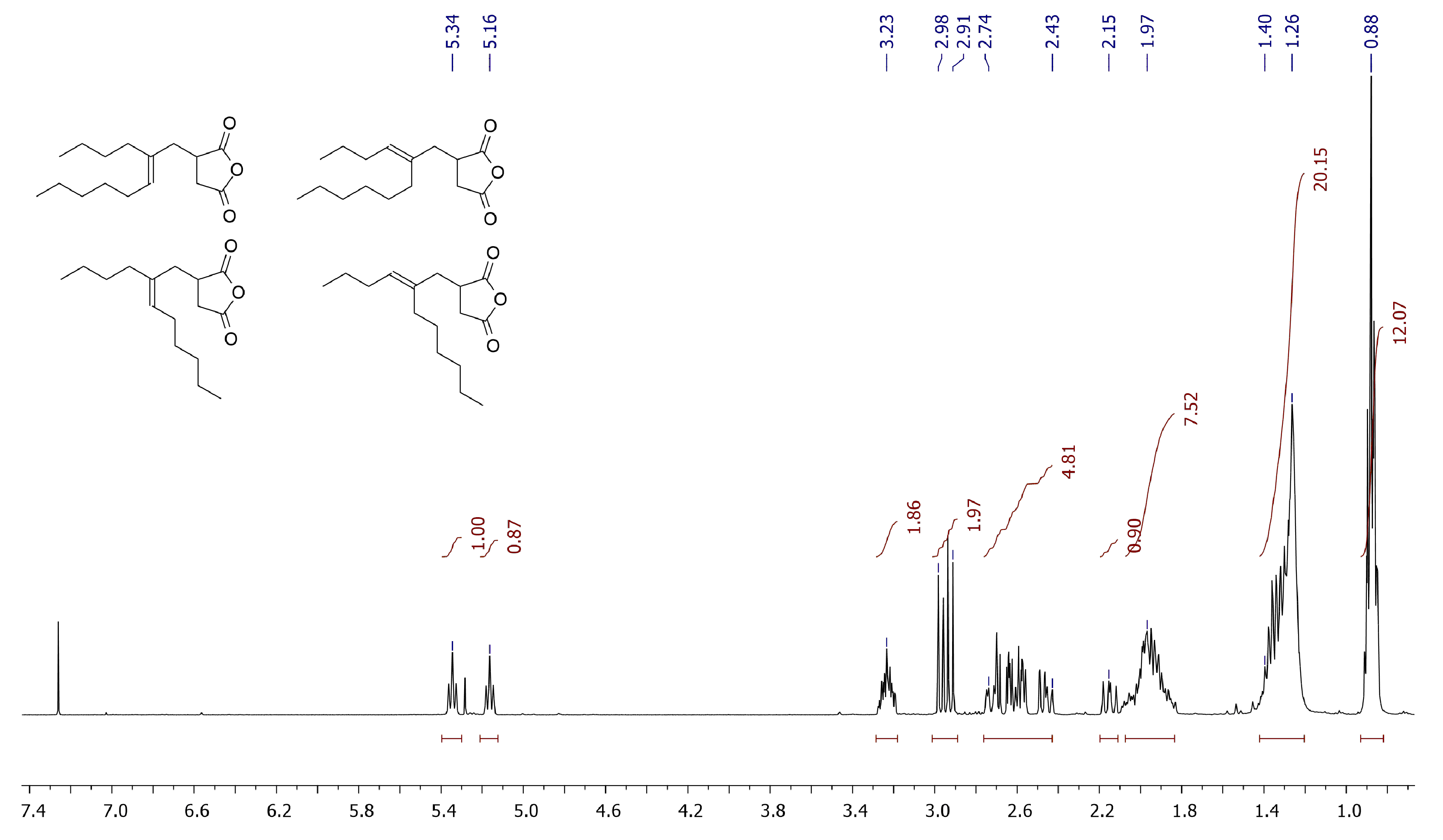
Our previous studies of free-radical copolymerization of MA with methylenealkanes [46] and comparative experiment (see Section 2.4) were conducted at 80 °C in order to provide high efficiency of the free-radical initiator AIBN. Our experiments with MA and 5-methyleneundecane (dimer of 1-hexene) in the absence of AIBN demonstrated that the temperature of 80 °C was not high enough to drive Alder-ene reaction (Section 2.4, Supplementary Figure S1). In fact, ~95% conversion of the reagents was achieved after 8 h heating at 130 °C, and the reaction resulted in the formation of the mixture of isomeric adducts (see Figure 3 for 1H NMR spectrum).
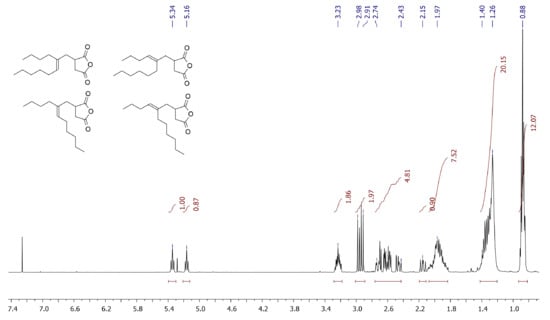
Figure 3.
1H NMR spectrum (CDCl3, 20 °C, 400 MHz) of the products of the Alder-ene reaction between MA and 5-methyleneundecane. Volatile product of the isomerization of the starting olefin and MA were preliminarily eliminated at 0.01 Torr and 100 °C.
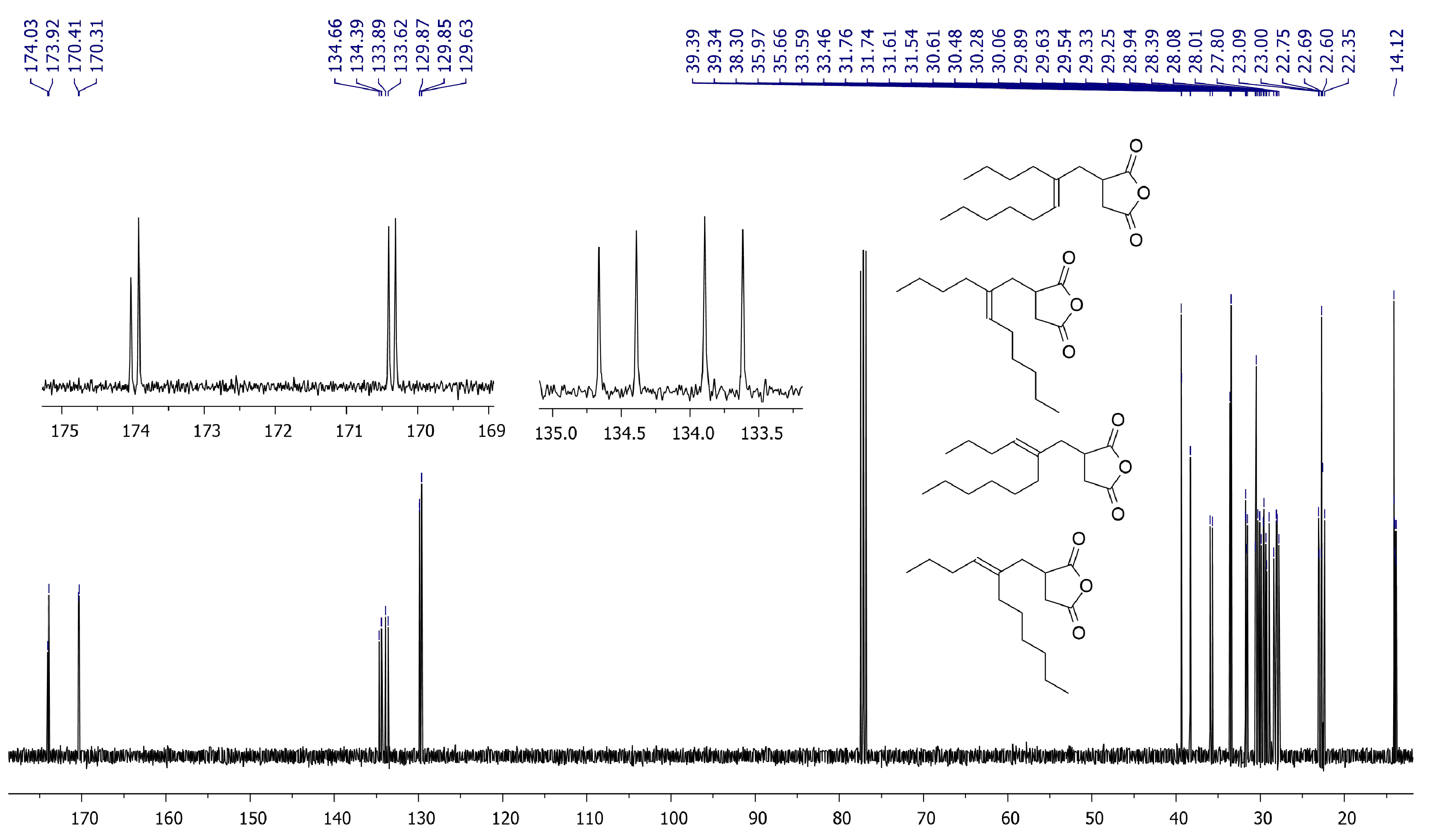
For non-symmetrical methylenealkanes, eight isomeric addicts can be formed in Alder-ene reaction. 13C NMR spectrum of the reaction mixture (Figure 4) indicated the presence of the four groups of signals that reflect the formation of all possible isomers. We optimized all configurations of the TS (Supplementary Section S3) and found that the gap of the free energies for these transition states did not exceed 1.8 kcal/mol.
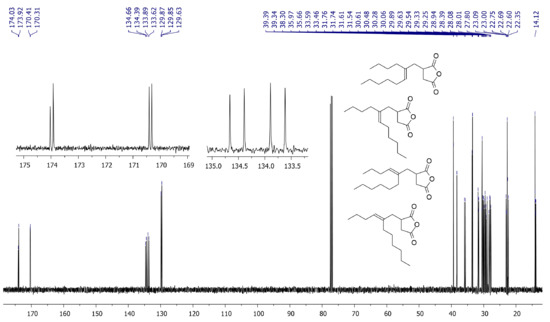
Figure 4.
13C NMR spectrum (CDCl3, 20 °C, 400 MHz) of the products of the Alder-ene reaction between MA and 5-methyleneundecane.
3.3. DFT Modeling of the Copolymerization of MA and Olefins
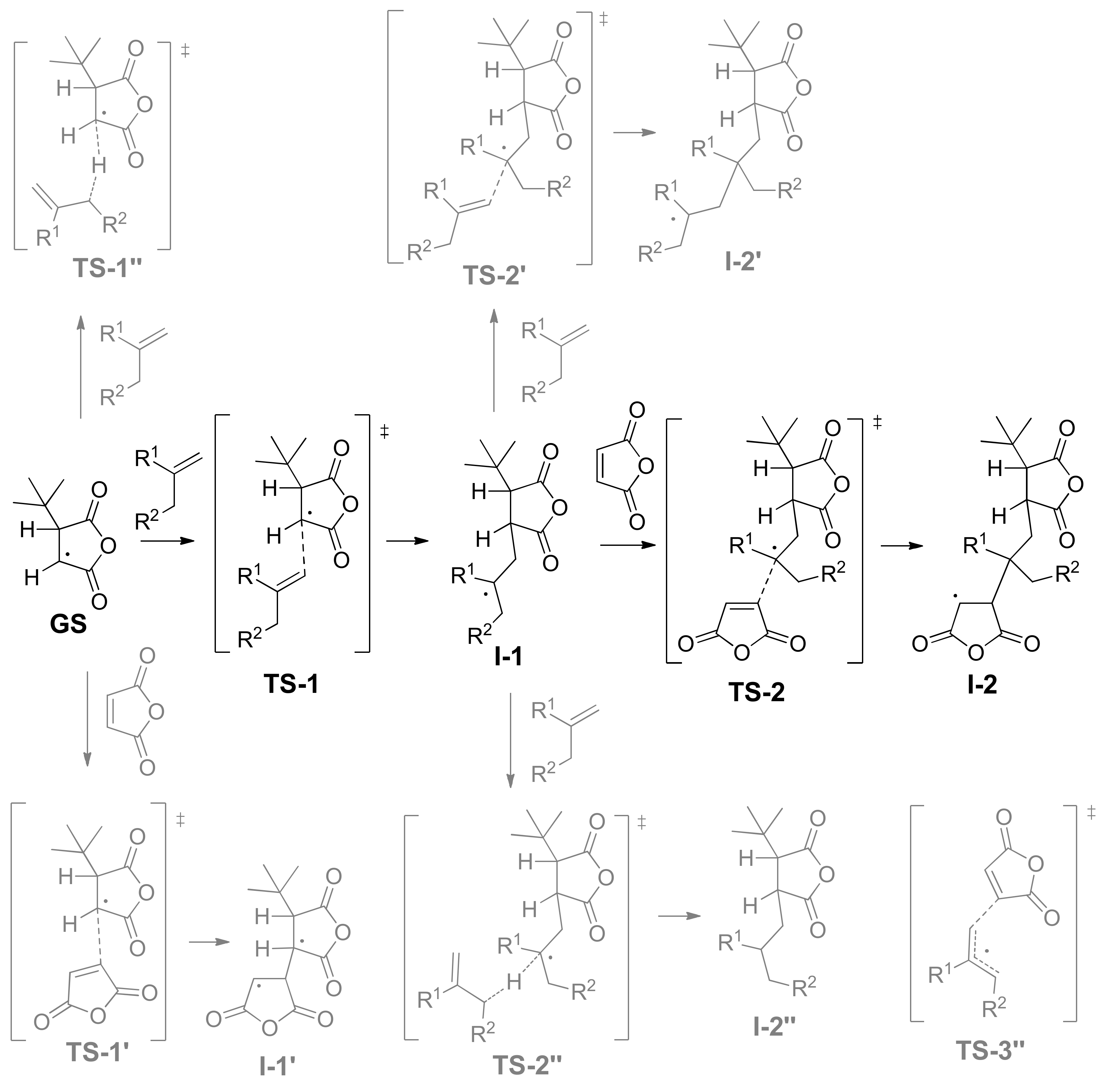
We used 3-(tert-butyl)dihydrofuran-2,5-dione radical (tBu-MA·), the product of the reaction of MA with tert-butyl radical, as a ground state (GS) for the complex analysis of the reaction profiles of the free-radical copolymerization of MA with olefins (Scheme 4). There are four possible reaction pathways for tBu-MA·:
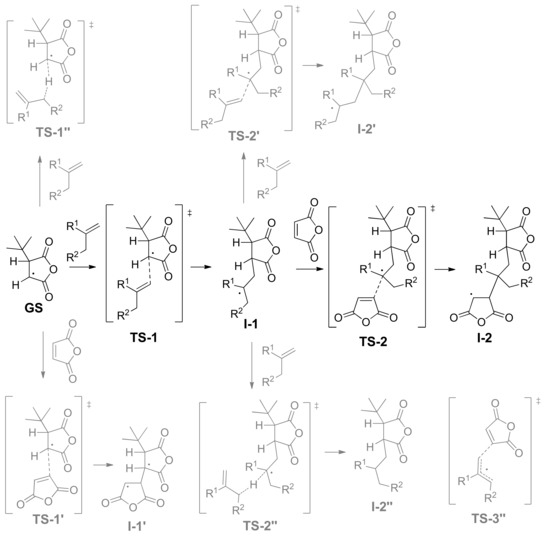
Scheme 4.
The main (marked in black) and side (marked in gray) model reactions for radical copolymerization of MA with olefins.
- The addition to olefin molecule via TS-1 with a formation of tBu-MA-S· species (I-1, Scheme 4). This reaction pathway represents the first step of alternating polymerization.
- The addition to MA molecule via TS-1’ with a formation of tBu-MA-MA· species (I-1’, Scheme 4). This reaction pathway mimics homopolymerization of MA. The calculated ΔG≠ and ΔH≠ for the formation of MA homopolymer were found to be 17.7 and 5.2 kcal/mol, respectively.
- The hydrogen transfer from tBu-MA· to MA molecule with a formation of 3-(tert-butyl)dihydrofuran-2,5-dione and MA-H·. The values of ΔG≠ and ΔH≠ for this reaction were found to be 32.9 and 20.8 kcal/mol, respectively.
- The transfer of the allyl hydrogen atom of the olefin molecule to tBu-MA· with a formation of allyl radical and tBu-MA-H. The energies of the corresponding TS-1’’ (Scheme 4) depend on the nature of the olefin (see Table 2). Evidently, the possibility of the further reactions of the allyl radicals should also be taken into account (see below).
 Table 2. Calculated free energies and free enthalpies of the key intermediated and transition states for MA–olefin copolymerization.
Table 2. Calculated free energies and free enthalpies of the key intermediated and transition states for MA–olefin copolymerization.
tBu-MA-S· species, formed at the first stage, can be involved into three different processes:
- The addition to MA molecule via TS-2 with a formation of tBu-MA-S-MA· species that are structurally similar to tBu-MA·. This model reaction mimics the second step of the polymerization with a formation of the product with alternating microstructure. Therefore, the sequence tBu-MA· – tBu-MA-S· – tBu-MA-S-MA· represents an adequate model of the alternating polymerization of MA with olefins.
- The addition to olefin molecule via TS-2’ with a formation of tBu-MA-S-S· species (I-2’, Scheme 4). This is the side reaction that reflects the possibility of the formation of oligoolefin blocks.
- The transfer of the allyl hydrogen atom of the olefin molecule to tBu-MA-S· (TS-2’’) with a formation of allyl radical and tBu-MA-SH.
The detailed analysis of the transition states and stationary points presented in Scheme 4 needed consideration of all possible orientations of the molecules of reagents. We optimized the structures of these TS and intermediates (the results of this analysis are given in the Supporting Information), and found the optimal orientations for all olefins studied. The values of the relative free energies and relative free enthalpies for such optimized species are presented in Table 2.
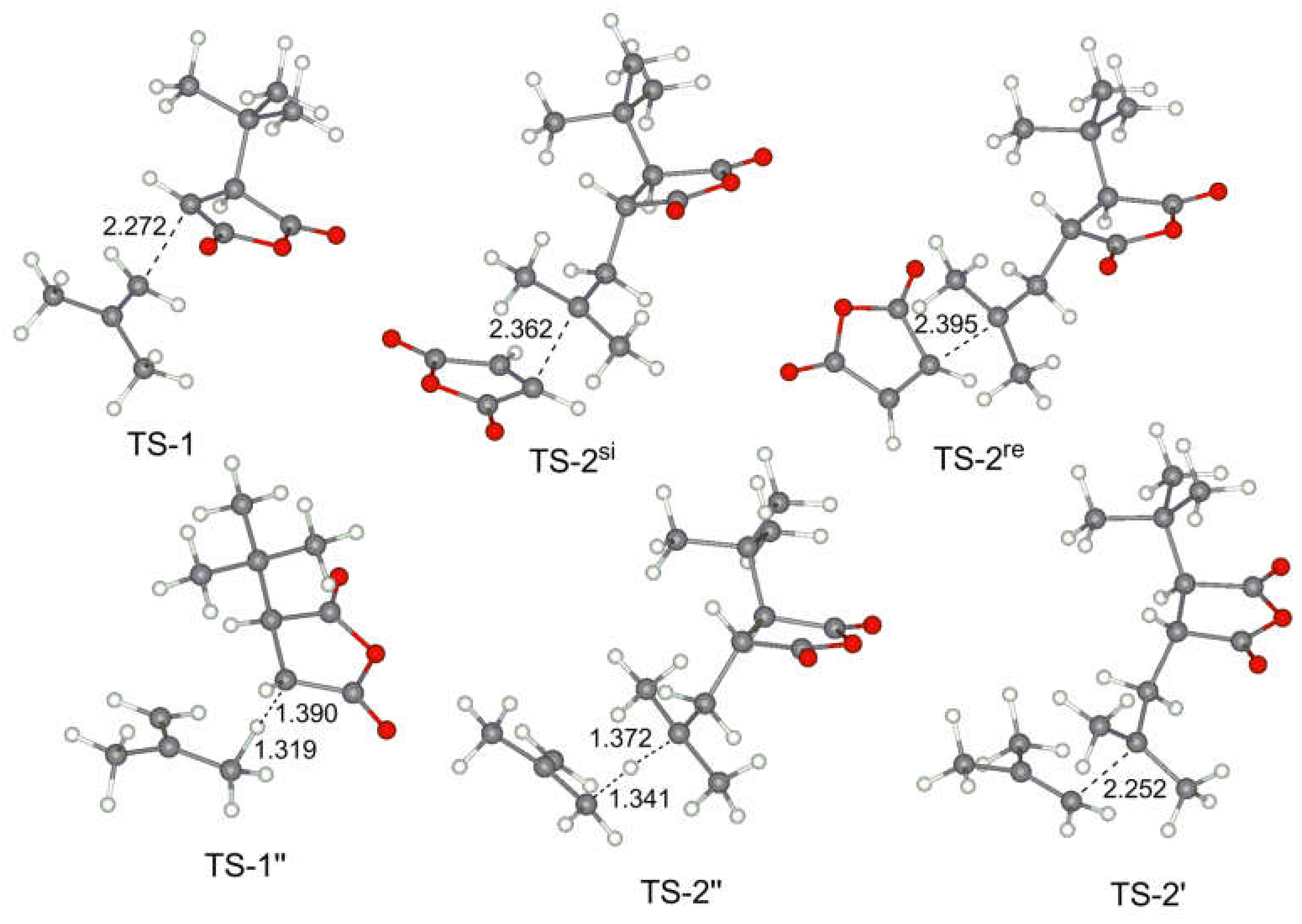
The geometries of the key transition states and stationary points for the reaction of MA with isobutylene IB are presented in Figure 5. We note that the interatomic distances in these TS correlate with relative energies, and the steric hindrance in the early transition states TS-1 with the participance of tert-alkyl radical are not significant.
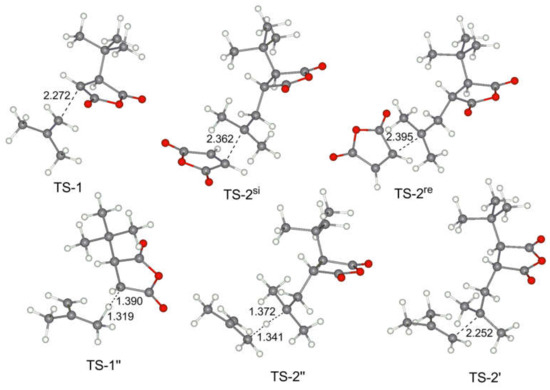
Figure 5.
Calculated geometries of the key transition states for MA–IB copolymerization.
The relative values of the transition states of hydrogen transfer from olefin to tBu-MA· (TS-1’’) and to tBu-MA-S· (TS-2’’) were only slightly higher than the values of TS-1 (Table 2), therefore, such transfer processes seem to be plausible pathways of the chain release. The calculated relative free energies of TS-3’’ for IB, OCT, and BU2 (16.4, 17.3 and 18.1 kcal/mol, respectively) indicate that allyl radical species also can initiate copolymerization.
3.4. DFT Modeling of the Copolymerization of MMI and Olefins
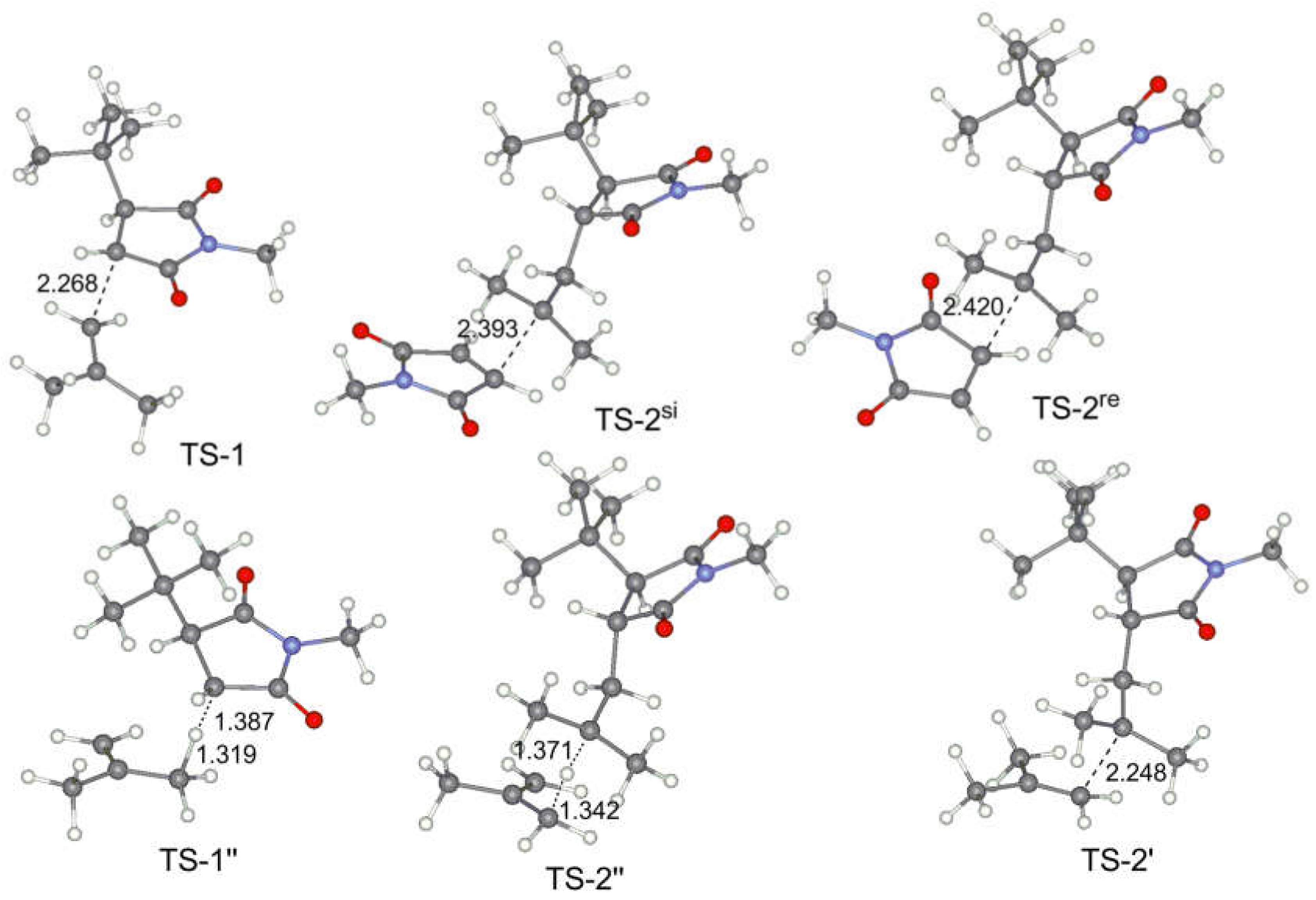
To compare the reaction ability of the close analog of maleic anhydride, N-methylmaleimide, we have chosen two olefins, namely, ethylene ET and isobutylene IB. tert-Butyl substituted radical (tBu-MMI·), closely related to tBu-MA·, was used as a model of the active polymer chain. The results of calculations are presented in Table 3 and in Figure 6.

Table 3.
Calculated free energies and free enthalpies of the key intermediated and transition states for MMI–olefin copolymerization.
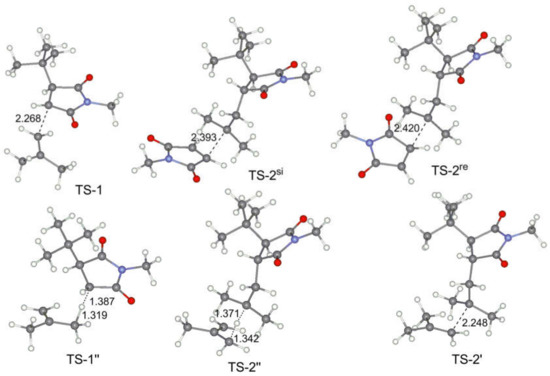
Figure 6.
Calculated geometries of the key transition states for MMI–IB copolymerization.
4. Discussion
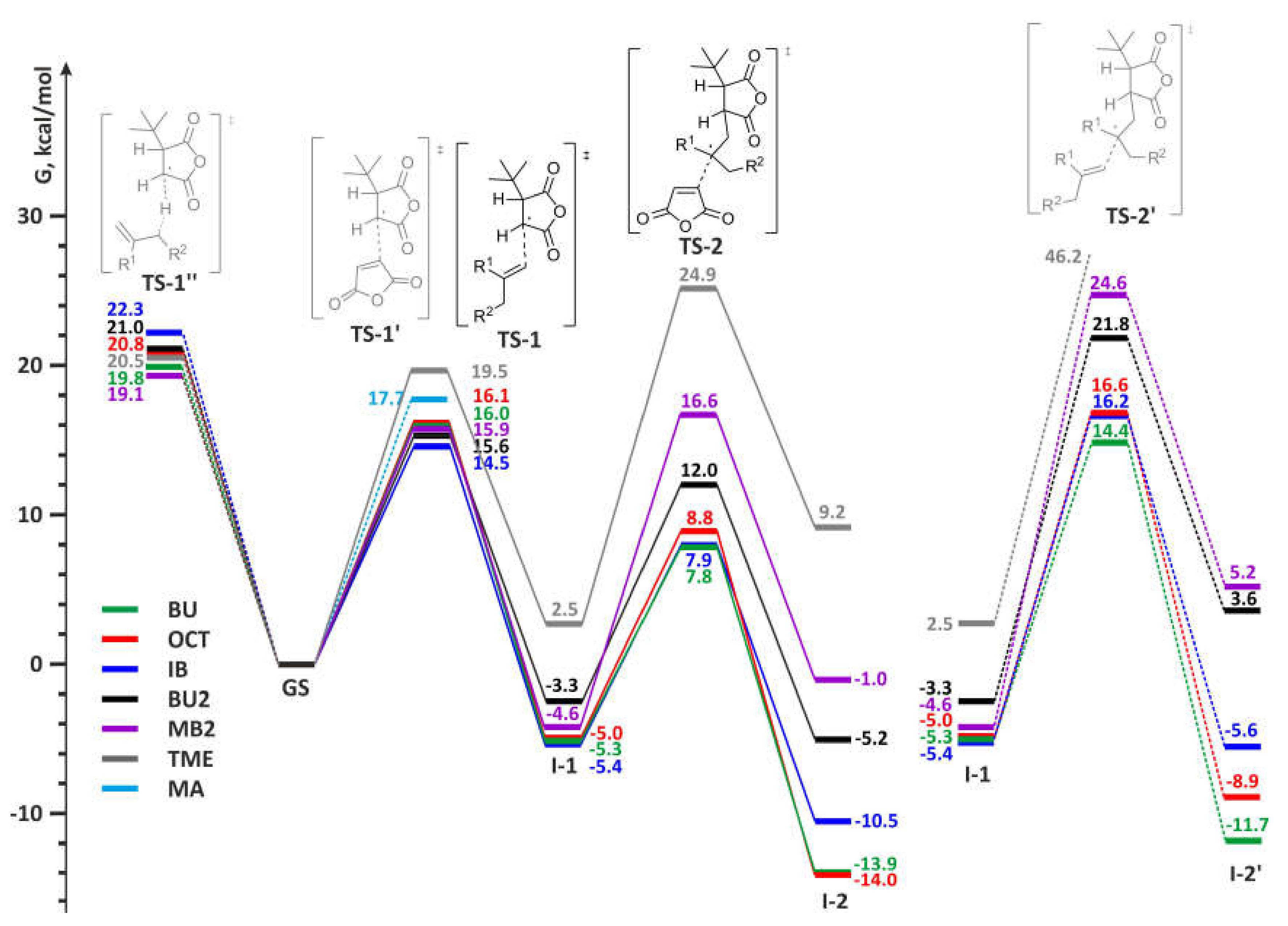
Some examples of the energy profiles for the main and side reactions during MA–olefin copolymerization are presented in Figure 7.
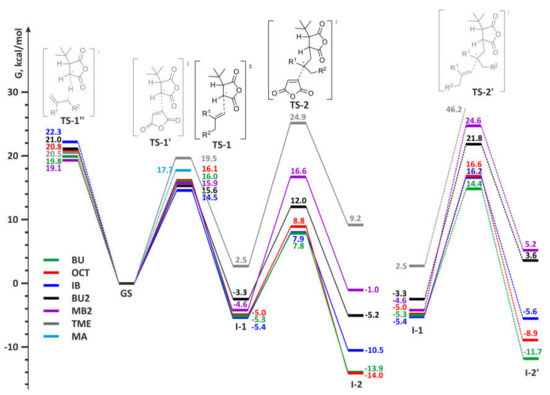
Figure 7.
Free energy profiles for main (left) and side (right) reactions during radical copolymerization of MA and olefins.
The free activation energies for the first step of alternating copolymerization, the reaction of tBu-MA· with olefins, substantially depend on the type of the olefin. This barrier is lower for isobutylene and methylenealkanes (14.5–15.9 kcal/mol), a little higher for α-olefins (16.0 kcal/mol) but considerably higher for internal olefins. For 2-butenes and tetramethylethylene the values of ΔG of TS-1 (~18.5 and 19.5 kcal/mol, respectively) exceeded the activation barrier of MA homopolymerization, therefore, the formation of copolymers for these olefins should be complicated by the formation of poly(MA) blocks. At the second stage of MA-olefin copolymerization, both MA and olefin molecule can react with alkyl radical formed at the first stage. For all olefins, the reaction with MA at the second stage is preferable, but the difference in free energies in chain propagation by MA and olefin is minimal for ethylene. The formation of 1,4-butylene fragments can therefore complicate MA-ethylene copolymerization under the high ethylene pressure. The result of the calculations are in agreement with early published data on the copolymerization of MA with different olefins [3,4,9,13,83,84], in particular, with low reactivity of 2-butenes and full inertness of TME towards MA in the presence of free-radical initiators [85].
In addition, calculated values of the activation barriers at 353 K for Alder-ene reaction (36.5 kcal/mol) and alternating polymerization for MA–BU2 pair (17.4 and 16.9 kcal/mol for BU2 and MA insertions, respectively), correspond the results of our experiments: at 80 °C, the reaction between MA and 5-methyleneundecane, close structural analog of BU2, was not observed. However, in the presence of AIBN, a copolymer with 1:1 MA/olefin ratio was formed with satisfactory yield.
From the data reported in Table 2 and Table 3 and Figure 5 and Figure 6, it can be concluded that there is no marked difference between MA and MMI in reactions with olefin molecules. However, a substantial difference was detected when the activation barriers of MA and MMI homopolymerization were compared. AS was mentioned above, for MA the addition of the olefin molecule to tBu-MA· is preferable for α-olefins and methylenealkanes, including isobutylene, and slightly preferable for ethylene and sterically hindered methylenealkane MB2. However, for MMI and IB the activation barriers of homopolymerization and copolymerization are close. Omayu and Matsumoto [75] estimated the activation barrier of the reaction of model radical Me-MMI· with IB by the value of 14.6 kcal/mol. Our estimation of the activation barrier of this stage for tBu-MMI· was 15.9 kcal/mol, but at the same time, the ΔG≠ for MMI homopolymerization was 15.8 kcal/mol.
Note that the calculated activation barrier for the reaction of tBu-MA· with IB was 14.5 kcal/mol, the activation barrier of the second step of the alternating copolymer formation was also substantially lower for MA–IB in comparison with MMI–IB (13.3 vs. 14.7 kcal/mol). Therefore, MMI fell behind MA as a comonomer in all respects.
5. Conclusions
The results of the comparative DFT calculations of the reaction profiles for maleic anhydride (MA) and different alkenes predicted the preference of the formation of alternating copolymer when MA was chosen in combination with α-olefins and methylenealkanes. For internal olefins, the formation of the alternating copolymer was found to be energetically unfavorable.
Bearing in mind the clear prospects of application for maleimide-based alternating copolymers with olefins, we analyzed the reaction profiles of the copolymerization of N-methylmaleimide (MMI) with ethylene and isobutylene. The results of our calculations predicted a higher propensity of maleimide to homopolymerization. Therefore, a more efficient and secure synthetic approach to such copolymers is, in our view, post-modification (imidization) of the alternating copolymers based on maleic anhydride.
The results of our calculations, performed for a wide range of olefins, might be used as a basis for the thorough theoretical analysis of other combinations of donor and acceptor monomers in the design of prospective macromolecules with alternating microstructures.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4360/12/4/744/s1, Figure S1: 1H NMR spectrum (CDCl3, 20 °C, 400 MHz) of the mixture of MA and 5-methylene-undecane after 2 h at 80 °C, Figure S2: 1H NMR spectrum (CDCl3, 20 °C, 400 MHz) of the copolymer of MA and 5-methyleneundecane, Figure S3: 1H NMR spectrum (CDCl3, 20 °C, 101 MHz) of the copolymer of MA and 5-methyleneundecane, Figure S4: 1H-13C correlation NMR spectrum (CDCl3, 20 °C) of the copolymer of MA and 5-methyleneundecane. Optimized geometries, energy parameters and cartesian coordinates for all stationary points and transition states.
Author Contributions
Conceptualization, P.I.; Methodology, I.N. and P.I.; Validation, A.V. (Alexander Vinogradov) and I.N.; Formal Analysis, P.I.; Investigation, A.V. (Alexander Vinogradov) and A.V. (Alexey Vinogradov); Resources, A.V. (Alexander Vinogradov); Data Curation, P.I.; Writing—Original Draft Preparation, A.V. (Alexander Vinogradov) and P.I.; Writing—Review & Editing, I.N. and P.I.; Visualization, P.I.; Supervision, I.N. and A.V. (Alexander Vinogradov); Project Administration, A.V. (Alexander Vinogradov); Funding Acquisition, A.V. (Alexander Vinogradov). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 18-73-10128, and partially supported by the TIPS RAS plan (experimental study of Alder-ene reaction and MA-olefin copolymerization).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Lutz, J.-F. Sequence-controlled polymerizations: The next Holy Grail in polymer science? Polym. Chem. 2010, 1, 55–62. [Google Scholar] [CrossRef]
- Machi, S.; Sakai, T.; Gotoda, M.; Kagiya, T. Alternating copolymerization of ethylene with maleic anhydride. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 821–828. [Google Scholar] [CrossRef]
- Komber, H. The 1H and 13C NMR spectra of an alternating ethene/maleic anhydride copolymer and the corresponding acid and sodium salt. Macromol. Chem. Phys. 1995, 196, 669–678. [Google Scholar] [CrossRef]
- Kellou, M.; Jenner, G. High pressure copolymerisation of maleic anhydride with cyclic olefins and substituted ethylenes. Makromol. Chem. 1978, 180, 1687–1696. [Google Scholar] [CrossRef]
- Kitano, T.; Kawaguchi, S.; Anazawa, N.; Minakata, A. Dissociation behavior of an alternating copolymer of isobutylene and maleic acid by potentiometric titration and intrinsic viscosity. Macromolecules 1987, 20, 2498–2506. [Google Scholar] [CrossRef]
- Chan, A.S.W.; Groves, M.; Malardier-Jugroot, C. Self-assembly of alternating copolymers and the role of hydrophobic interactions: Characterisation by molecular modelling. Mol. Simul. 2011, 37, 701–709. [Google Scholar] [CrossRef]
- Zeng, W.; Shirota, Y. Studies on alternating radical copolymerization: Analysis of microstructures of styrene-maleic anhydride, styrene-acrylonitrile, and styrene-methyl methacrylate copolymers by fluorescence spectroscopy. Macromolecules 1989, 22, 4204–4208. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Recent advances in alternating copolymers: The synthesis, modification, and applications of precision polymers. Polymer 2017, 116, 572–586. [Google Scholar] [CrossRef]
- Komber, H. The 1H and 13C NMR spectra of alternating isobutene/maleic anhydride copolymer and the corresponding acid and sodium salt—A stereochemical analysis. Macromol. Chem. Phys. 1996, 197, 343–353. [Google Scholar] [CrossRef]
- Schoukens, G.; Martins, J.; Samyn, P. Insights in the molecular structure of low- and high-molecular weight poly (styrene-maleic anhydride) from vibrational and resonance spectroscopy. Polymer 2013, 54, 349–362. [Google Scholar] [CrossRef]
- Lappalainen, E.; Koskimies, S. Preparation of alternating olefine maleic anhydride copolymers from C4–C6 hydrocarbon mixtures formed in petrochemical cracking units. J. Polym. Sci. Part C Polym. Lett. 1986, 24, 17–23. [Google Scholar] [CrossRef]
- Davis, F.; Hodge, P.; Towns, C.R.; Ali-Adib, Z. Langmuir and Langmuir-Blodgett films of derivatives of alternating copolymers of straight-chain α-olefins and maleic anhydride. Macromolecules 1991, 24, 5695–5703. [Google Scholar] [CrossRef]
- Martínez, F.; Uribe, E.; Olea, A.F. Copolymerization of Maleic Anhydride with Styrene and α-Olefins. Molecular and Thermal Characterization. J. Macromol. Sci. Part A Pure Appl. Chem. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Davies, M.C.; Dawkins, J.V.; Hourston, D.J.; Meehan, E. Molar mass determination of poly(octadecene-alt-maleic anhydride) copolymers by size exclusion chromatography and dilute solution viscometry. Polymer 2002, 43, 4311–4314. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Tinsley, J.; Adamson, D.H.; Pethica, B.A.; Huang, J.S.; Prud’homme, R.K.; Guo, X. Improvement of oil flowability by assembly of comb-type copolymers with paraffin and asphaltene. AIChE J. 2012, 58, 2254–2261. [Google Scholar] [CrossRef]
- Mazi, H.; Kibarer, G.; Emregul, E.; Rzaev, Z.M.O. Bioengineering Functional Copolymers. IX. Poly (maleic anhydride-co-hexene-1)-g-poly(ethylene oxide). Macromol. Biosci. 2006, 6, 311–321. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Hamouly, S.H.; Al Sabagh, A.M.; Gabr, M.M. Crosslinking of Reactive a-Olefins and Maleic Anhydride Copolymers as Oil Sorbers. J. Appl. Polym. Sci. 2007, 104, 871–881. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; Atta, A.M.; Kabel, K.I. Modified maleic anhydride-co-octadecene copolymers as flow improver for waxy Egyptian crude oil. J. Petrol. Sci. Eng. 2014, 122, 411–419. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Belavadi, G.; Prabhu, K.N. Preparation, Characterization and Performance Study of Poly (isobutylene-alt-maleic anhydride) [PIAM] and Polysulfone [PSf] Composite Membranes before and after Alkali Treatment. Ind. Eng. Chem. Res. 2011, 50, 6234–6528. [Google Scholar] [CrossRef]
- Osaki, T.; Werner, C. Ionization Characteristics and Structural Transitions of Alternating Maleic Acid Copolymer Films. Langmuir 2003, 19, 5787–5793. [Google Scholar] [CrossRef]
- Ku, J.-H.; Hwang, S.-S.; Ham, D.-J.; Song, M.-S.; Shon, J.-K.; Ji, S.-M.; Choi, J.-M.; Doo, S.-G. Poly (isobutylene-alt-maleic anhydride) binders containing lithium for high-performance Li-ion batteries. J. Power Sources 2015, 287, 36–42. [Google Scholar] [CrossRef]
- Li, M.-J.; Bertocchi, M.J.; Weiss, R.G. Photophysics of Pyrenyl-Functionalized Poly(isobutylene-alt-maleic anhydride) and Poly(isobutylene-alt-maleic N-alkylimide). Influence of Solvent, Degree of Substitution, and Temperature. Macromolecules 2017, 50, 1919–1929. [Google Scholar] [CrossRef]
- Thoma, J.L.; Duhamel, J.; Li, M.-J.; Bertocchi, M.J.; Weiss, R.G. Long-Range, Polymer Chain Dynamics of a Stiff Polymer. Fluorescence from Poly (isobutylene-alt-maleic anhydride) with N-(1-Pyrenylmethyl)succinimide Groups. Macromolecules 2017, 50, 3396–3403. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Parak, W.J. Design of pyridyl-modified amphiphilic polymeric ligands: Towards better passivation of water-soluble colloidal quantum dots for improved optical performance. J. Colloid Interface Sci. 2016, 478, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yao, P.; Jiang, M. Reversibility of Structural Transition of Cytochrome c on Interacting with and Releasing from Alternating Copolymers of Maleic Acid and Alkene. Biomacromolecules 2006, 7, 1829–1835. [Google Scholar] [CrossRef]
- Gao, G.; Yao, P. Structure and activity transition of lysozyme on interacting with and releasing from polyelectrolyte with different hydrophobicity. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4681–4690. [Google Scholar] [CrossRef]
- Williams, E.G.L.; Fairbanks, B.; Moad, G.; Mulder, R.J.; Rizzardo, E.; Thang, S.H. Preparation of 1:1 alternating, nucleobase-containing copolymers for use in sequence-controlled polymerization. Polym. Chem. 2017, 6, 228–232. [Google Scholar] [CrossRef]
- Paraskar, A.; Soni, S.; Basu, S.; Amarasiriwardena, C.J.; Lupoli, N.; Srivats, S.; Roy, R.S.; Sengupta, S. Rationally engineered polymeric cisplatin nanoparticles for improved antitumor efficacy. Nanotechnology 2011, 22, 265101. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Akkapeddi, P.; Manjunath, G.B.; Yarlagadda, V.; Hoque, J.; Haldar, J. Polymers with tunable side-chain amphiphilicity as non-hemolytic antibacterial agents. Chem. Commun. 2013, 49, 9389–9391. [Google Scholar] [CrossRef]
- Khan, M.; Beniah, G.; Wiradharma, N.; Guo, X.D.; Yang, Y.Y. Brush-Like Amphoteric Poly[isobutylene-alt-(maleic acid)-graft-oligoethyleneamine)]/DNA Complexes for Efficient Gene Transfection. Macromol. Rapid Commun. 2010, 31, 1142–1147. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Honold, T.; Carregal-Romero, S.; Kantner, K.; Karg, M.; Parak, W.J. Influence of Temperature on the Colloidal Stability of Polymer-Coated Gold Nanoparticles in Cell Culture Media. Small 2016, 12, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yao, P.; Jiang, M.; Zhang, G.; Yan, Y. Interactions of Apo Cytochrome c with Alternating Copolymers of Maleic Acid and Alkene. Langmuir 2005, 21, 10662–10670. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Sun, J.; Li, L.; Guo, X. How comb-type poly (maleic acid alkylamide-co-a-olefin) assemble in waxy oils and improve flowing ability. Asia Pac. J. Chem. Eng. 2009, 4, 551–556. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Noor El-Din, M.R.; Morsi, R.E.; Elsabee, M.Z. Styrene-maleic anhydride copolymer esters as flow improvers of waxy crude oil. J. Petrol. Sci. Eng. 2009, 65, 139–146. [Google Scholar] [CrossRef]
- Xu, J.; Qian, H.; Xing, S.; Li, L.; Guo, X. Synthesis of Poly(maleic acid alkylamide-co-?-olefin-co-styrene) Co-polymers and Their Effect on the Yield Stress and Morphology of Waxy Gels with Asphaltenes. Energy Fuels 2011, 25, 573–579. [Google Scholar] [CrossRef]
- Abdel-Azim, A.; Nasser, A.M.; Ahmed, N.S.; Kamal, R.S. Multifunctional Lube Oil Additives Based on Octadecene-Maleic Anhydride Copolymer. Pet. Sci. Technol. 2011, 29, 97–107. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Sun, J.; Xing, S.; Li, L.; Guo, X. Synthesis of poly (maleic acid alkylamide-co-α-olefin-co-styrene) and their effect on flow ability of oils. Front. Chem. Sci. Eng. 2011, 5, 74–78. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M. Multifunctional lube oil additives based on maleic anhydride. Pet. Sci. Technol. 2016, 34, 1761–1767. [Google Scholar] [CrossRef]
- Soni, H.P.; Kiranbala; Agrawal, K.; Nagar, A.; Bharambe, D.P. Designing maleic anhydride-α-olifin copolymeric combs as wax crystal growth nucleators. Fuel Proc. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Noor El-Din, M.R.; Morsi, R.E.; Elsabee, M.Z. Styrene-Maleic Anhydride Copolymer Esters as Flow Improvers of Waxy Crude Oil. J. Dispers. Sci. Technol. 2009, 30, 420–426. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Hamouly, S.H.; Khidr, T.T.; El-Ghazawy, R.A.; Higazy, S.A. Preparation the esters of oleic acid-maleic anhydride copolymer and their evaluation as flow improvers for waxy crude oil. J. Dispers. Sci. Technol. 2013, 34, 1585–1596. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Q.; Wei, X.; Yu, Y.; Yao, Z. Influences of the Molecular Weight and Its Distribution of Poly(styrene-alt-octadecyl maleimide) as a Flow Improver for Crude Oils. Energy Fuels 2016, 30, 2721–2728. [Google Scholar] [CrossRef]
- Cao, K.; Wei, X.; Li, B.; Zhang, J.; Yao, Z. Study of the Influence of Imidization Degree of Poly(styrene-co-octadecyl maleimide) as Waxy Crude Oil Flow Improvers. Energy Fuels 2013, 27, 640–645. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, G.; Yang, F.; Li, C.; Dong, G. Modified Maleic Anhydride Co-polymers as Pour-Point Depressants and Their Effects on Waxy Crude Oil Rheology. Energy Fuels 2012, 26, 995–1001. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Q.; Wei, X.; Yao, Z. Study on the Influence of the Imidization Degree of Poly(styrene-co-octadecyl maleimide) as a Flow Improver in Waxy Crude Oils with Asphaltenes. Energy Fuels 2015, 29, 993–1000. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Bondarenko, G.N.; Korchagina, S.A.; Shlyakhtin, A.V.; Roznyatovsky, V.A.; Ivchenko, P.V. Copolymers of Maleic Anhydride and Methylene Alkanes: Synthesis, Modification, and Pour Point Depressant Properties. Polym. Sci. Ser. B 2018, 60, 469–480. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene dimer. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Coote, M.L. Quantum-Chemical Modeling of Free-Radical Polymerization. Macromol. Theory Simul. 2009, 18, 388–400. [Google Scholar] [CrossRef]
- Dossi, M.; Storti, G.; Moscatelli, D. Quantum Chemistry: A Powerful Tool in Polymer Reaction Engineering. Macromol. Symp. 2011, 302, 16–25. [Google Scholar] [CrossRef]
- Mavroudakis, E.; Cuccato, D.; Moscatelli, D. On the Use of Quantum Chemistry for the Determination of Propagation, Copolymerization, and Secondary Reaction Kinetics in Free Radical Polymerization. Polymers 2015, 7, 9. [Google Scholar] [CrossRef]
- Megiel, E.; Kaim, A. Molecular geometry and electronic structure of molecules in free-radical copolymerization of styrene and methyl methacrylate derived from density functional calculations. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3761–3769. [Google Scholar] [CrossRef]
- Cieplak, P.; Kaim, A. Theoretical study of the free-radical copolymerization of styrene with methyl methacrylate: Comparative study to the styrene–acrylonitrile monomer system. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1557–1565. [Google Scholar] [CrossRef]
- Günaydin, H.; Salman, S.; Tüzün, N.Ş.; Avci, D.; Avïyente, V. Modeling the free radical polymerization of acrylates. Int. J. Quantum Chem. 2005, 103, 176–189. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; Brittain, D.R.B.; Hodgson, J.L.; Krenske, E.H.; Lin, C.Y.; Namazian, M.; Coote, M.L. Should Contemporary Density Functional Theory Methods Be Used to Study the Thermodynamics of Radical Reactions? J. Phys. Chem. A 2007, 111, 10754–10768. [Google Scholar] [CrossRef] [PubMed]
- Deǧirmenci, İ.; Avci, D.; Aviyente, V.; Van Cauter, K.; Van Speybroeck, V.; Waroquier, M. Density Functional Theory Study of Free-Radical Polymerization of Acrylates and Methacrylates: Structure−Reactivity Relationship. Macromolecules 2007, 40, 9590–9602. [Google Scholar] [CrossRef]
- Değirmenci, İ.; Aviyente, V.; Van Speybroeck, V.; Waroquier, M. DFT Study on the Propagation Kinetics of Free-Radical Polymerization of α-Substituted Acrylates. Macromolecules 2009, 42, 3033–3041. [Google Scholar] [CrossRef]
- Miller, M.D.; Holder, A.J. A Quantum Mechanical Study of Methacrylate Free-Radical Polymerizations. J. Phys. Chem. A 2010, 114, 10988–10996. [Google Scholar] [CrossRef]
- Değirmenci, İ.; Eren, Ş.; Aviyente, V.; De Sterck, B.; Hemelsoet, K.; Van Speybroeck, V.; Waroquier, M. Modeling the Solvent Effect on the Tacticity in the Free Radical Polymerization of Methyl Methacrylate. Macromolecules 2010, 43, 5602–5610. [Google Scholar] [CrossRef]
- Lin, C.Y.; Izgorodina, E.I.; Coote, M.L. First Principles Prediction of The Propagation Rate Coefficients of Acrylic and Vinyl Esters: Are We There Yet? Macromolecules 2010, 43, 553–560. [Google Scholar] [CrossRef]
- Yavuz, I.; Çiftçioğlu, G.A.A. DFT characterization of the first step of methyl acrylate polymerization: Performance of modern functionals in the complete basis limit. Comput. Theor. Chem. 2011, 978, 88–97. [Google Scholar] [CrossRef]
- Cuccato, D.; Mavroudakis, E.; Dossi, M.; Moscatelli, D. A Density Functional Theory Study of Secondary Reactions in n-Butyl Acrylate Free Radical Polymerization. Macromol. Theory Simul. 2013, 22, 127–135. [Google Scholar] [CrossRef]
- Dogan, B.; Catak, S.; Van Speybroeck, V.; Waroquier, M.; Aviyente, V. Free radical polymerization of ethyl methacrylate and ethyl α-hydroxy methacrylate: A computational approach to the propagation kinetics. Polymer 2012, 53, 3211–3219. [Google Scholar] [CrossRef]
- Karahan, Ö.; Aviyente, V.; Avci, D.; Zijlstra, H.; Bickelhaupt, F.M. A computational study on the reactivity enhancement in the free radical polymerization of alkyl α-hydroxymethacrylate and acrylate derivatives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 880–889. [Google Scholar] [CrossRef]
- Furuncuoğlu Özaltın, T.; Dereli, B.; Karahan, Ö.; Salman, S.; Aviyente, V. Solvent effects on free-radical copolymerization of styrene and 2-hydroxyethyl methacrylate: A DFT study. New J. Chem. 2014, 38, 170–178. [Google Scholar] [CrossRef]
- Liu, S.; Srinivasan, S.; Grady, M.C.; Soroush, M.; Rappe, A.M. Backbiting and β-scission reactions in free-radical polymerization of methyl acrylate. Int. J. Quantum Chem. 2014, 114, 345–360. [Google Scholar] [CrossRef]
- Cinar, S.A.; De Proft, F.; Avci, D.; Aviyente, V.; De Vleeschouwer, F. Relationship between the Free Radical Polymerization Rates of Methacrylates and the Chemical Properties of their Monomeric Radicals. Macromol. Chem. Phys. 2015, 216, 334–343. [Google Scholar] [CrossRef]
- Akman, B. Experimental and theoretical investigation of molecular structure, vibrational analysis, chemical reactivity, electrostatic potential of benzyl methacrylate monomer and homopolymer. Can. J. Phys. 2016, 94, 853–864. [Google Scholar] [CrossRef]
- Carlson, R.K.; Lee, R.A.; Assam, J.H.; King, R.A.; Nagel, M.L. Free-radical copolymerisation of acrylamides, acrylates, and α-olefins. J. Mol. Phys. 2015, 113, 1809–1822. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, L.; Gao, H.; Konstantinov, I.A.; Arturo, S.G.; Yu, D.; Torkelson, J.M.; Broadbelt, L.J. A Combined Computational and Experimental Study of Copolymerization Propagation Kinetics for 1-Ethylcyclopentyl methacrylate and Methyl methacrylate. Macromol. Theory Simul. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Kayık, G.; Tüzün, N.Ş. Stereoselective propagation in free radical polymerization of acrylamides: A DFT study. J. Mol. Graph. Model. 2014, 49, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kayık, G.; Tüzün, N.Ş. A Quantum Mechanical Study on the Propagation Kinetics of N-methylacrylamide: Comparison With N,N-Dimethylacrylamide in Free Radical Polymerization. Macromol. Theory Simul. 2015, 24, 218–231. [Google Scholar] [CrossRef]
- Kaim, A.; Megiel, E. Transition structures and reaction barriers in the styrene–acrylonitrile copolymerization system according to quantum mechanical calculations. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1827–1844. [Google Scholar] [CrossRef]
- Omayu, A.; Matsumoto, A. Synthesis and Thermal Properties of Alternating Copolymers of N-Methylmaleimide with Olefins Including Cyclic and Polar Groups. Macromol. Chem. Phys. 2008, 209, 2312–2319. [Google Scholar] [CrossRef]
- Tsujii, A.; Namba, M.; Okamura, H.; Matsumoto, A. Radical Alternating Copolymerization of Twisted 1,3-Butadienes with Maleic Anhydride as a New Approach for Degradable Thermosetting Resin. Macromolecules 2014, 47, 6619–6626. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Graeper, J.; Paolucci, G.; Fischer, R.D. Zirconocenophane dichlorides with di- and trisiloxane-bridged ring ligands: Crystal structure of rac-[1,1,3,3-tetramethyldisiloxane-diyl-bis(3-tert-butyl-η5- cyclopentadienyl)zirconium(IV) dichloride]. J. Organomet. Chem. 1995, 501, 211–218. [Google Scholar] [CrossRef]
- Cincu, C.; Chatxopoulos, F.; Monthéard, J.-P. Alternating Copolymers: Maleic Anhydride with Cyclic Olefins. Influence of the Cyclic Olefin on the Copolymerization Process and Properties of the Copolymers. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 83–91. [Google Scholar] [CrossRef]
- Mazi, H.; Emregul, E.; Rzaev, Z.M.O.; Kibarer, G. Preparation and properties of invertase immobilized on a poly(maleic anhydride-hexen-1) membrane. J. Biomater. Sci. Polym. Ed. 2006, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Chitanu, G.-C.; Zaharia, L.-I.; Carpov, A. Review: Analysis and Characterization of Maleic Copolymers. Int. J. Polym. Anal. Charact. 1997, 4, 1–20. [Google Scholar] [CrossRef]
- Murahashi, S.; Nozakura, S.; Yasufuku, K. The Copolymerization of Trimethylethylene and Tetramethylethylene with Maleic Anhydride. Bull. Chem. Soc. Jpn. 1966, 39, 1338–1339. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).