DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins
Abstract
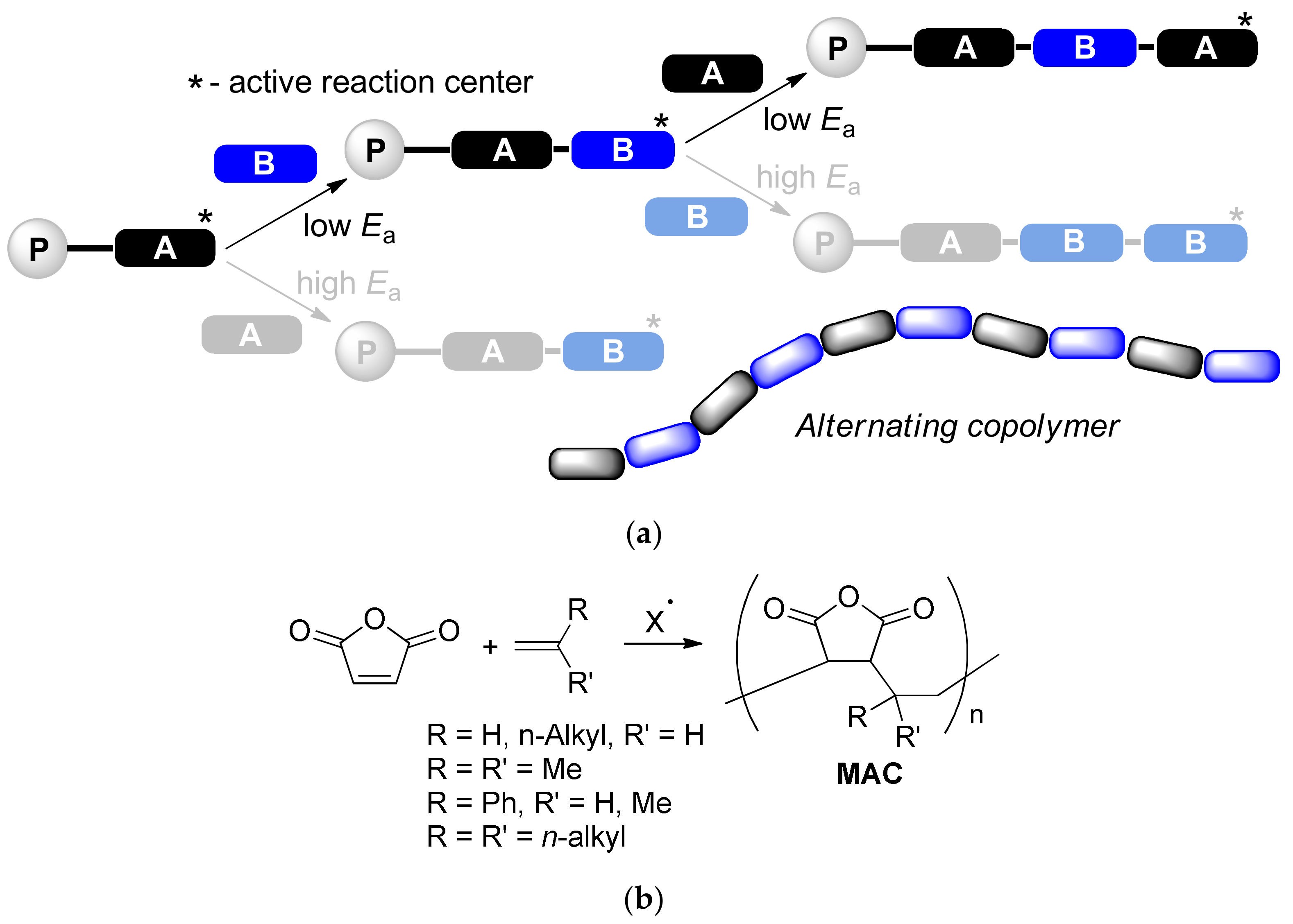
:1. Introduction
2. Materials and Methods
2.1. DFT Calculations
2.2. General Experimentsl Remarks
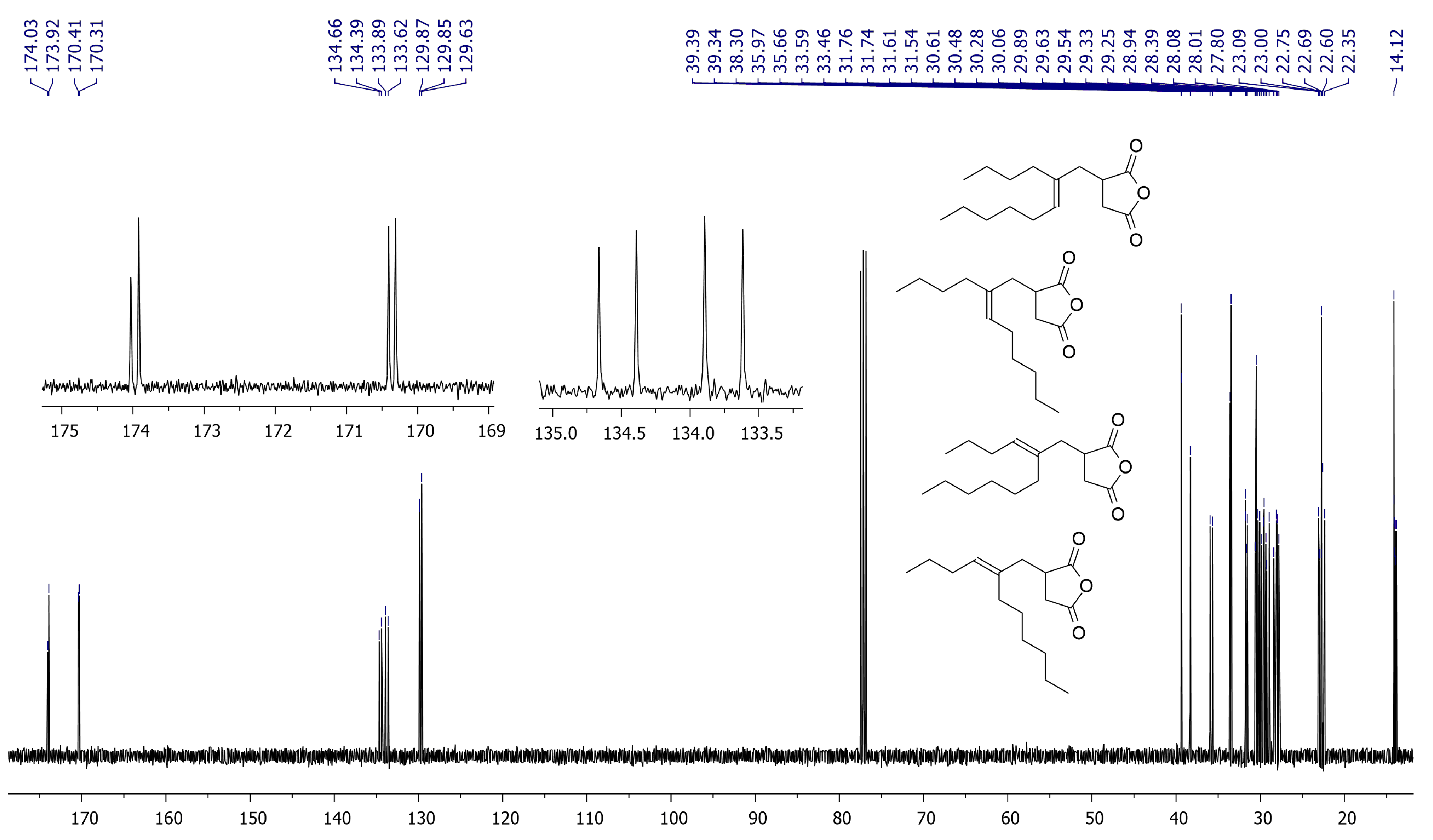
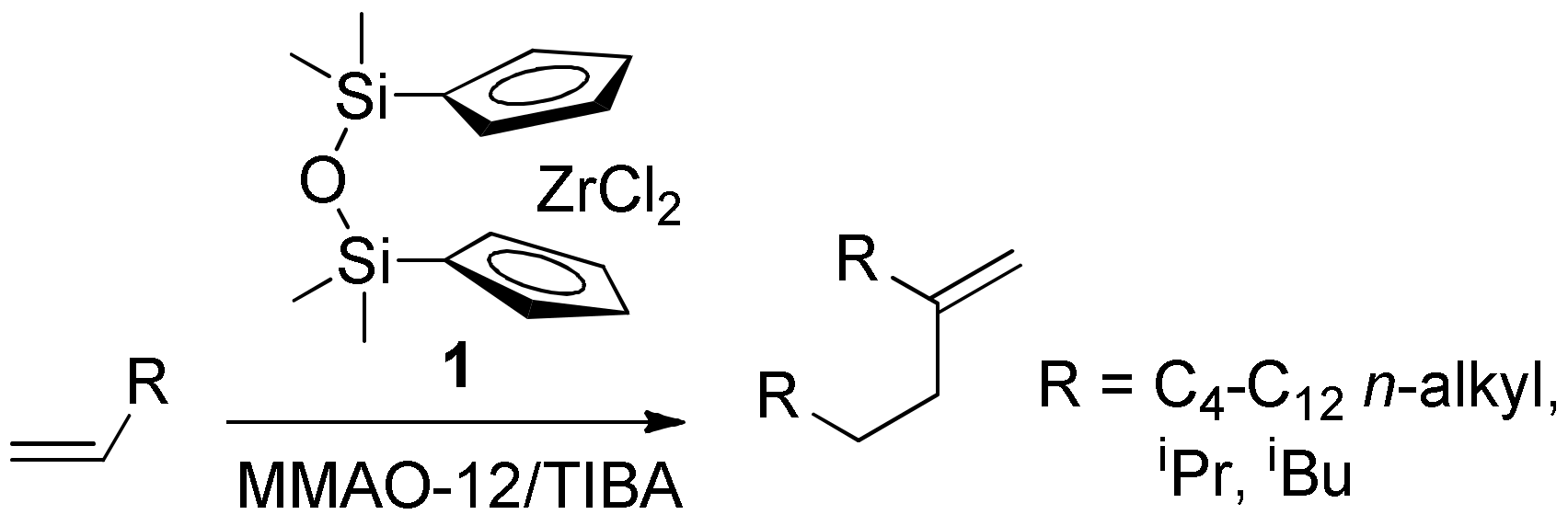
2.3. The Alder-Ene Reaction of MA with 5-Methyleneundecane
2.4. Copolymerization of MA and 5-Methyleneundecane
3. Results
3.1. MA–Olefin Association
3.2. The Alder-Ene Reaction between MA and Methylenealkanes
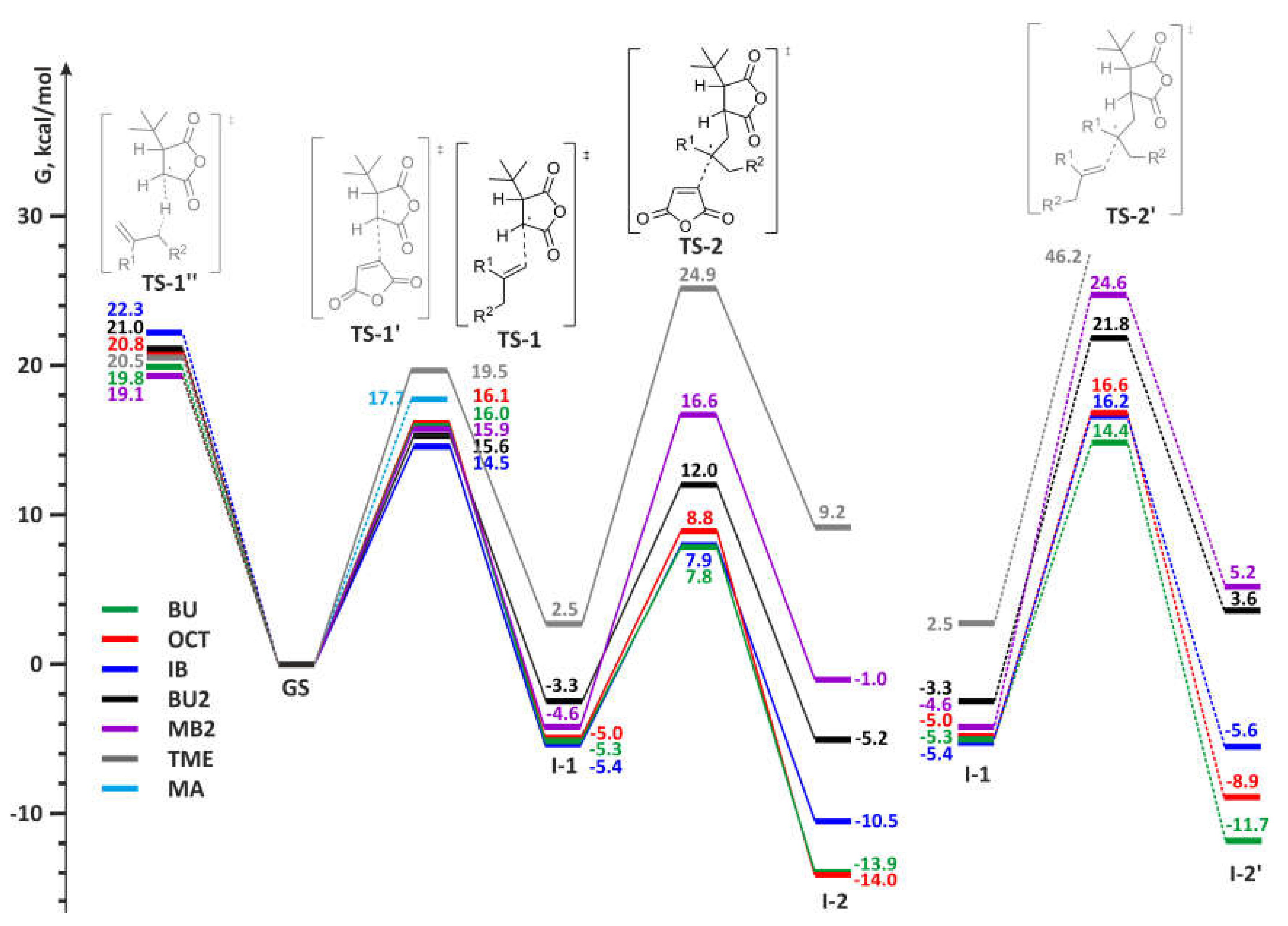
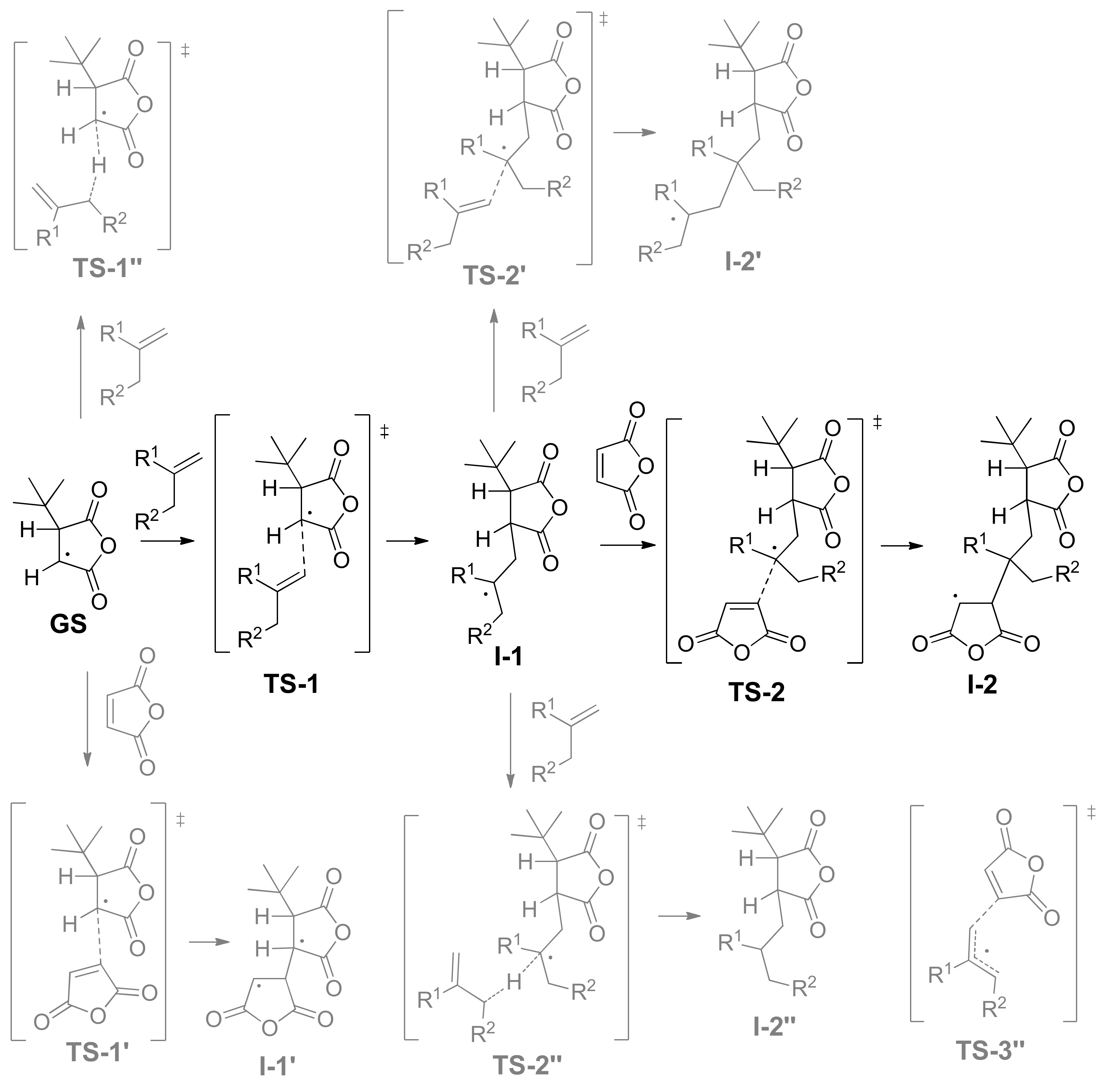
3.3. DFT Modeling of the Copolymerization of MA and Olefins
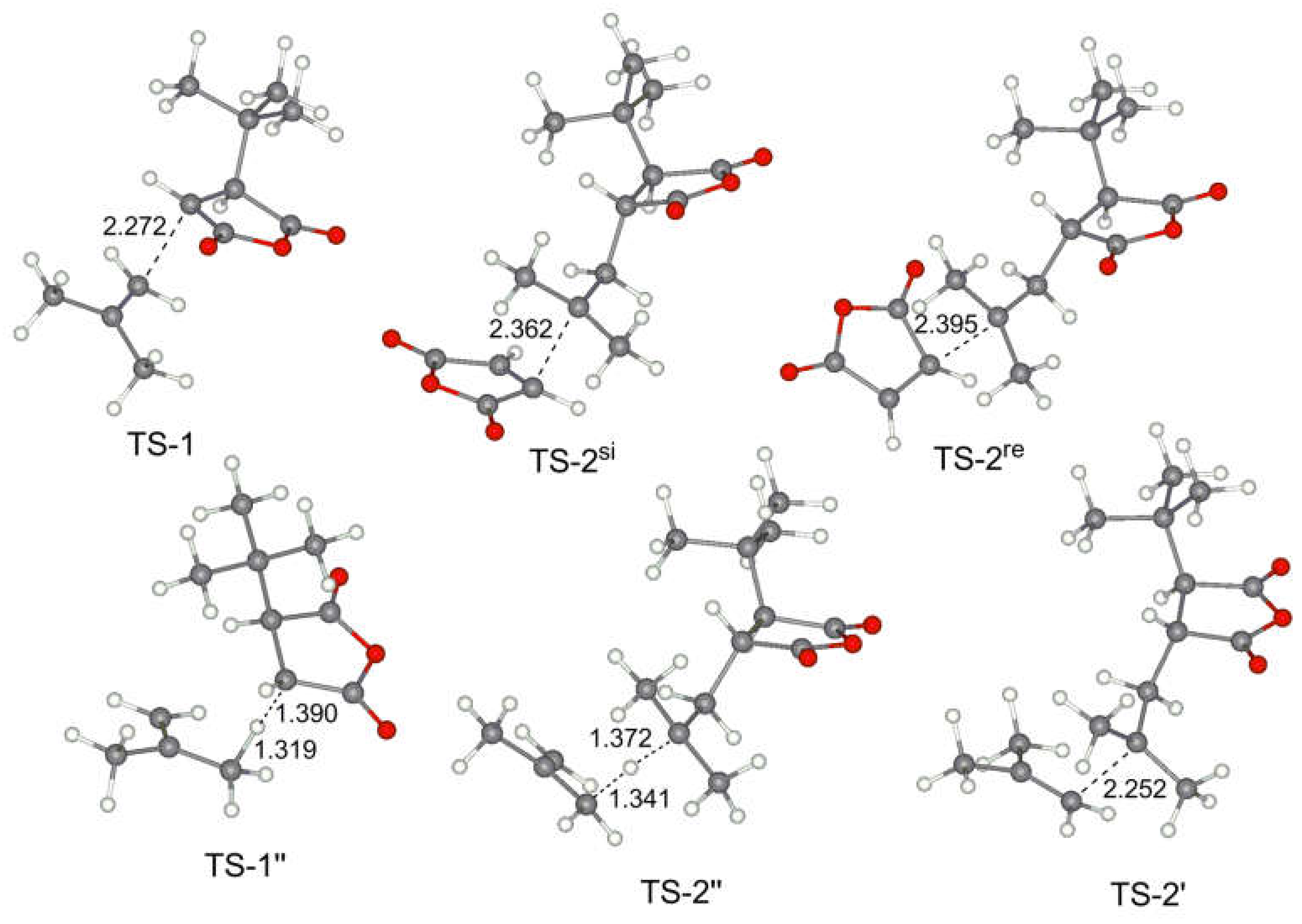
- The addition to olefin molecule via TS-1 with a formation of tBu-MA-S· species (I-1, Scheme 4). This reaction pathway represents the first step of alternating polymerization.
- The addition to MA molecule via TS-1’ with a formation of tBu-MA-MA· species (I-1’, Scheme 4). This reaction pathway mimics homopolymerization of MA. The calculated ΔG≠ and ΔH≠ for the formation of MA homopolymer were found to be 17.7 and 5.2 kcal/mol, respectively.
- The hydrogen transfer from tBu-MA· to MA molecule with a formation of 3-(tert-butyl)dihydrofuran-2,5-dione and MA-H·. The values of ΔG≠ and ΔH≠ for this reaction were found to be 32.9 and 20.8 kcal/mol, respectively.
- The transfer of the allyl hydrogen atom of the olefin molecule to tBu-MA· with a formation of allyl radical and tBu-MA-H. The energies of the corresponding TS-1’’ (Scheme 4) depend on the nature of the olefin (see Table 2). Evidently, the possibility of the further reactions of the allyl radicals should also be taken into account (see below).
- The addition to MA molecule via TS-2 with a formation of tBu-MA-S-MA· species that are structurally similar to tBu-MA·. This model reaction mimics the second step of the polymerization with a formation of the product with alternating microstructure. Therefore, the sequence tBu-MA· – tBu-MA-S· – tBu-MA-S-MA· represents an adequate model of the alternating polymerization of MA with olefins.
- The addition to olefin molecule via TS-2’ with a formation of tBu-MA-S-S· species (I-2’, Scheme 4). This is the side reaction that reflects the possibility of the formation of oligoolefin blocks.
- The transfer of the allyl hydrogen atom of the olefin molecule to tBu-MA-S· (TS-2’’) with a formation of allyl radical and tBu-MA-SH.
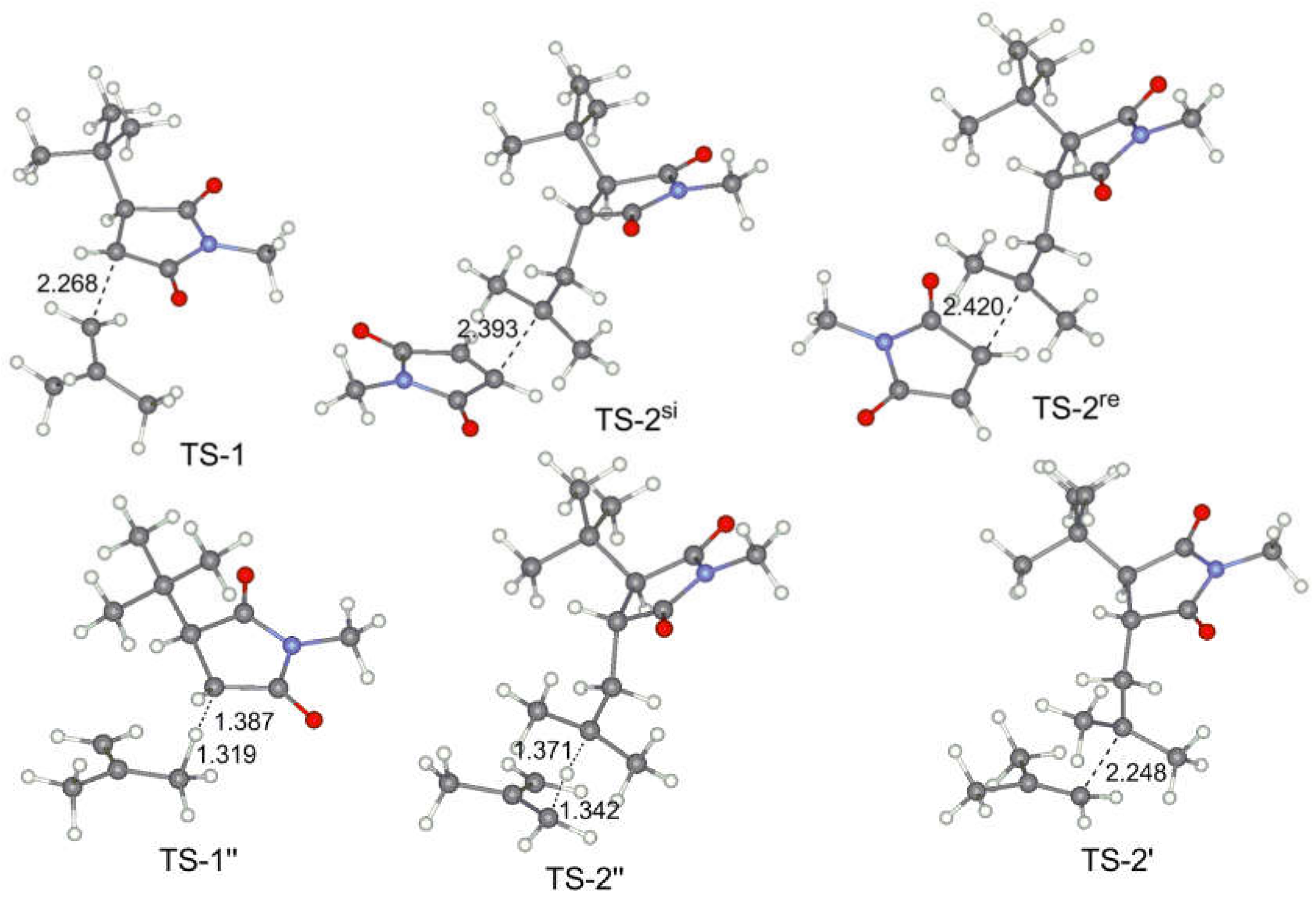
3.4. DFT Modeling of the Copolymerization of MMI and Olefins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lutz, J.-F. Sequence-controlled polymerizations: The next Holy Grail in polymer science? Polym. Chem. 2010, 1, 55–62. [Google Scholar] [CrossRef]
- Machi, S.; Sakai, T.; Gotoda, M.; Kagiya, T. Alternating copolymerization of ethylene with maleic anhydride. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 821–828. [Google Scholar] [CrossRef]
- Komber, H. The 1H and 13C NMR spectra of an alternating ethene/maleic anhydride copolymer and the corresponding acid and sodium salt. Macromol. Chem. Phys. 1995, 196, 669–678. [Google Scholar] [CrossRef]
- Kellou, M.; Jenner, G. High pressure copolymerisation of maleic anhydride with cyclic olefins and substituted ethylenes. Makromol. Chem. 1978, 180, 1687–1696. [Google Scholar] [CrossRef]
- Kitano, T.; Kawaguchi, S.; Anazawa, N.; Minakata, A. Dissociation behavior of an alternating copolymer of isobutylene and maleic acid by potentiometric titration and intrinsic viscosity. Macromolecules 1987, 20, 2498–2506. [Google Scholar] [CrossRef]
- Chan, A.S.W.; Groves, M.; Malardier-Jugroot, C. Self-assembly of alternating copolymers and the role of hydrophobic interactions: Characterisation by molecular modelling. Mol. Simul. 2011, 37, 701–709. [Google Scholar] [CrossRef]
- Zeng, W.; Shirota, Y. Studies on alternating radical copolymerization: Analysis of microstructures of styrene-maleic anhydride, styrene-acrylonitrile, and styrene-methyl methacrylate copolymers by fluorescence spectroscopy. Macromolecules 1989, 22, 4204–4208. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Recent advances in alternating copolymers: The synthesis, modification, and applications of precision polymers. Polymer 2017, 116, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Komber, H. The 1H and 13C NMR spectra of alternating isobutene/maleic anhydride copolymer and the corresponding acid and sodium salt—A stereochemical analysis. Macromol. Chem. Phys. 1996, 197, 343–353. [Google Scholar] [CrossRef]
- Schoukens, G.; Martins, J.; Samyn, P. Insights in the molecular structure of low- and high-molecular weight poly (styrene-maleic anhydride) from vibrational and resonance spectroscopy. Polymer 2013, 54, 349–362. [Google Scholar] [CrossRef]
- Lappalainen, E.; Koskimies, S. Preparation of alternating olefine maleic anhydride copolymers from C4–C6 hydrocarbon mixtures formed in petrochemical cracking units. J. Polym. Sci. Part C Polym. Lett. 1986, 24, 17–23. [Google Scholar] [CrossRef]
- Davis, F.; Hodge, P.; Towns, C.R.; Ali-Adib, Z. Langmuir and Langmuir-Blodgett films of derivatives of alternating copolymers of straight-chain α-olefins and maleic anhydride. Macromolecules 1991, 24, 5695–5703. [Google Scholar] [CrossRef]
- Martínez, F.; Uribe, E.; Olea, A.F. Copolymerization of Maleic Anhydride with Styrene and α-Olefins. Molecular and Thermal Characterization. J. Macromol. Sci. Part A Pure Appl. Chem. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Davies, M.C.; Dawkins, J.V.; Hourston, D.J.; Meehan, E. Molar mass determination of poly(octadecene-alt-maleic anhydride) copolymers by size exclusion chromatography and dilute solution viscometry. Polymer 2002, 43, 4311–4314. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Tinsley, J.; Adamson, D.H.; Pethica, B.A.; Huang, J.S.; Prud’homme, R.K.; Guo, X. Improvement of oil flowability by assembly of comb-type copolymers with paraffin and asphaltene. AIChE J. 2012, 58, 2254–2261. [Google Scholar] [CrossRef]
- Mazi, H.; Kibarer, G.; Emregul, E.; Rzaev, Z.M.O. Bioengineering Functional Copolymers. IX. Poly (maleic anhydride-co-hexene-1)-g-poly(ethylene oxide). Macromol. Biosci. 2006, 6, 311–321. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Hamouly, S.H.; Al Sabagh, A.M.; Gabr, M.M. Crosslinking of Reactive a-Olefins and Maleic Anhydride Copolymers as Oil Sorbers. J. Appl. Polym. Sci. 2007, 104, 871–881. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; Atta, A.M.; Kabel, K.I. Modified maleic anhydride-co-octadecene copolymers as flow improver for waxy Egyptian crude oil. J. Petrol. Sci. Eng. 2014, 122, 411–419. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Belavadi, G.; Prabhu, K.N. Preparation, Characterization and Performance Study of Poly (isobutylene-alt-maleic anhydride) [PIAM] and Polysulfone [PSf] Composite Membranes before and after Alkali Treatment. Ind. Eng. Chem. Res. 2011, 50, 6234–6528. [Google Scholar] [CrossRef]
- Osaki, T.; Werner, C. Ionization Characteristics and Structural Transitions of Alternating Maleic Acid Copolymer Films. Langmuir 2003, 19, 5787–5793. [Google Scholar] [CrossRef]
- Ku, J.-H.; Hwang, S.-S.; Ham, D.-J.; Song, M.-S.; Shon, J.-K.; Ji, S.-M.; Choi, J.-M.; Doo, S.-G. Poly (isobutylene-alt-maleic anhydride) binders containing lithium for high-performance Li-ion batteries. J. Power Sources 2015, 287, 36–42. [Google Scholar] [CrossRef]
- Li, M.-J.; Bertocchi, M.J.; Weiss, R.G. Photophysics of Pyrenyl-Functionalized Poly(isobutylene-alt-maleic anhydride) and Poly(isobutylene-alt-maleic N-alkylimide). Influence of Solvent, Degree of Substitution, and Temperature. Macromolecules 2017, 50, 1919–1929. [Google Scholar] [CrossRef]
- Thoma, J.L.; Duhamel, J.; Li, M.-J.; Bertocchi, M.J.; Weiss, R.G. Long-Range, Polymer Chain Dynamics of a Stiff Polymer. Fluorescence from Poly (isobutylene-alt-maleic anhydride) with N-(1-Pyrenylmethyl)succinimide Groups. Macromolecules 2017, 50, 3396–3403. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Carrion, C.; Parak, W.J. Design of pyridyl-modified amphiphilic polymeric ligands: Towards better passivation of water-soluble colloidal quantum dots for improved optical performance. J. Colloid Interface Sci. 2016, 478, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yao, P.; Jiang, M. Reversibility of Structural Transition of Cytochrome c on Interacting with and Releasing from Alternating Copolymers of Maleic Acid and Alkene. Biomacromolecules 2006, 7, 1829–1835. [Google Scholar] [CrossRef]
- Gao, G.; Yao, P. Structure and activity transition of lysozyme on interacting with and releasing from polyelectrolyte with different hydrophobicity. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4681–4690. [Google Scholar] [CrossRef]
- Williams, E.G.L.; Fairbanks, B.; Moad, G.; Mulder, R.J.; Rizzardo, E.; Thang, S.H. Preparation of 1:1 alternating, nucleobase-containing copolymers for use in sequence-controlled polymerization. Polym. Chem. 2017, 6, 228–232. [Google Scholar] [CrossRef]
- Paraskar, A.; Soni, S.; Basu, S.; Amarasiriwardena, C.J.; Lupoli, N.; Srivats, S.; Roy, R.S.; Sengupta, S. Rationally engineered polymeric cisplatin nanoparticles for improved antitumor efficacy. Nanotechnology 2011, 22, 265101. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Akkapeddi, P.; Manjunath, G.B.; Yarlagadda, V.; Hoque, J.; Haldar, J. Polymers with tunable side-chain amphiphilicity as non-hemolytic antibacterial agents. Chem. Commun. 2013, 49, 9389–9391. [Google Scholar] [CrossRef]
- Khan, M.; Beniah, G.; Wiradharma, N.; Guo, X.D.; Yang, Y.Y. Brush-Like Amphoteric Poly[isobutylene-alt-(maleic acid)-graft-oligoethyleneamine)]/DNA Complexes for Efficient Gene Transfection. Macromol. Rapid Commun. 2010, 31, 1142–1147. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Honold, T.; Carregal-Romero, S.; Kantner, K.; Karg, M.; Parak, W.J. Influence of Temperature on the Colloidal Stability of Polymer-Coated Gold Nanoparticles in Cell Culture Media. Small 2016, 12, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Yao, P.; Jiang, M.; Zhang, G.; Yan, Y. Interactions of Apo Cytochrome c with Alternating Copolymers of Maleic Acid and Alkene. Langmuir 2005, 21, 10662–10670. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Sun, J.; Li, L.; Guo, X. How comb-type poly (maleic acid alkylamide-co-a-olefin) assemble in waxy oils and improve flowing ability. Asia Pac. J. Chem. Eng. 2009, 4, 551–556. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Noor El-Din, M.R.; Morsi, R.E.; Elsabee, M.Z. Styrene-maleic anhydride copolymer esters as flow improvers of waxy crude oil. J. Petrol. Sci. Eng. 2009, 65, 139–146. [Google Scholar] [CrossRef]
- Xu, J.; Qian, H.; Xing, S.; Li, L.; Guo, X. Synthesis of Poly(maleic acid alkylamide-co-?-olefin-co-styrene) Co-polymers and Their Effect on the Yield Stress and Morphology of Waxy Gels with Asphaltenes. Energy Fuels 2011, 25, 573–579. [Google Scholar] [CrossRef]
- Abdel-Azim, A.; Nasser, A.M.; Ahmed, N.S.; Kamal, R.S. Multifunctional Lube Oil Additives Based on Octadecene-Maleic Anhydride Copolymer. Pet. Sci. Technol. 2011, 29, 97–107. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Sun, J.; Xing, S.; Li, L.; Guo, X. Synthesis of poly (maleic acid alkylamide-co-α-olefin-co-styrene) and their effect on flow ability of oils. Front. Chem. Sci. Eng. 2011, 5, 74–78. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M. Multifunctional lube oil additives based on maleic anhydride. Pet. Sci. Technol. 2016, 34, 1761–1767. [Google Scholar] [CrossRef]
- Soni, H.P.; Kiranbala; Agrawal, K.; Nagar, A.; Bharambe, D.P. Designing maleic anhydride-α-olifin copolymeric combs as wax crystal growth nucleators. Fuel Proc. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Noor El-Din, M.R.; Morsi, R.E.; Elsabee, M.Z. Styrene-Maleic Anhydride Copolymer Esters as Flow Improvers of Waxy Crude Oil. J. Dispers. Sci. Technol. 2009, 30, 420–426. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Hamouly, S.H.; Khidr, T.T.; El-Ghazawy, R.A.; Higazy, S.A. Preparation the esters of oleic acid-maleic anhydride copolymer and their evaluation as flow improvers for waxy crude oil. J. Dispers. Sci. Technol. 2013, 34, 1585–1596. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Q.; Wei, X.; Yu, Y.; Yao, Z. Influences of the Molecular Weight and Its Distribution of Poly(styrene-alt-octadecyl maleimide) as a Flow Improver for Crude Oils. Energy Fuels 2016, 30, 2721–2728. [Google Scholar] [CrossRef]
- Cao, K.; Wei, X.; Li, B.; Zhang, J.; Yao, Z. Study of the Influence of Imidization Degree of Poly(styrene-co-octadecyl maleimide) as Waxy Crude Oil Flow Improvers. Energy Fuels 2013, 27, 640–645. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, G.; Yang, F.; Li, C.; Dong, G. Modified Maleic Anhydride Co-polymers as Pour-Point Depressants and Their Effects on Waxy Crude Oil Rheology. Energy Fuels 2012, 26, 995–1001. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Q.; Wei, X.; Yao, Z. Study on the Influence of the Imidization Degree of Poly(styrene-co-octadecyl maleimide) as a Flow Improver in Waxy Crude Oils with Asphaltenes. Energy Fuels 2015, 29, 993–1000. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Bondarenko, G.N.; Korchagina, S.A.; Shlyakhtin, A.V.; Roznyatovsky, V.A.; Ivchenko, P.V. Copolymers of Maleic Anhydride and Methylene Alkanes: Synthesis, Modification, and Pour Point Depressant Properties. Polym. Sci. Ser. B 2018, 60, 469–480. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene dimer. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Coote, M.L. Quantum-Chemical Modeling of Free-Radical Polymerization. Macromol. Theory Simul. 2009, 18, 388–400. [Google Scholar] [CrossRef]
- Dossi, M.; Storti, G.; Moscatelli, D. Quantum Chemistry: A Powerful Tool in Polymer Reaction Engineering. Macromol. Symp. 2011, 302, 16–25. [Google Scholar] [CrossRef]
- Mavroudakis, E.; Cuccato, D.; Moscatelli, D. On the Use of Quantum Chemistry for the Determination of Propagation, Copolymerization, and Secondary Reaction Kinetics in Free Radical Polymerization. Polymers 2015, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Megiel, E.; Kaim, A. Molecular geometry and electronic structure of molecules in free-radical copolymerization of styrene and methyl methacrylate derived from density functional calculations. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3761–3769. [Google Scholar] [CrossRef]
- Cieplak, P.; Kaim, A. Theoretical study of the free-radical copolymerization of styrene with methyl methacrylate: Comparative study to the styrene–acrylonitrile monomer system. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1557–1565. [Google Scholar] [CrossRef]
- Günaydin, H.; Salman, S.; Tüzün, N.Ş.; Avci, D.; Avïyente, V. Modeling the free radical polymerization of acrylates. Int. J. Quantum Chem. 2005, 103, 176–189. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; Brittain, D.R.B.; Hodgson, J.L.; Krenske, E.H.; Lin, C.Y.; Namazian, M.; Coote, M.L. Should Contemporary Density Functional Theory Methods Be Used to Study the Thermodynamics of Radical Reactions? J. Phys. Chem. A 2007, 111, 10754–10768. [Google Scholar] [CrossRef] [PubMed]
- Deǧirmenci, İ.; Avci, D.; Aviyente, V.; Van Cauter, K.; Van Speybroeck, V.; Waroquier, M. Density Functional Theory Study of Free-Radical Polymerization of Acrylates and Methacrylates: Structure−Reactivity Relationship. Macromolecules 2007, 40, 9590–9602. [Google Scholar] [CrossRef]
- Değirmenci, İ.; Aviyente, V.; Van Speybroeck, V.; Waroquier, M. DFT Study on the Propagation Kinetics of Free-Radical Polymerization of α-Substituted Acrylates. Macromolecules 2009, 42, 3033–3041. [Google Scholar] [CrossRef]
- Miller, M.D.; Holder, A.J. A Quantum Mechanical Study of Methacrylate Free-Radical Polymerizations. J. Phys. Chem. A 2010, 114, 10988–10996. [Google Scholar] [CrossRef]
- Değirmenci, İ.; Eren, Ş.; Aviyente, V.; De Sterck, B.; Hemelsoet, K.; Van Speybroeck, V.; Waroquier, M. Modeling the Solvent Effect on the Tacticity in the Free Radical Polymerization of Methyl Methacrylate. Macromolecules 2010, 43, 5602–5610. [Google Scholar] [CrossRef]
- Lin, C.Y.; Izgorodina, E.I.; Coote, M.L. First Principles Prediction of The Propagation Rate Coefficients of Acrylic and Vinyl Esters: Are We There Yet? Macromolecules 2010, 43, 553–560. [Google Scholar] [CrossRef]
- Yavuz, I.; Çiftçioğlu, G.A.A. DFT characterization of the first step of methyl acrylate polymerization: Performance of modern functionals in the complete basis limit. Comput. Theor. Chem. 2011, 978, 88–97. [Google Scholar] [CrossRef]
- Cuccato, D.; Mavroudakis, E.; Dossi, M.; Moscatelli, D. A Density Functional Theory Study of Secondary Reactions in n-Butyl Acrylate Free Radical Polymerization. Macromol. Theory Simul. 2013, 22, 127–135. [Google Scholar] [CrossRef]
- Dogan, B.; Catak, S.; Van Speybroeck, V.; Waroquier, M.; Aviyente, V. Free radical polymerization of ethyl methacrylate and ethyl α-hydroxy methacrylate: A computational approach to the propagation kinetics. Polymer 2012, 53, 3211–3219. [Google Scholar] [CrossRef]
- Karahan, Ö.; Aviyente, V.; Avci, D.; Zijlstra, H.; Bickelhaupt, F.M. A computational study on the reactivity enhancement in the free radical polymerization of alkyl α-hydroxymethacrylate and acrylate derivatives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 880–889. [Google Scholar] [CrossRef]
- Furuncuoğlu Özaltın, T.; Dereli, B.; Karahan, Ö.; Salman, S.; Aviyente, V. Solvent effects on free-radical copolymerization of styrene and 2-hydroxyethyl methacrylate: A DFT study. New J. Chem. 2014, 38, 170–178. [Google Scholar] [CrossRef]
- Liu, S.; Srinivasan, S.; Grady, M.C.; Soroush, M.; Rappe, A.M. Backbiting and β-scission reactions in free-radical polymerization of methyl acrylate. Int. J. Quantum Chem. 2014, 114, 345–360. [Google Scholar] [CrossRef]
- Cinar, S.A.; De Proft, F.; Avci, D.; Aviyente, V.; De Vleeschouwer, F. Relationship between the Free Radical Polymerization Rates of Methacrylates and the Chemical Properties of their Monomeric Radicals. Macromol. Chem. Phys. 2015, 216, 334–343. [Google Scholar] [CrossRef]
- Akman, B. Experimental and theoretical investigation of molecular structure, vibrational analysis, chemical reactivity, electrostatic potential of benzyl methacrylate monomer and homopolymer. Can. J. Phys. 2016, 94, 853–864. [Google Scholar] [CrossRef]
- Carlson, R.K.; Lee, R.A.; Assam, J.H.; King, R.A.; Nagel, M.L. Free-radical copolymerisation of acrylamides, acrylates, and α-olefins. J. Mol. Phys. 2015, 113, 1809–1822. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, L.; Gao, H.; Konstantinov, I.A.; Arturo, S.G.; Yu, D.; Torkelson, J.M.; Broadbelt, L.J. A Combined Computational and Experimental Study of Copolymerization Propagation Kinetics for 1-Ethylcyclopentyl methacrylate and Methyl methacrylate. Macromol. Theory Simul. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Kayık, G.; Tüzün, N.Ş. Stereoselective propagation in free radical polymerization of acrylamides: A DFT study. J. Mol. Graph. Model. 2014, 49, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kayık, G.; Tüzün, N.Ş. A Quantum Mechanical Study on the Propagation Kinetics of N-methylacrylamide: Comparison With N,N-Dimethylacrylamide in Free Radical Polymerization. Macromol. Theory Simul. 2015, 24, 218–231. [Google Scholar] [CrossRef]
- Kaim, A.; Megiel, E. Transition structures and reaction barriers in the styrene–acrylonitrile copolymerization system according to quantum mechanical calculations. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1827–1844. [Google Scholar] [CrossRef]
- Omayu, A.; Matsumoto, A. Synthesis and Thermal Properties of Alternating Copolymers of N-Methylmaleimide with Olefins Including Cyclic and Polar Groups. Macromol. Chem. Phys. 2008, 209, 2312–2319. [Google Scholar] [CrossRef]
- Tsujii, A.; Namba, M.; Okamura, H.; Matsumoto, A. Radical Alternating Copolymerization of Twisted 1,3-Butadienes with Maleic Anhydride as a New Approach for Degradable Thermosetting Resin. Macromolecules 2014, 47, 6619–6626. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Graeper, J.; Paolucci, G.; Fischer, R.D. Zirconocenophane dichlorides with di- and trisiloxane-bridged ring ligands: Crystal structure of rac-[1,1,3,3-tetramethyldisiloxane-diyl-bis(3-tert-butyl-η5- cyclopentadienyl)zirconium(IV) dichloride]. J. Organomet. Chem. 1995, 501, 211–218. [Google Scholar] [CrossRef]
- Cincu, C.; Chatxopoulos, F.; Monthéard, J.-P. Alternating Copolymers: Maleic Anhydride with Cyclic Olefins. Influence of the Cyclic Olefin on the Copolymerization Process and Properties of the Copolymers. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 83–91. [Google Scholar] [CrossRef]
- Mazi, H.; Emregul, E.; Rzaev, Z.M.O.; Kibarer, G. Preparation and properties of invertase immobilized on a poly(maleic anhydride-hexen-1) membrane. J. Biomater. Sci. Polym. Ed. 2006, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Chitanu, G.-C.; Zaharia, L.-I.; Carpov, A. Review: Analysis and Characterization of Maleic Copolymers. Int. J. Polym. Anal. Charact. 1997, 4, 1–20. [Google Scholar] [CrossRef]
- Murahashi, S.; Nozakura, S.; Yasufuku, K. The Copolymerization of Trimethylethylene and Tetramethylethylene with Maleic Anhydride. Bull. Chem. Soc. Jpn. 1966, 39, 1338–1339. [Google Scholar] [CrossRef] [Green Version]
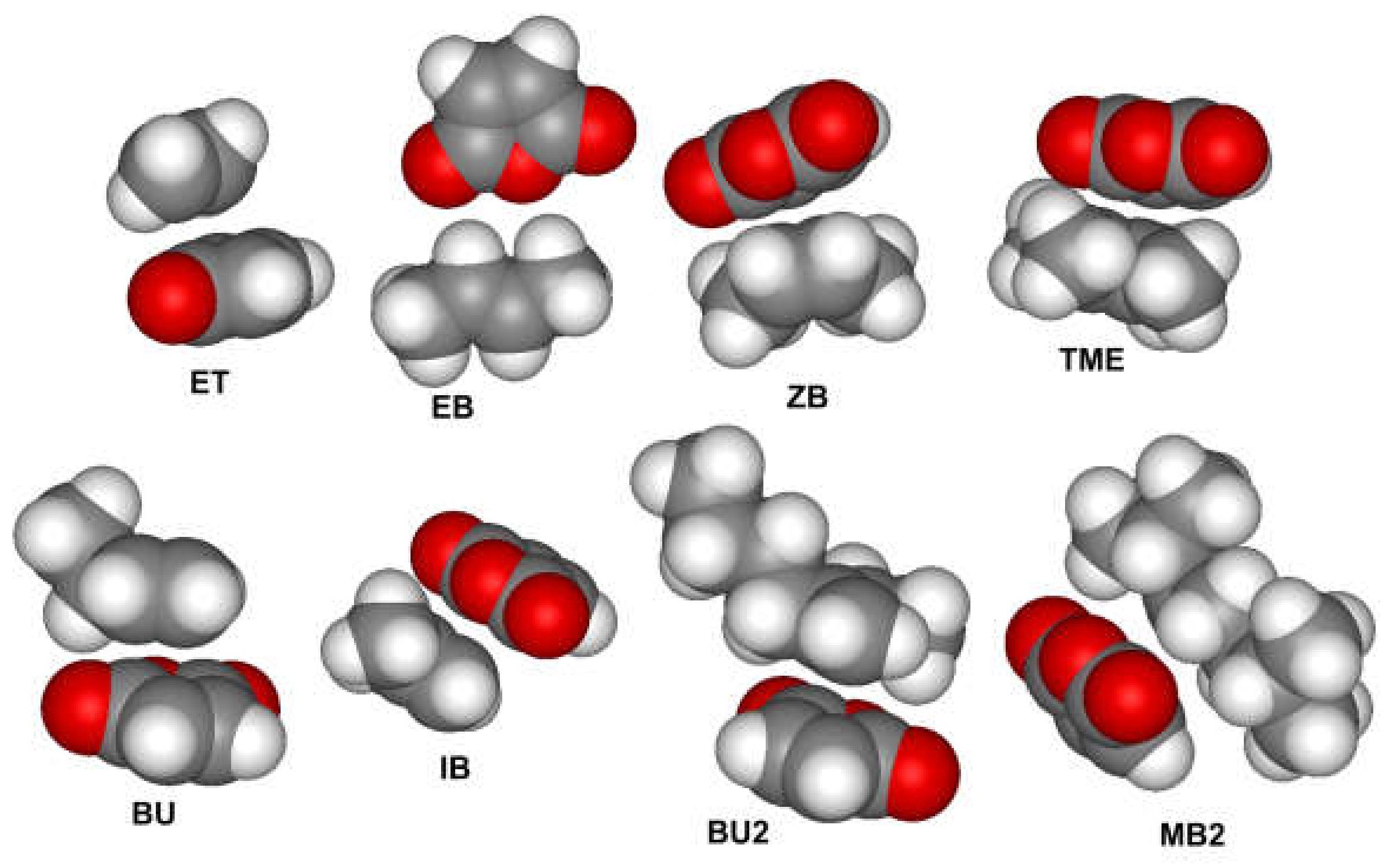



| Olefin | ΔGf | ΔHf |
|---|---|---|
| ET | 5.80 | –0.22 |
| Bu | 6.97 | –0.61 |
| EB | 6.79 | –0.81 |
| ZB | 7.02 | –0.42 |
| IB | 7.09 | –1.05 |
| TME | 8.10 | –0.46 |
| BU2 | 7.67 | –1.05 |
| MB2 | 6.52 | –0.82 |
| Olefin | TS-1 | I-1 | TS-2 | I-2 | TS-2’ | I-2’ | TS-1’’ | TS-2’’ | |
|---|---|---|---|---|---|---|---|---|---|
| ET | ΔG | 17.21 | –5.14 | 7.21 | –22.84 | 10.77 | –19.78 | – | – |
| ΔH | 5.77 | –16.98 | –16.27 | –47.70 | –11.71 | –42.64 | – | – | |
| BU | ΔG | 16.05 | –5.34 | 7.82 | –13.95 | 14.49 | –11.69 | 19.84 | 16.79 |
| ΔH | 4.49 | –16.97 | –16.40 | –39.87 | -9.48 | –35.86 | 8.51 | –6.99 | |
| OCT | ΔG | 16.10 | –4.96 | 8.75 | –13.98 | 16.58 | –8.94 | 20.76 | 19.59 |
| ΔH | 4.35 | –16.94 | –15.99 | --40.05 | –8.43 | –36.57 | 8.64 | –5.06 | |
| ZB | ΔG | 18.70 | –0.52 | 11.72 | –11.11 | 20.12 | –1.89 | 20.80 | – |
| ΔH | 5.83 | –13.86 | –14.36 | –37.71 | –3.11 | –28.33 | 9.08 | – | |
| EB | ΔG | 18.47 | –1.24 | 12.15 | –12.15 | 21.82 | –0.40 | 20.90 | – |
| ΔH | 6.15 | –13.70 | –13.38 | –38.45 | –3.49 | –26.98 | 9.56 | – | |
| IB | ΔG | 14.50 | –5.41 | 7.86 | –10.50 | 16.20 | –5.57 | 22.25 | 20.39 |
| ΔH | 2.96 | –17.26 | –17.95 | –37.20 | –9.24 | –31.30 | 11.13 | –3.92 | |
| BU2 | ΔG | 15.57 | –3.33 | 11.98 | –5.15 | 21.76 | 3.56 | 21.03 | 24.85 |
| ΔH | 3.03 | –16.30 | –14.86 | –32.71 | –4.31 | –24.61 | 8.90 | –1.49 | |
| MB2 | ΔG | 15.93 | –4.60 | 16.41 | –1.00 | 24.57 | 5.19 | 19.06 | – |
| ΔH | 3.22 | –16.76 | –10.45 | –28.30 | –2.53 | –22.73 | 7.58 | – | |
| TME | ΔG | 19.47 | 2.49 | 23.94 | 9.17 | 46.60 | 34.28 | 20.47 | – |
| ΔH | 6.80 | –11.81 | –4.52 | –20.80 | 16.33 | 3.01 | 9.86 | – | |
| MA | ΔG | 17.71 1 | –5.70 1 | – | – | 32.88 2 | – | – | – |
| ΔH | 5.54 1 | –18.97 1 | – | – | 20.78 2 | – | – | – |
| Olefin | TS-1 | I-1 | TS-2 | I-2 | TS-1’’ | TS-2’ | I-2’ | |
|---|---|---|---|---|---|---|---|---|
| ET | ΔG | 18.00 | –5.22 | 7.57 | –25.98 | – | 11.19 | –19.50 |
| ΔH | 6.68 | –17.53 | –16.42 | –51.07 | – | –12.05 | –42.99 | |
| IB | ΔG | 15.92 | –5.48 | 9.21 | –12.95 | 23.39 | 15.54 | –6.30 |
| ΔH | 4.52 | –17.48 | –17.41 | -40.15 | 11.99 | –9.46 | –31.42 | |
| MMI | ΔG | 15.82 1 | –10.27 1 | – | – | – | 32.76 2 | – |
| ΔH | 4.34 1 | –23.50 1 | – | – | – | 20.72 2 | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Ivchenko, P. DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins. Polymers 2020, 12, 744. https://doi.org/10.3390/polym12040744
Nifant’ev I, Vinogradov A, Vinogradov A, Ivchenko P. DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins. Polymers. 2020; 12(4):744. https://doi.org/10.3390/polym12040744
Chicago/Turabian StyleNifant’ev, Ilya, Alexander Vinogradov, Alexey Vinogradov, and Pavel Ivchenko. 2020. "DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins" Polymers 12, no. 4: 744. https://doi.org/10.3390/polym12040744
APA StyleNifant’ev, I., Vinogradov, A., Vinogradov, A., & Ivchenko, P. (2020). DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins. Polymers, 12(4), 744. https://doi.org/10.3390/polym12040744








