Biofunctionalization of Textile Materials. 2. Antimicrobial Modification of Poly(lactide) (PLA) Nonwoven Fabricsby Fosfomycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
2.1.2. Chemical Agents
2.1.3. Finishing Agents
2.1.4. Bacterial Strains
2.2. Methods
2.2.1. Nonwoven Fabrics
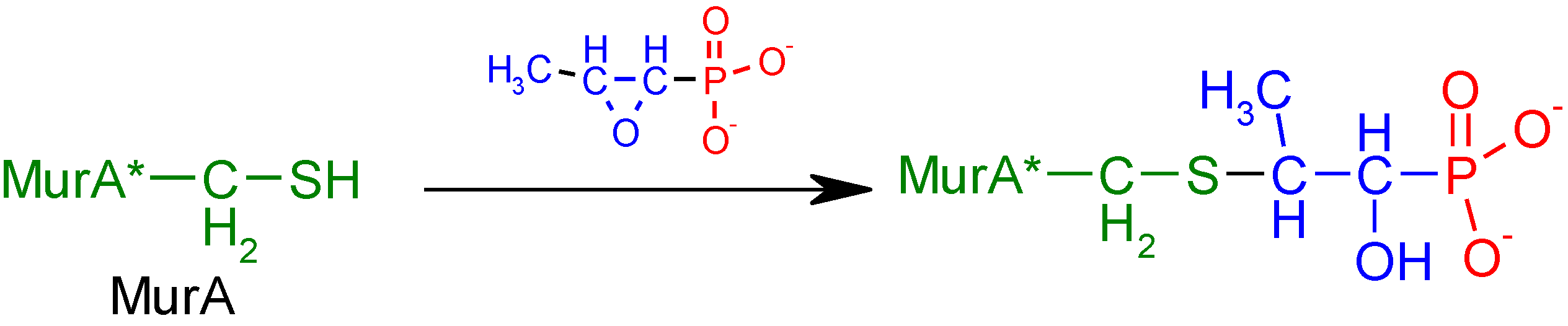
2.2.2. Dip-Coating Modification of Poly(lactic acid) Nonwoven
2.2.3. SEM—Scanning Electron Microscopy
2.2.4. ATR-FTIR—Attenuated Total Reflection Fourier Transform Infrared Spectroscopy
2.2.5. UV-VIS Analysis
2.2.6. Filtration Parameters
2.2.7. Tensile Testing
2.2.8. Microbial Activity
3. Resultsand Discussion
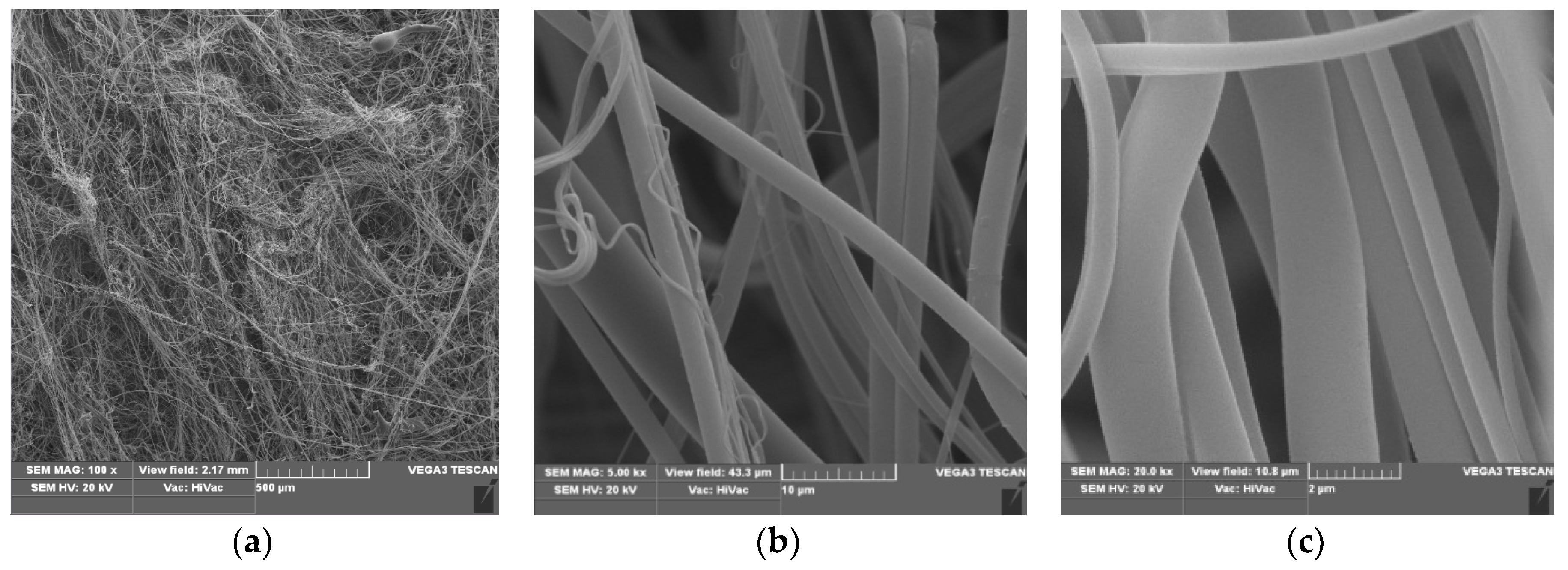
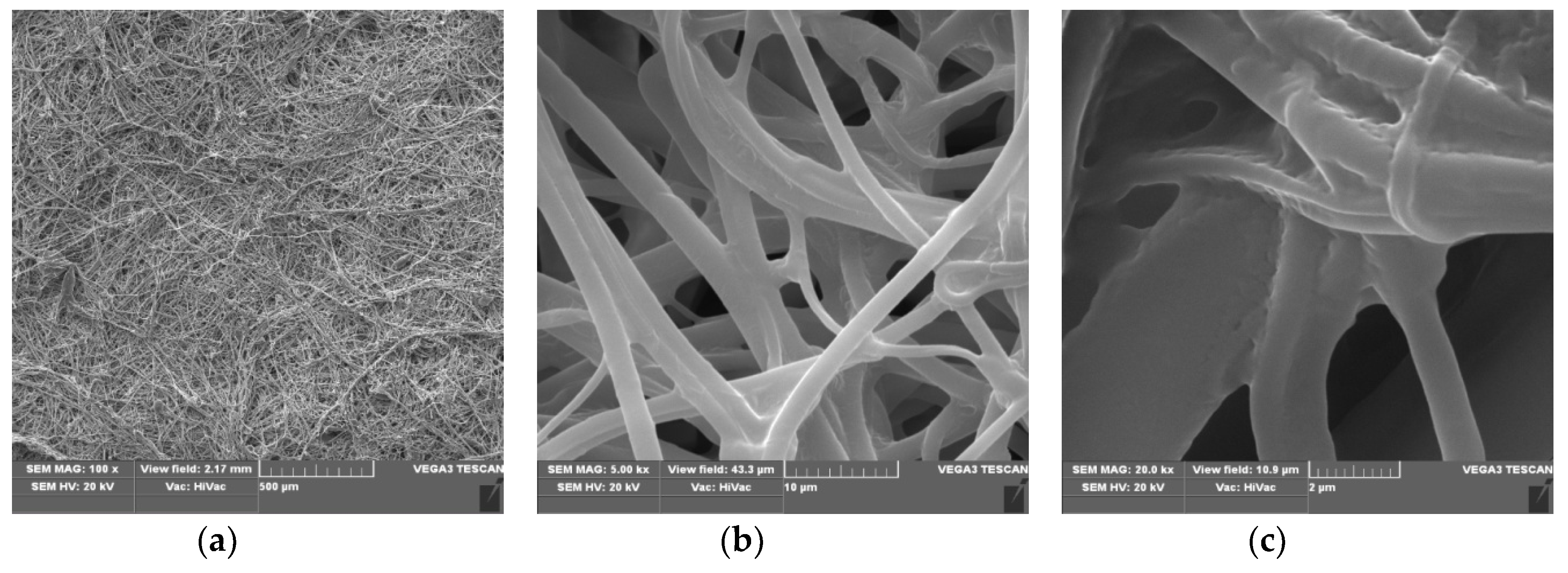
3.1. Scanning Electron Microscopy
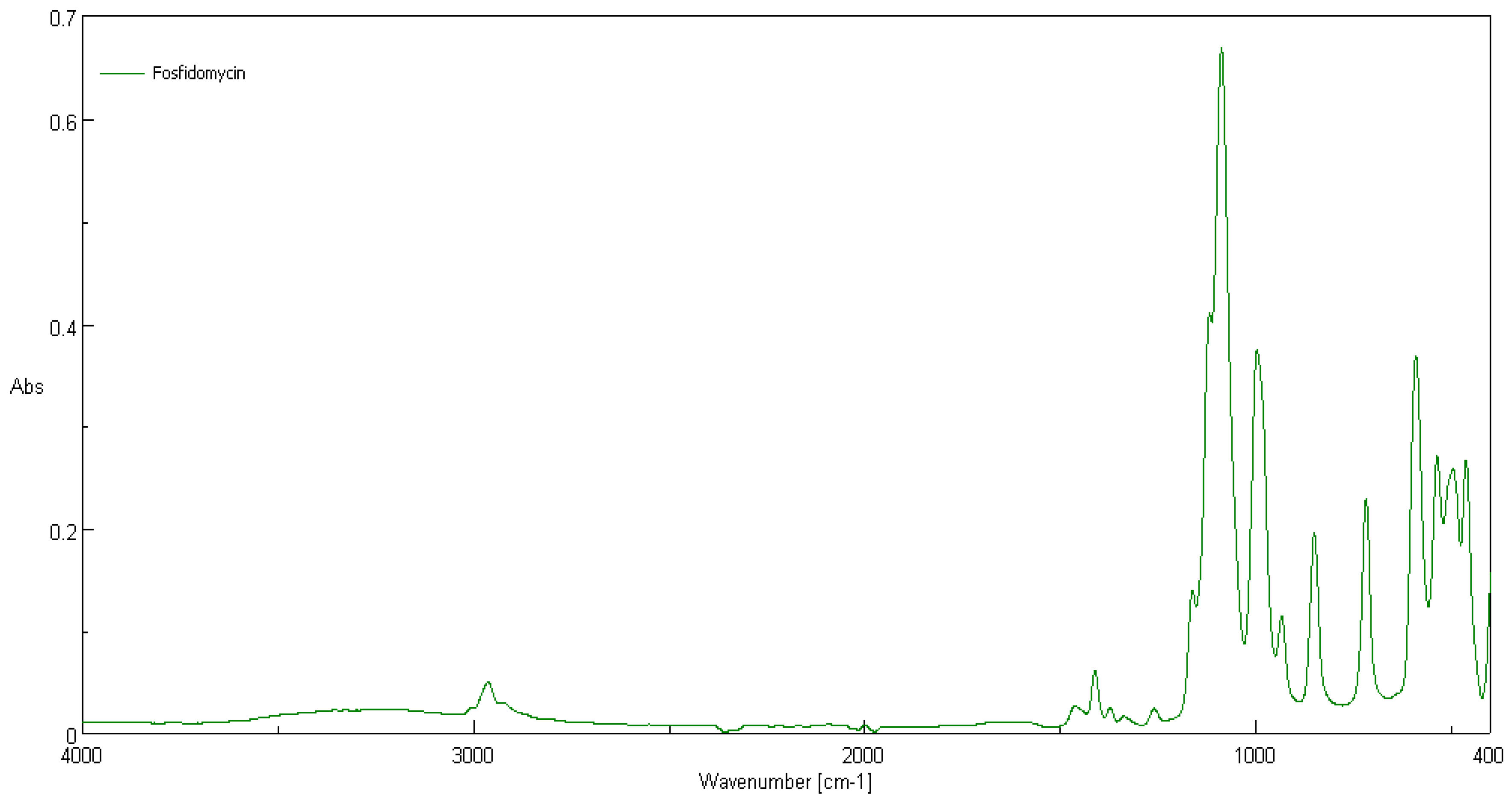
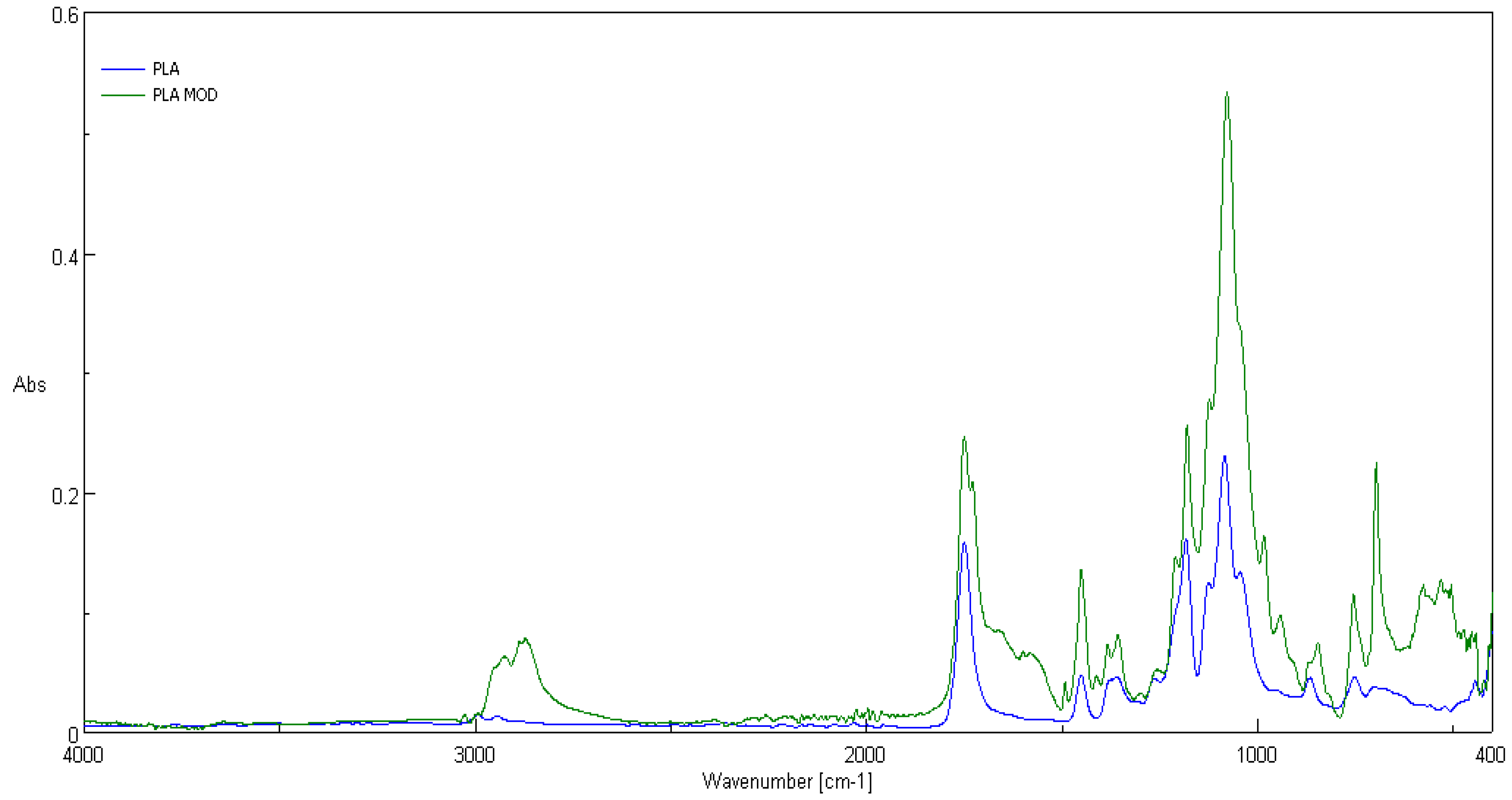
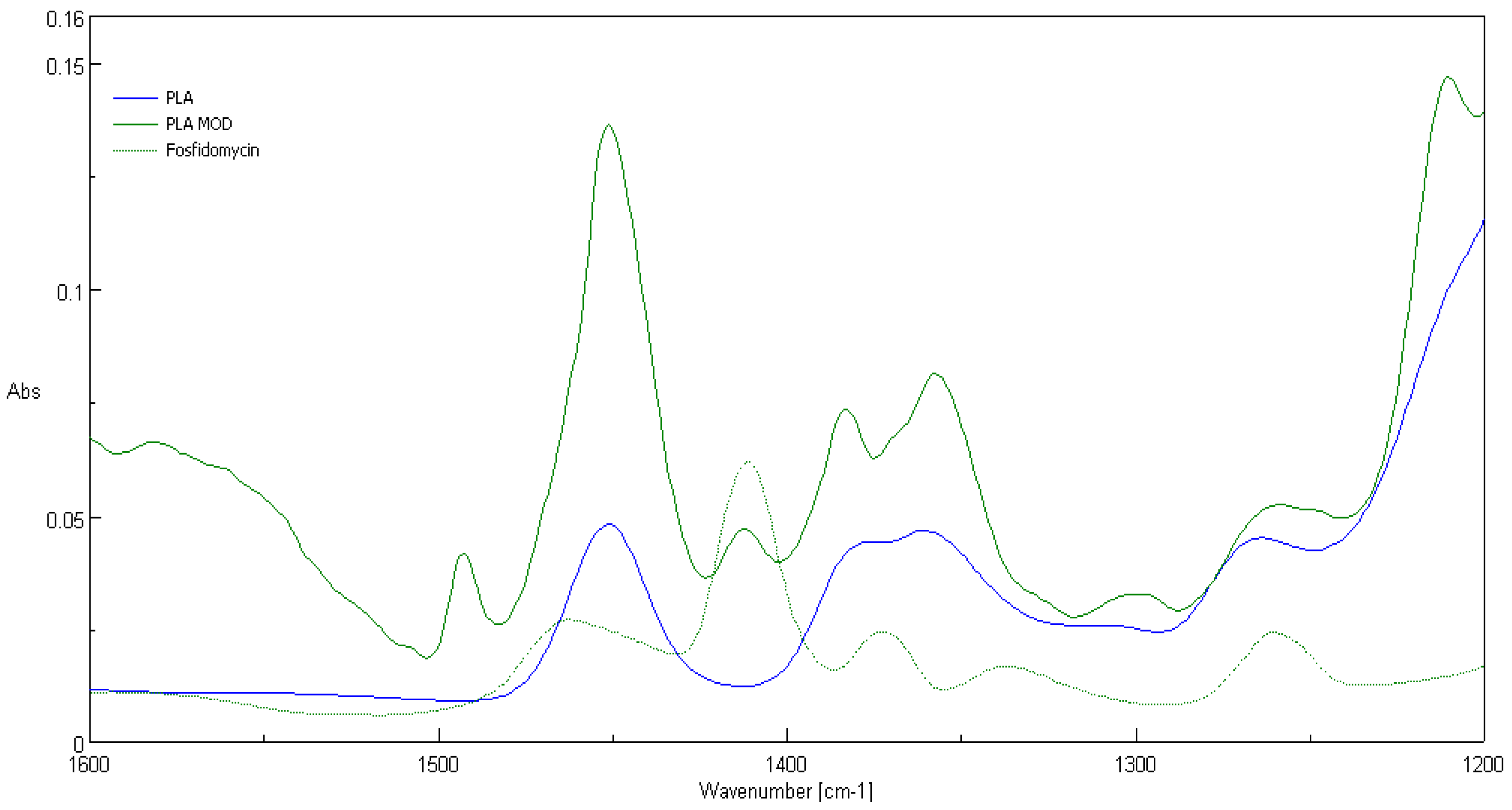
3.2. ATR-FTIR Spectra
3.3. UV/VIS Transmittance Spectra
3.4. Technical Parameters
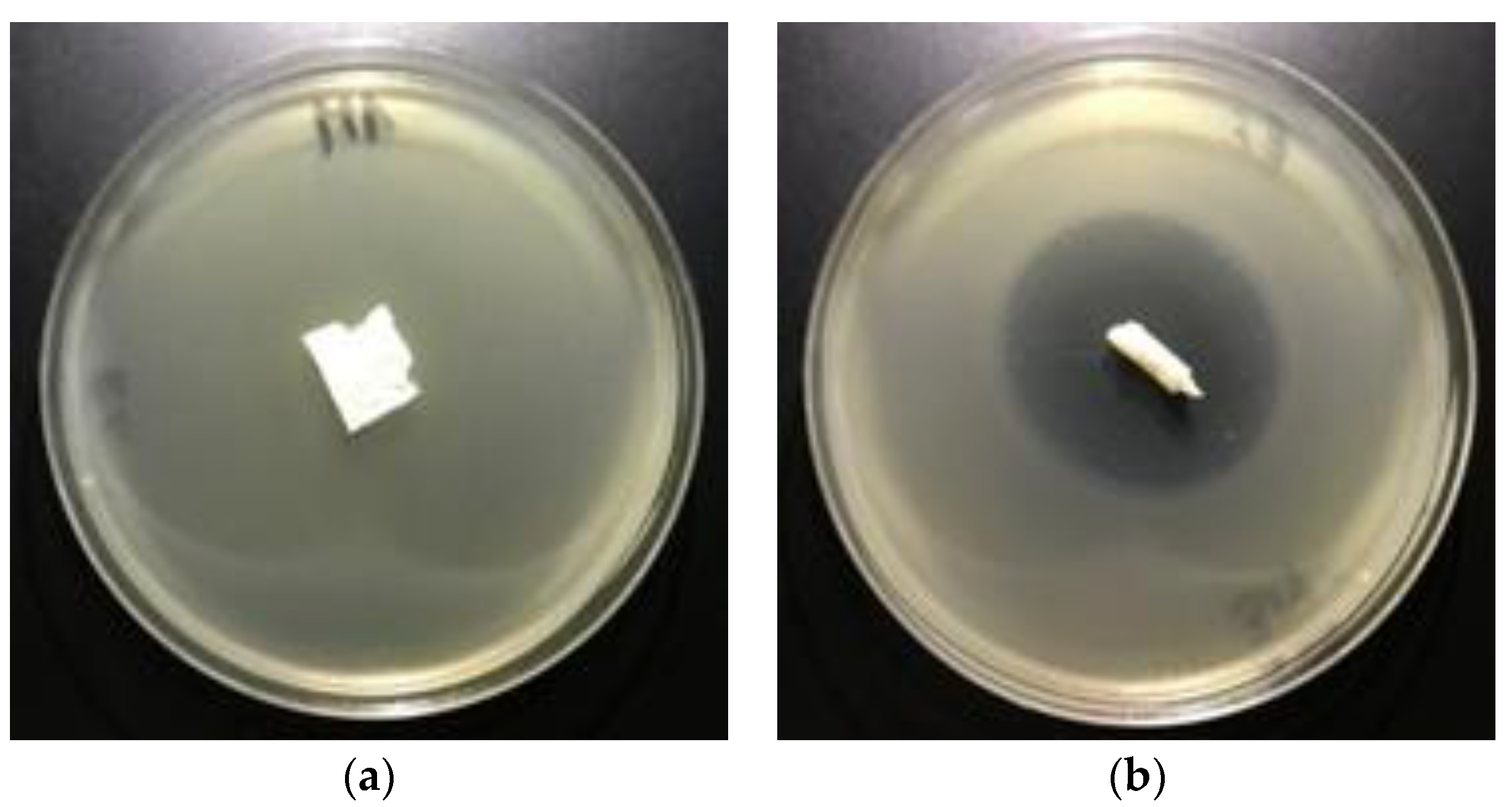
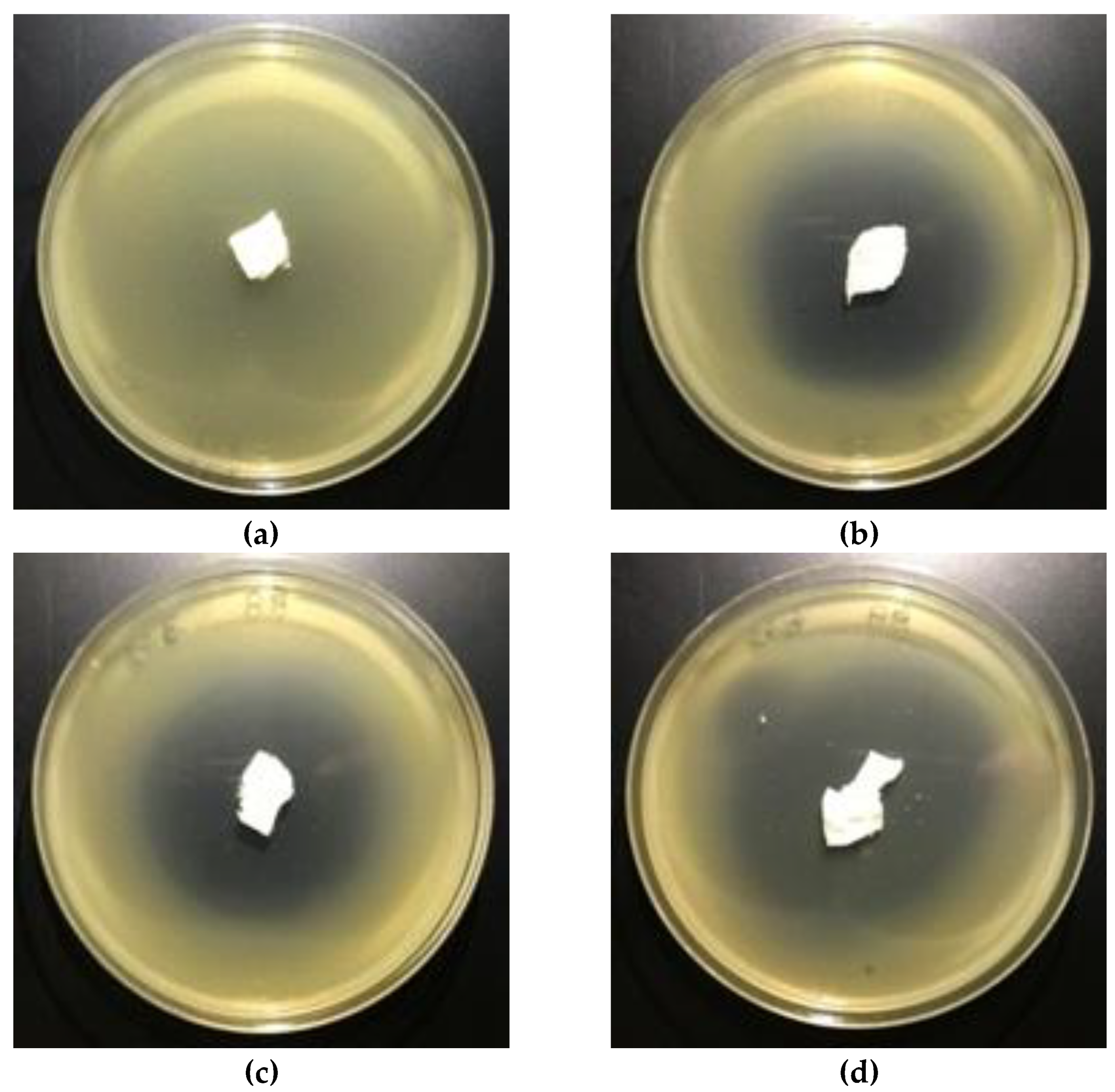
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. (Eds.) Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Valle, L.J.; Camps, R.; Díaz, A.; Alfonso, L.F.; Rodríguez-Galán, A.; Puiggalí, J. Electrospinning of polylactide and polycaprolactone mixtures for preparation of materials with tunable drug release properties. J. Polym. Res. 2011, 18, 1903–1917. [Google Scholar] [CrossRef]
- Reise, M.; Wyrwa, R.; Müller, U.; Zylinski, M.; Voelpel, A.; Schnabelrauch, M.; Berg, A.; Jandt, K.D.; Watts, D.C.; Sigusch, B.W. Release of metronidazole from electrospun poly(l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent. Mater. 2012, 28, 179–188. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, M.; Li, L.; Li, W.; Xue, J. Formulation and evaluation of in situ forming PLA implant containing tinidazole for the treatment of periodontitis. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2197–2202. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled delivery of gentamicin antibiotic from bioactive electrospun polylactide-based ultrathin fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Kau, Y.C.; Chen, D.W.C.; Hsieh, Y.T.; Lee, F.Y.; Liu, S.J. Compression molding of biodegradable drug-eluting implants for sustained release of metronidazole and doxycycline. J. Appl. Polym. Sci. 2013, 127, 554–560. [Google Scholar] [CrossRef]
- Zhou, J.; Han, S.; Dou, Y.; Lu, J.; Wang, C.; He, H.; Li, X.; Zhang, J. Nanostructured poly(l-lactide) matrix as novel platform for drug delivery. Int. J. Pharm. 2013, 448, 175–188. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Wu, T.; Zhao, X.; Liu, S.; Li, G.; Cui, W.; Fan, C. Silver nanoparticles/ibuprofen-loaded poly(L-lactide) fibrous membrane: Anti-infection and anti-adhesion effects. Int. J. Mol. Sci. 2014, 15, 14014–14025. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Madrigal, M.M.; Llorens, E.; Del Valle, L.J.; Puiggali, J.; Armelin, E.; Alemán, C. Semiconducting, biodegradable and bioactive fibers for drug delivery. Express Polym. Lett. 2016, 10, 628–646. [Google Scholar] [CrossRef]
- Schkarpetkin, D.; Reise, M.; Wyrwa, R.; Völpel, A.; Berg, A.; Schweder, M.; Schnabelrauch, M.; Watts, D.C.; Sigusch, B.W. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent. Mater. 2016, 32, 951–960. [Google Scholar] [CrossRef]
- Yakub, G.; Toncheva, A.; Manolova, N.; Rashkov, I.; Danchev, D.; Kussovski, V. Electrospun polylactide-based materials for curcumin release: Photostability, antimicrobial activity, and anticoagulant effect. J. Appl. Polym. Sci. 2016, 133, 42940. [Google Scholar] [CrossRef]
- Herrera, M.T.; Artunduaga, J.J.; Ortiz, C.C.; Torres, R.G. Synthesis of antibiotic loaded polylactic acid nanoparticles and their antibacterial activity against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus. Biomedica 2017, 37, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaszewska, A.; Kamińska, I.; Kiwała, M.; Gadzinowski, M.; Gosecki, M.; Słomkowski, S. Preparation and properties of textile materials modified with triclosan-loaded polylactide microparticles. Polym. Adv. Technol. 2017, 28, 1185–1193. [Google Scholar] [CrossRef]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly(lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 104–114. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mat. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Zhu, T.; Feng, N.; Sun, Y.; Song, Z.; Li, S.; Liu, J.; Ding, J. Electrospun polylactide-nano-hydroxyapatite-vancomycin composite scaffolds for advanced osteomyelitis therapy. J. Biomed. Nanotechnol. 2019, 16, 1213–1222. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B.; Zamanian, A.; Torabinejad, B.; Ziaeirad, Z. Investigating composite systems based on poly L-lactide and poly L-lactide/triclosan nanoparticles for tissue engineering and medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 294–309. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordoño, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggalí, J.; Engel, E.; del Valle, L.J.; Alemán, C. Electrospun conducting and biocompatible uniaxial and core–shell fibers having poly(lactic acid), poly(ethylene glycol), and polyaniline for cardiac tissue engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Lakalayeh, G.A.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline hydrochloride-containing poly (ε-caprolactone)/poly lactic acid scaffold for bone tissue engineering application: In vitro and in vivo study. Int. J. Polym. Mater. Polym. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Q.; Wang, X.; Gao, W.; Zhao, X. Antibacterial properties of PLA nonwoven medical dressings coated with nanostructured silver. Fiber. Polym. 2008, 9, 556–560. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of electrospun polylactic acid/ Cinnamaldehyde/β-cyclodextrin fibers as an antimicrobial wound dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Foong, C.Y.; Hamzah, M.S.A.; Razak, S.I.A.; Saidin, S.; Nayan, N.H.M. Influence of poly(lactic acid) layer on the physical and antibacterial properties of dry bacterial cellulose sheet for potential acute wound healing materials. Fiber. Polym. 2018, 19, 263–271. [Google Scholar] [CrossRef]
- Vakilian, S.; Norouzi, M.; Soufi-Zomorrod, M.; Shabani, I.; Hosseinzadeh, S.; Soleimani, M.L. Inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Moslem, Z.; Sadri, M.; Pebdeni, A.B. Antimicrobial and cellular activity of poly(L-lactide)/ chitosan/Imipenem antibiotic composite nanofibers. Fiber. Polym. 2016, 17, 1336–1342. [Google Scholar] [CrossRef]
- Li, W.; Yu, Q.; Yao, H.; Zhu, Y.; Topham, P.D.; Yue, K.; Ren, L.; Wang, L. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019, 92, 60–70. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. (Eds.) Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Wiley&Sons Ltd.: New York, NY, USA, 2000. [Google Scholar]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethylphosphonic acid inhibits growth and metastasis of human prostate cancer in an orthotopic xenograft mouse model. Oncotarget 2016, 7, 10616–10626. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, K.R.; Zhang, Q.; Liu, S.; You, Z. Aminomethyl phosphonic acid and methoxyacetic acid induce apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2015, 16, 11750–11765. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature 1959, 184 (Suppl. 12), 901–902. [Google Scholar] [CrossRef]
- Tan, S.A.; Tan, L.G. Distribution of ciliatine (2-aminoethylphosphonic acid) and phosphonoalanine (2-amino-3-phosphonopropionic acid) in human tissues. Clin. Physiol. Biochem. 1989, 7, 303–309. [Google Scholar] [PubMed]
- Watts, M. Glufosinate-Ammonium Monograph. 2008. Available online: http://www.pananz.net/wp-content/uploads/2013/04/Glufosinate-monograph-12-Dec-2008.pdf (accessed on 18 June 2019).
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, development, applications, and properties. In Glyphosate Resistance in Crops and Weeds. History, Development and Management; Nandula, V.K., Ed.; John Wiley& Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassal, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature 1978, 272, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Holmes, S.W.; Lambert, R.W.; Nisbet, L.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Alaphosphin and related phosphono-peptides. Antimicrob. Agents Chemother. 1979, 15, 684–695. [Google Scholar] [CrossRef] [Green Version]
- Atherton, F.R.; Hall, M.J.; Hassall, C.H.; Lambert, R.W.; Lloyd, W.J.; Ringrose, P.S. Phosphonopeptides as antibacterial agents: Mechanism of action of alaphosphin. Antimicrob. Agents Chemother. 1979, 15, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Atherton, F.R.; Hassall, C.H.; Lambert, R.W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)-phosphonic acid. J. Med. Chem. 1986, 29, 29–40. [Google Scholar] [CrossRef]
- Lejczak, B.; Kafarski, P.; Sztajer, H.; Mastalerz, P. Antibacterial activity of phosphono dipeptides related to alafosfalin. J. Med. Chem. 1986, 29, 2212–2217. [Google Scholar] [CrossRef]
- Neu, H.C.; Kamimura, T. In vitro and in vivo antibacterial activity of FR-31564, a phosphonic acid antimicrobial agent. Antimicrob.Agents Chemother. 1981, 19, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Jomaa, H.; Wiesner, J.; Sanderbrand, S.; Altincicek, B.; Weidemeyer, C.; Hintz, M.; Türbachova, I.; Eberl, M.; Zeidler, J.; Lichtenthaler, H.K.; et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 1999, 285, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, J.; Borrmann, S.; Jomaa, H. Fosmidomycin for the treatment of malaria. Parasitol. Res. 2003, 90 (Suppl. 2), S71–S76. [Google Scholar] [CrossRef]
- Zhang, B.; Watts, K.M.; Hodge, D.; Kemp, L.M.; Hunstad, D.A.; Hicks, L.M.; Odom, A.R. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 2011, 50, 3570–3577. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.; Staines, H.M. Non-Antifolate Antibiotics: Clindamycin, Doxycycline, Azithromycin and Fosmidomycin. In Treatment and Prevention of Malaria. Milestones in Drug Therapy; Staines, H., Krishna, S., Eds.; Springer: Basel, Switzerland, 2011; Volume 41, pp. 141–156. [Google Scholar] [CrossRef]
- Jane, D. Neuroactive aminophosphonic and aminophosphinic acid derivatives. In Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; Wiley& Sons Ltd.: New York, NY, USA, 2000; Chpt. 14; pp. 483–536. [Google Scholar]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef]
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88 (Suppl. 12), 2961–2978. [Google Scholar] [CrossRef]
- Russell, R.G.G.; Croucher, P.I.; Rogers, M.J. Bisphosphonates: Pharmacology, mechanisms of action and clinical uses. Osteoporos. Int. 1999, 9 (Suppl. 2), S66–S80. [Google Scholar] [CrossRef]
- De Clercq, E.; Holý, A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Rev. Drug Discov. 2005, 4, 928–940. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin Tromethamine. A Review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 1997, 53, 537–656. [Google Scholar] [CrossRef]
- Falagas, M.E.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [Green Version]
- Díez-Aguilar, M.; Cantón, R. New microbiological aspects of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. 1), 8–18. [Google Scholar]
- Gulcu, A.; Akman, A.; Demirkan, A.F.; Yorukoglu, A.C.; Kaleli, I.; Bir, F. Fosfomycin Addition to Poly(D,L-Lactide) Coating does not affect prophylaxis efficacy in rat implant-related infection model, but that of gentamicin does. PLoS ONE 2016, 11, e0165544. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. Nomenclature of aminoalkylphosphonic acids and derivatives. Evolution of the code system. Acta Biochim. Pol. 2015, 62, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-chloroacetylamino)-alkylphosphonic acids - Synthetic precursors of phosphonopeptides. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J. Thioureidoalkylphosphonates in the synthesis of 1-aminoalkylphosphonic acids. The Ptc-aminophosphonate method. Arkivoc 2011, 2011, 227–269. [Google Scholar] [CrossRef] [Green Version]
- Drabowicz, J.; Jordan, F.; Kudzin, M.H.; Kudzin, Z.H.; Stevens, C.V.; Urbaniak, P. Reactivity of aminophosphonic acids. Oxidative dephosphonylation of 1-aminoalkylphosphonic acids by aqueous halogens. Dalton Trans. 2016, 45, 2308–2317. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Chruściel, J.J.; Kudzin, M.H.; Łatwińska, M.; Kiwała, M. Antimicrobial functionalization of textile materials with copper silicate. Fibres Text. East. Eur. 2016, 24, 151–156. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Walawska, A.; Sójka-Ledakowicz, J. Biofunctionalization of textile materials.1. Biofunctionalization of poly(propylene) (PP) nonwovens fabrics by alafosfalin. Coatings 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Stem cell differentiation on electrospun nanofibrous substrates for vascular tissue engineering. Mater. Sci. Eng. C 2013, 33, 4640–4650. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Pachón, E.Y.; Vera-Graziano, R.; Campos, R.M. Structure of poly(lactic-acid) PLA nanofibers scaffolds prepared by electrospinning. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 012003. [Google Scholar] [CrossRef] [Green Version]
- Piccirillo, G.; Bochicchio, B.; Pepe, A.; Schenke-Layland, K.; Hinderer, S. Electrospun poly-L-lactide scaffold for the controlled and targeted delivery of a synthetically obtained Diclofenac prodrug to treat actinic keratosis. Acta Biomater. 2017, 52, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, L.; Xiang, C.; Li, L. Electrospun porous PLLA and poly(LLA-co-CL) fibers by phase separation. New J. Chem. 2018, 42, 5102. [Google Scholar] [CrossRef]
- Matysiak, W.; Kapica, A.; Tański, T.; Dubiel, A. Analysis of the influence of electro spinning process parameters on the morphology of poly(Lactic acid) fibres. Arch. Mater. Sci. Eng. 2019, 96, 73–78. [Google Scholar] [CrossRef]
- Krucińska, I.; Surma, B.; Chrzanowski, M.; Skrzetuska, E.; Puchalski, M. Application of melt-blown technology in the manufacturing of a solvent vapor-sensitive, non-woven fabric composed of poly(lactic acid) loaded with multi-walled carbon nanotubes. Text. Res. J. 2013, 83, 859–870. [Google Scholar] [CrossRef]
- Chrzanowska, O.; Struszczyk, M.H.; Krucinska, I. Small diameter tubular structure design using solvent-free textile techniques. J. Appl. Polym. Sci. 2014, 131, 40147. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Brochocka, A.; Brycki, B. Biocidal agent for modification of poly(lactic acid) high-efficiency filtering nonwovens. Fibres Text. East. Eur. 2015, 23, 88–95. [Google Scholar] [CrossRef]
- Łatwińska, M.; Sójka-Ledakowicz, J.; Chruściel, J.; Piórkowski, M. PLA and PP composite nonwoven with antimicrobial activity for filtration applications. Int. J. Polym. Sci. 2016, 2016, 2510372. [Google Scholar] [CrossRef] [Green Version]
- Szuman, K.; Krucińska, I.; Boguń, M.; Draczyński, Z. PLA/PHA- biodegradable blends for pneumothermic fabrication of nonwovens. Autex Res. J. 2016, 16, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Feng, J. Preparation and properties of poly(lactic acid) fiber melt blown non-woven disordered mats. Mater. Lett. 2017, 189, 180–183. [Google Scholar] [CrossRef]
- Yu, B.; Wang, M.; Sun, H.; Zhu, F.; Han, J.; Bhat, G. Preparation and properties of poly (lactic acid)/magnetic Fe3O4 composites and nonwovens. RSC Adv. 2017, 7, 41929–41935. [Google Scholar] [CrossRef] [Green Version]
- Vadas, D.; Kmetykó, D.; Marosi, G.; Bocz, K. Application of melt-blown Poly(lactic acid) fibres in self-reinforced composites. Polymers 2018, 10, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chen, G.; Bhat, G.S.; Azari, H.; Pen, H. Electret characteristics of melt-blown polylactic acid fabrics for air filtration application. J. Appl. Polym. Sci. 2020, 137, 48309. [Google Scholar] [CrossRef]
- Zhu, F.; Yu, B.; Su, J.; Han, J. Study on PLA/PA11 bio-based toughening melt-blown nonwovens. Autex Res. J. 2020, 20. in press. [Google Scholar] [CrossRef] [Green Version]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly( lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Carstenn-Lichterfelde, C.; Fernandez-Ibanez, M.; Gálvez-Ruano, E.; Bellanato, J. Structural study of fosfomycin [(–)-cis-1,2-epoxypropylphosphonic acid] salts and related compounds. J. Chem. Soc. Perkin Trans. 2 1983, 943–947. [Google Scholar] [CrossRef]
- ENISO 20645. 2006–Textile Fabrics–Determination of Antibacterial Activity—Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2012, 114, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]


| Name (Abbreviation) /a | Structure /b | Origin | Action/Application /c | Ref. |
|---|---|---|---|---|
| Phospho-glycine (GlyP) |  | Primary PMG metabolite | Inhibition of prostate cancer cell growth (in vitro), phytotoxin | [30,31] |
| β-AlaP (β-phosphono-alanine, 2-AEP, ciliatine) |  | The first and most abundant natural AAP | [32,33] | |
| Phosphino-thricin GluγP(Me) (phosphino-thricin, PPT) |  | Produced by strains of Streptomyces herbicide | Inhibition of glutamine synthetase (E.C. 6.3.1.2) | [34] |
| PMG (Phosphono-Methyl-Glycine; Glyphosate) |  | Artificial herbicide | inhibition of 5-enolpyruvyl-shikimic acid-3-phosphate synthase | [35,36] |
| Alafosfalin Ala-AlaP (alaphosphin; alafosfalin) |  | Artificial antibiotic, against gram-negative and gram-positive bacteria. | selective inhibition of alanine racemase (EC 5.1.1.1). | [37,38,39,40,41] |
| Fosmidomycin ((3-(Formyl-hydroxy-amino)-propyl)-phosphonic acid; The phosphonate antibiotic FR-31564 |  | Produced by strains of
Streptomyces A broad-spectrum antimicrobial agent | Inhibition of DXR (in vitro). A broad-spectrum antimicrobial agent currently applied for the malaria treatment. | [42,43,44,45,46] |
| ϖ-Aminoalkyl-phosphonic acids (ϖ-AAP) |  | Artificial | Neuroactive acids | [47] |
| Aminoalkyl-bisphosphonic acids (ϖ-AAP,P) |  X = H, OH, halogen | Artificial | Inhibition of osteoclastic bone resorption | [48,49,50,51] |
| Acyclic Nucleoside Phosphonates (ANPs) Cidovir [HPMPC] (B = Cyt; X = OH; R = H); Adefovir [PMEA] (B = Ade; X = H; R = POM); Tenovir [PMPA] (B = Ade; X = H; R = POC) |  | Artificial | Treatment of various DNA virus infections (cidofovir), hepatitis B (adefovir), and AIDS (HIV infections, tenofovir) | [52,53] |
| Fosfomycin (phosphomycin/phosphono-mycin) | 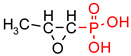 | Fosfomycin - a broad-spectrum antibiotic produced by Streptomyces species. | Oral UTIs treatment. provide a useful option for the treatment of patients with pathogens with advanced resistance infections. | [54,55,56,57,58] |
| Processing Parameters | |
|---|---|
| Temperature of the extruder in zone 1 | 195 °C |
| Temperature of the extruder in zone 2 | 245 °C |
| Temperature of the extruder in zone 3 | 260 °C |
| Head temperature | 260 °C |
| Air heater temperature | 260 °C |
| Air flow rate | 7–8 m³/h |
| Mass per unit area of nonwovens | 102 g/m2 |
| Polymer yields | 6 g/min |
| Components | g | % |
|---|---|---|
| styrene-acrylic resin | 6 | 6 |
| thickening agent | 1 | 1 |
| wetting agent | 3 | 3 |
| water | 90 | 90 |
| PLA [82] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IR [ν/cm−1]; Intensity | 2997; M | 2947; M | 1760; VS | 1452; S | 1348–1388; S | 1368–1360; S | 1270; S | 1215–1185; VS | 1130; S | 1100–1090; VS, sh | 1045; S |
| Assign- ment | νas CH3 | νs CH3 | ν C=O | δas CH3 | δs CH3 | δ1 CH + δs CH3 | δCH+ ν COC | νas COC +rasCH3 | rasCH3 | νs COC | ν C-CH3 |
| Fosfomycin (Fosfm) [83] | |||||||||||
| FosfmNa2 (Nujol) | |||||||||||
| IR [ν/cm−1]; Intensity | 3010 | 1414 w | 1270 w; 1260 w | 1125 s; 1096 vs | 1008 m | ||||||
| Assign- ment | ν(C–H) (ring) | δ (CH3) | Ring breath | νa (PO32−) | νa (PO32−) | ||||||
| FosfmCa × H2O (KBr) | |||||||||||
| IR [ν/cm−1]; Intensity | 3000 w | 1423 w; 1419 sh | 1262 vw | 1095 vs | 1017 m | ||||||
| Assign- ment | ν(C–H) (ring) | δ (CH3) | Ring breath | νa (PO32−) | νa (PO32−) | ||||||
| Parameter | PLA | PLA-Fosfomycin [% Fosfomycin paste concentr.] | |||
|---|---|---|---|---|---|
| 0.005% | 0.01% | 0.1% | |||
| Average air permeability [mm/s], pressure decrease: | 100 Pa | 910 | 442 | 440 | 449 |
| 200 Pa | 1677 | 880 | 876 | 891 | |
| Parameter | PLA | PLA-Fosfomycin [% fosfomycin paste concentr.] | ||
|---|---|---|---|---|
| 0.005% | 0.01% | 0.1% | ||
| Tensile strength[kN/m] | 0.032 | 0.117 | 0.120 | 0.115 |
| Relative elongation at maximum load [%] | 10.0 | 9.720 | 9.930 | 10.102 |
| Sample No. | Fosfomycinon PLAnonwovens | Bacterial Average Inhibition ZoneGrowth for Bacteria (mm) | |
|---|---|---|---|
| Fosfomycin Coating Pastes Concentrations (%) | E. coli | S. aureus | |
| 1 | 0 | 0 | 0 |
| 2 | 0.005 | 3–4 | 5 |
| 3 | 0.01 | 4–5 | 6 |
| 4 | 0.1 | 5–6 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of Textile Materials. 2. Antimicrobial Modification of Poly(lactide) (PLA) Nonwoven Fabricsby Fosfomycin. Polymers 2020, 12, 768. https://doi.org/10.3390/polym12040768
Kudzin MH, Mrozińska Z. Biofunctionalization of Textile Materials. 2. Antimicrobial Modification of Poly(lactide) (PLA) Nonwoven Fabricsby Fosfomycin. Polymers. 2020; 12(4):768. https://doi.org/10.3390/polym12040768
Chicago/Turabian StyleKudzin, Marcin H., and Zdzisława Mrozińska. 2020. "Biofunctionalization of Textile Materials. 2. Antimicrobial Modification of Poly(lactide) (PLA) Nonwoven Fabricsby Fosfomycin" Polymers 12, no. 4: 768. https://doi.org/10.3390/polym12040768





