A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CS-Alg Nanoparticles and MET-CS-AlgNPs Nanocomposites
2.3. Methodology
2.3.1. Modeling of Different Responses
2.3.2. Full Factorial Design
2.4. MET Loading Efficiency
2.5. Particle Size and Zeta Potential of Nanocomposites
2.6. Controlled Release Study of the MET from the Nanocomposites
2.7. Instrumentation
3. Results and Discussion
3.1. MultipleLinear Regression Analysis
3.2. Evaluation of the Models
3.2.1. Pareto Chart of Responses of Standardized Effects and Normal Plot of the Standardized Effects
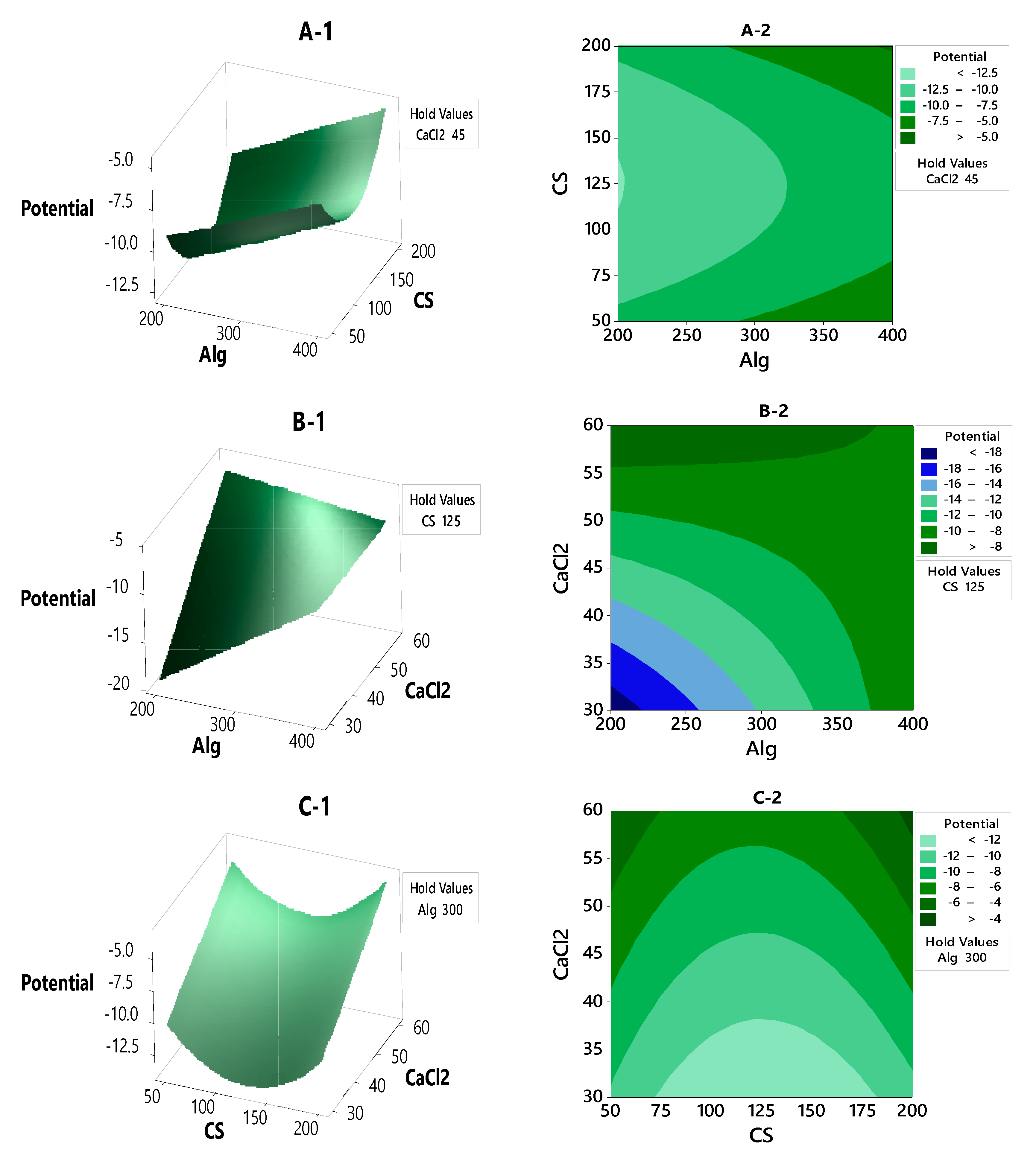
3.2.2. Contour Plot and Surface Plot of LE, Particle Size and Zeta Potential Against Selected Independent Variables
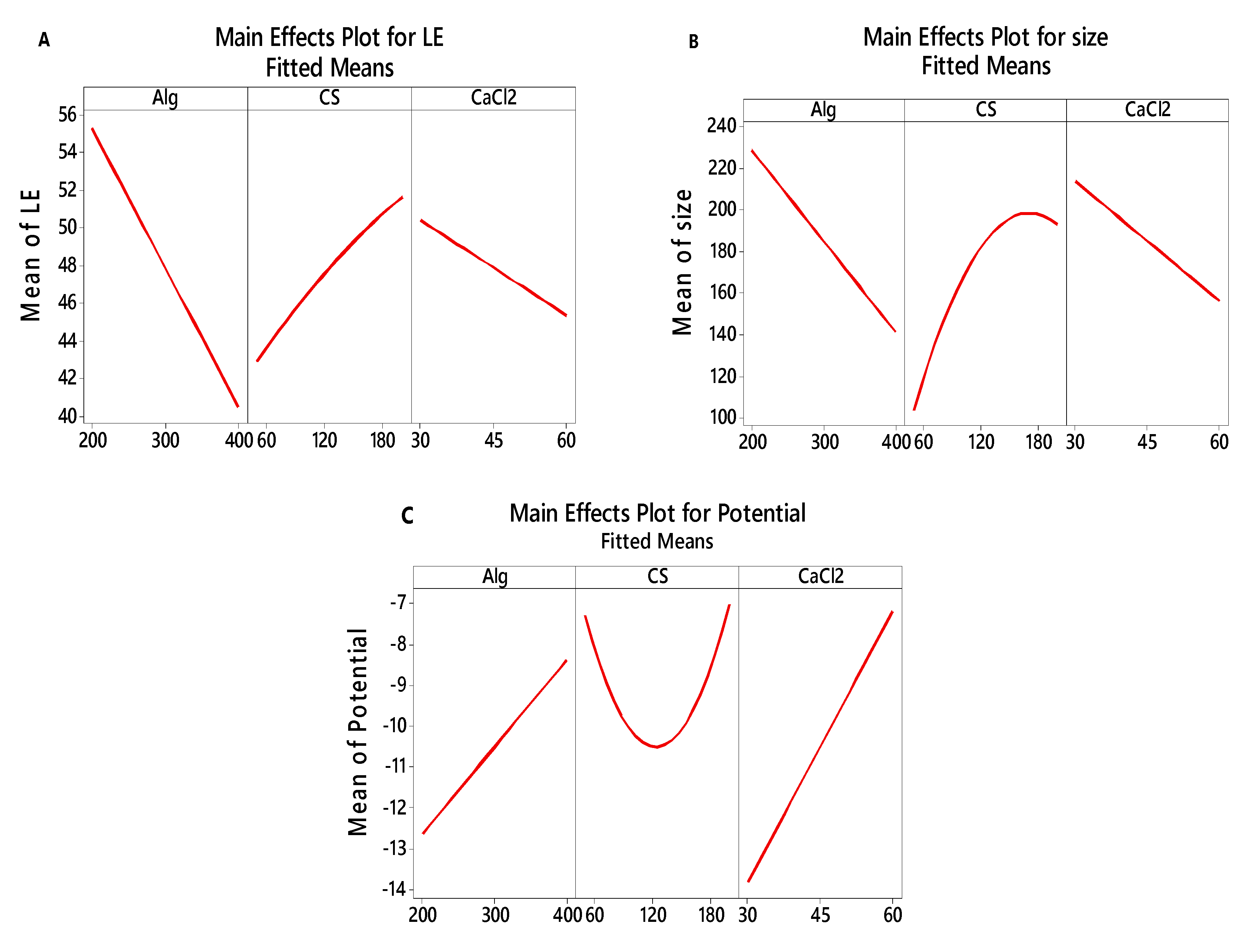
3.2.3. Main effects plot for LE, particle size and zeta potential
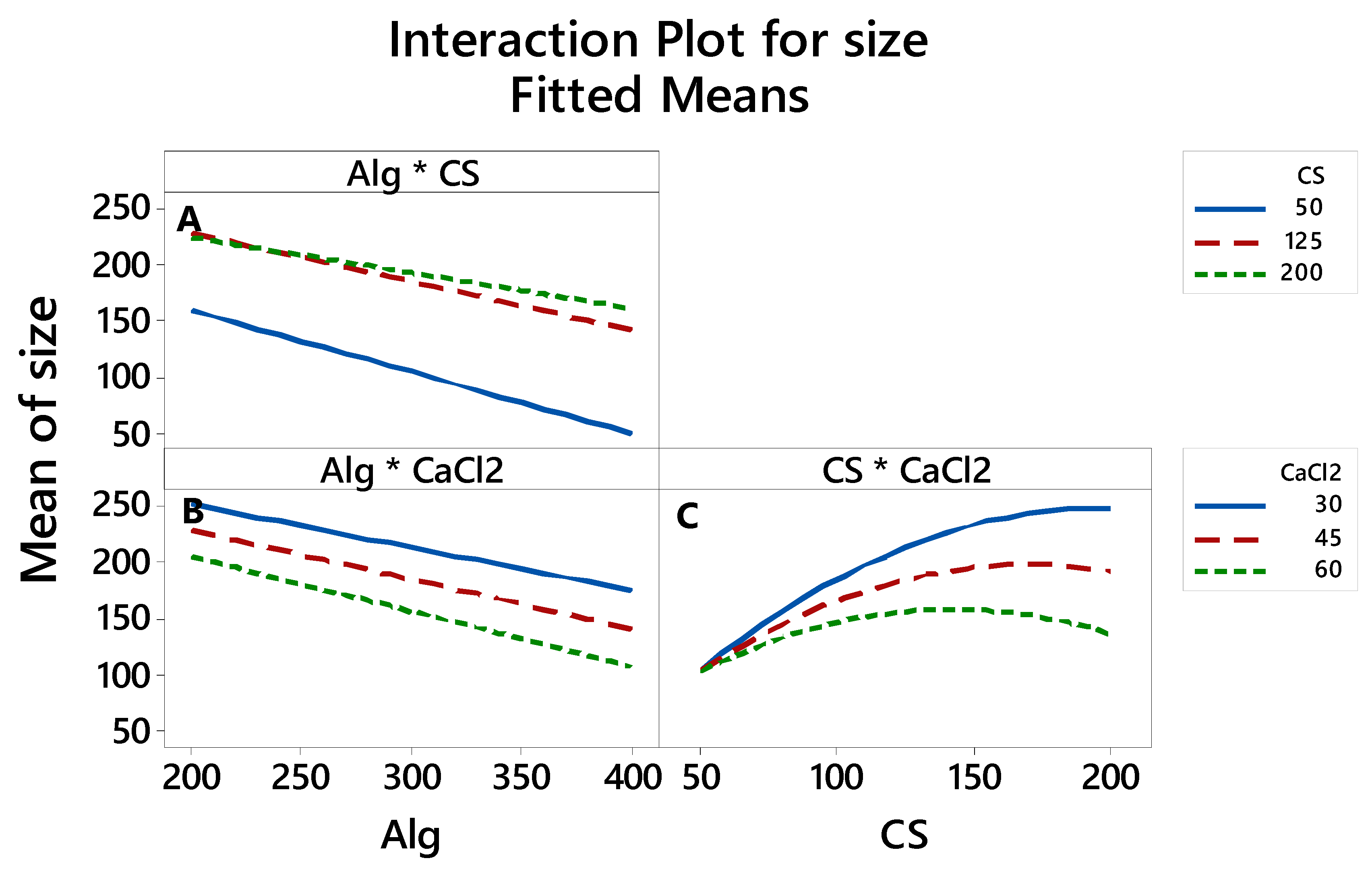
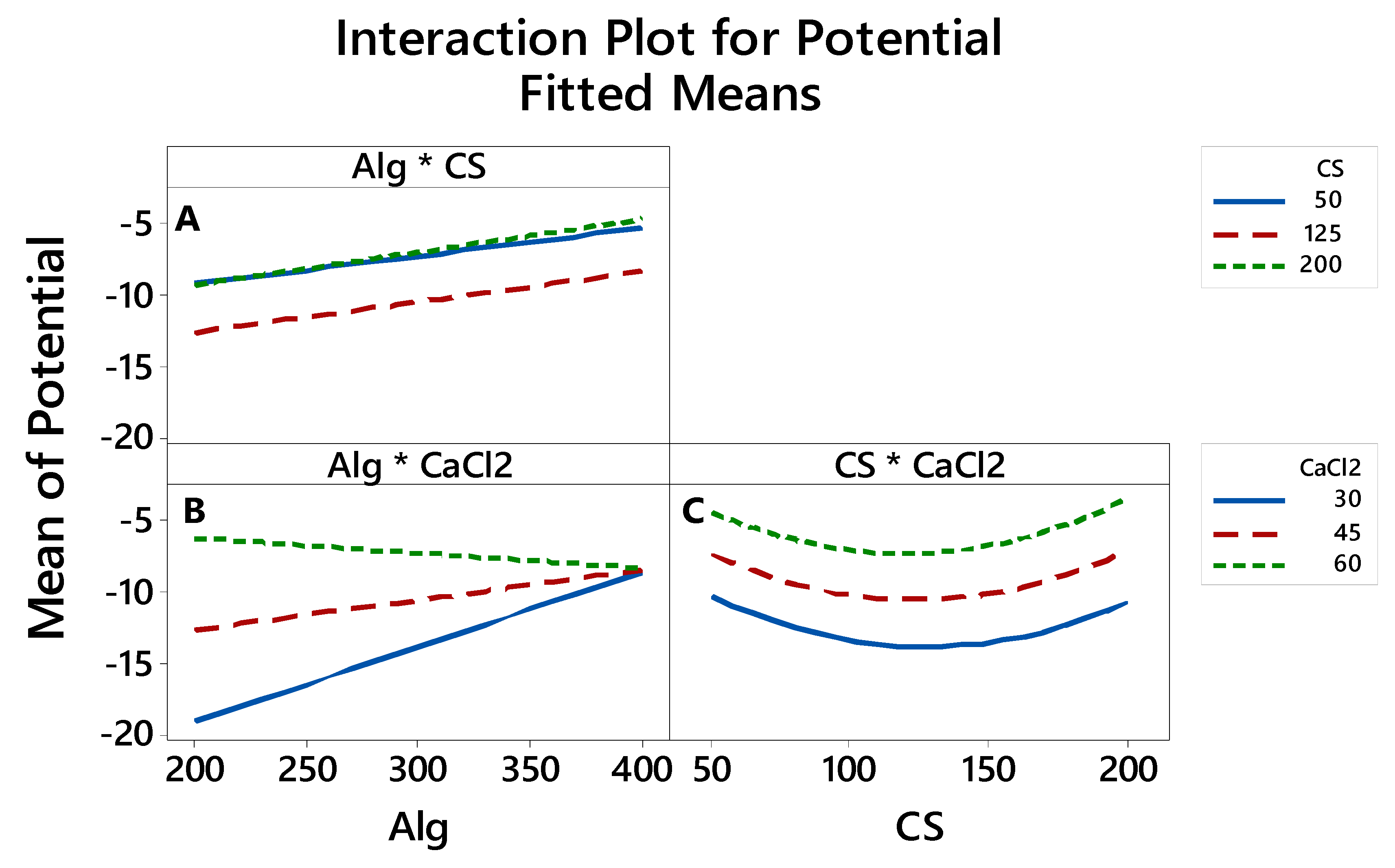
3.2.4. The Interaction between the Factors thatAffects the LE, Particle Size and Zeta Potential
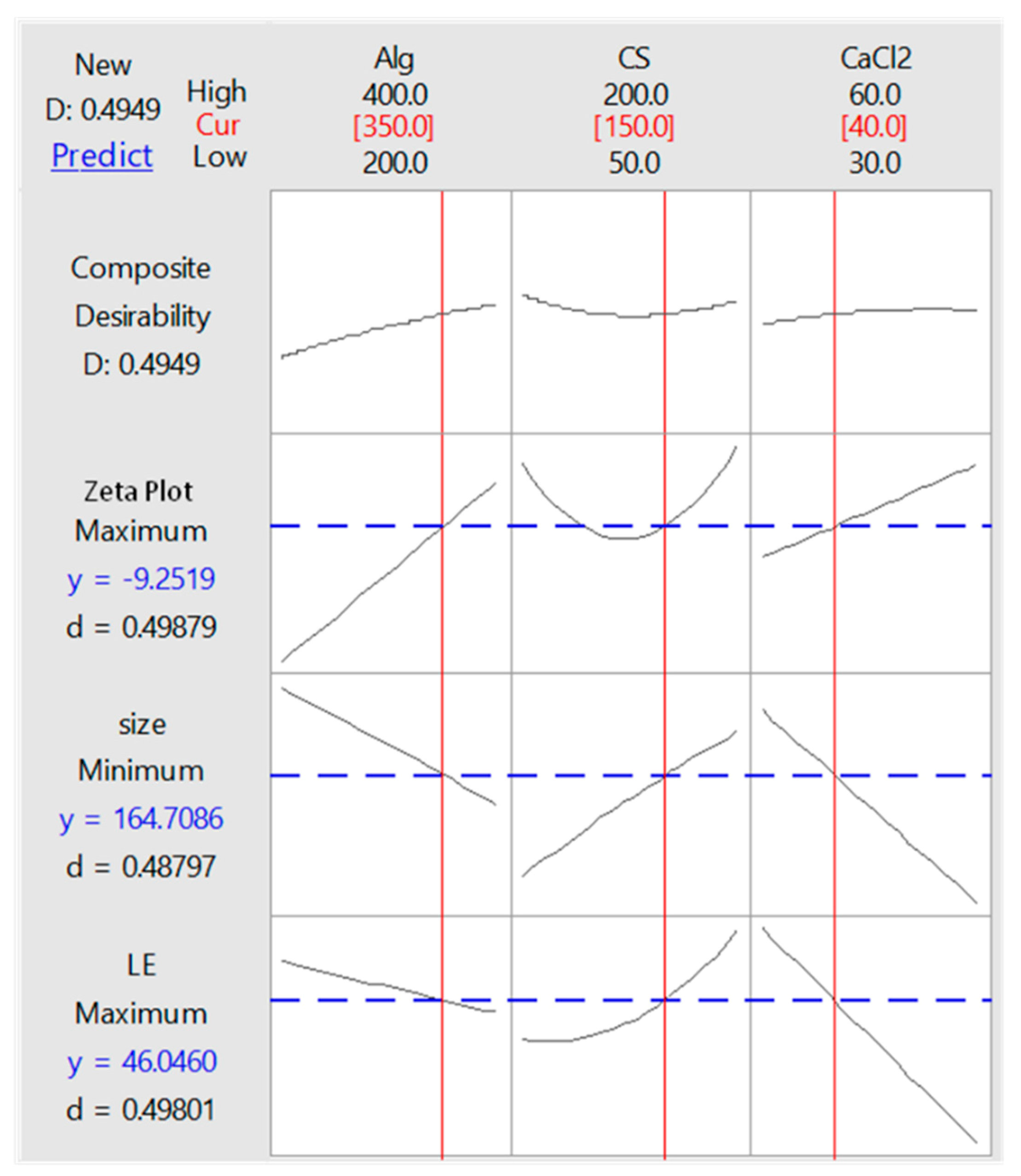
3.3. Optimization of LE, Particle Size and Zeta Potential
3.4. Validation Test for Building Model
3.5. X-Ray Diffraction of MET-CS-AlgNPs Nanocomposites
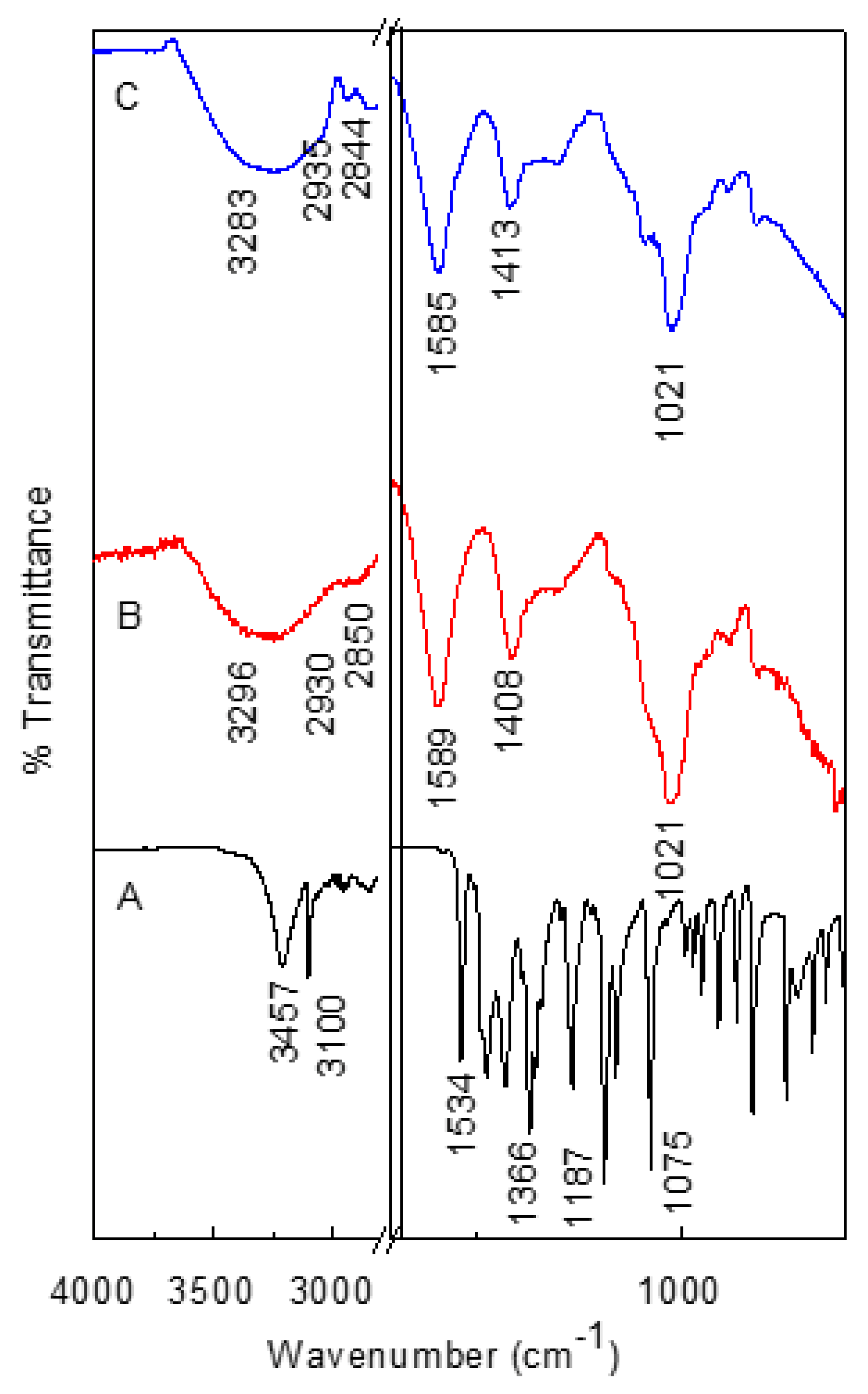
3.6. FTIR Spectroscopic Analysis of CS-AlgNPs and MET-CS-AlgNPs
3.7. Thermogravimetric Analysis of MET-CS-AlgNPs Nanocomposites
3.8. Scanning Electron Microscopy
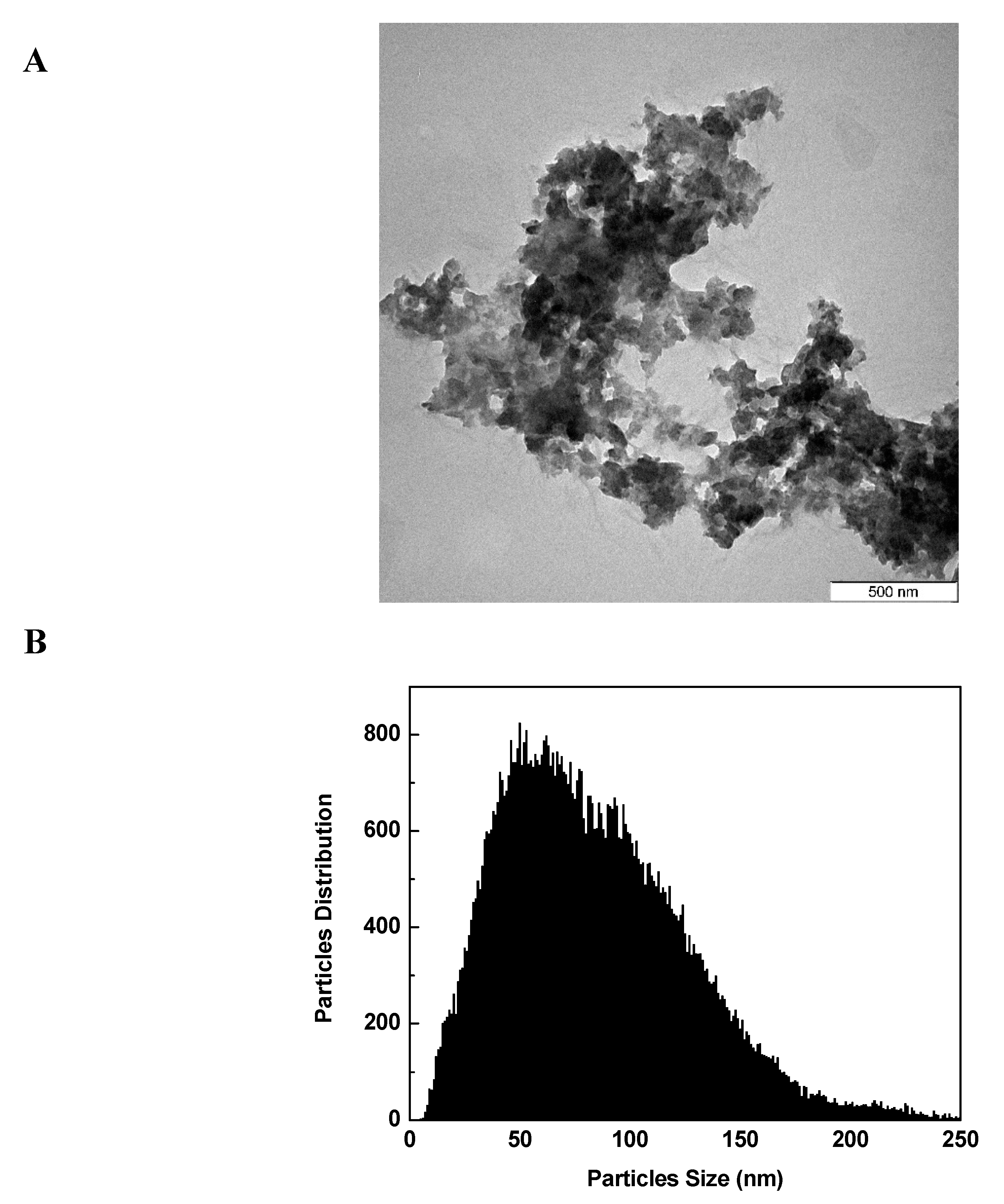
3.9. Transmission Electron Microscopy
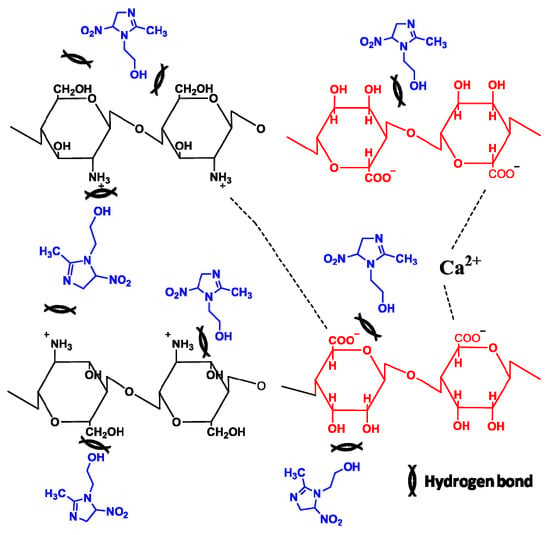
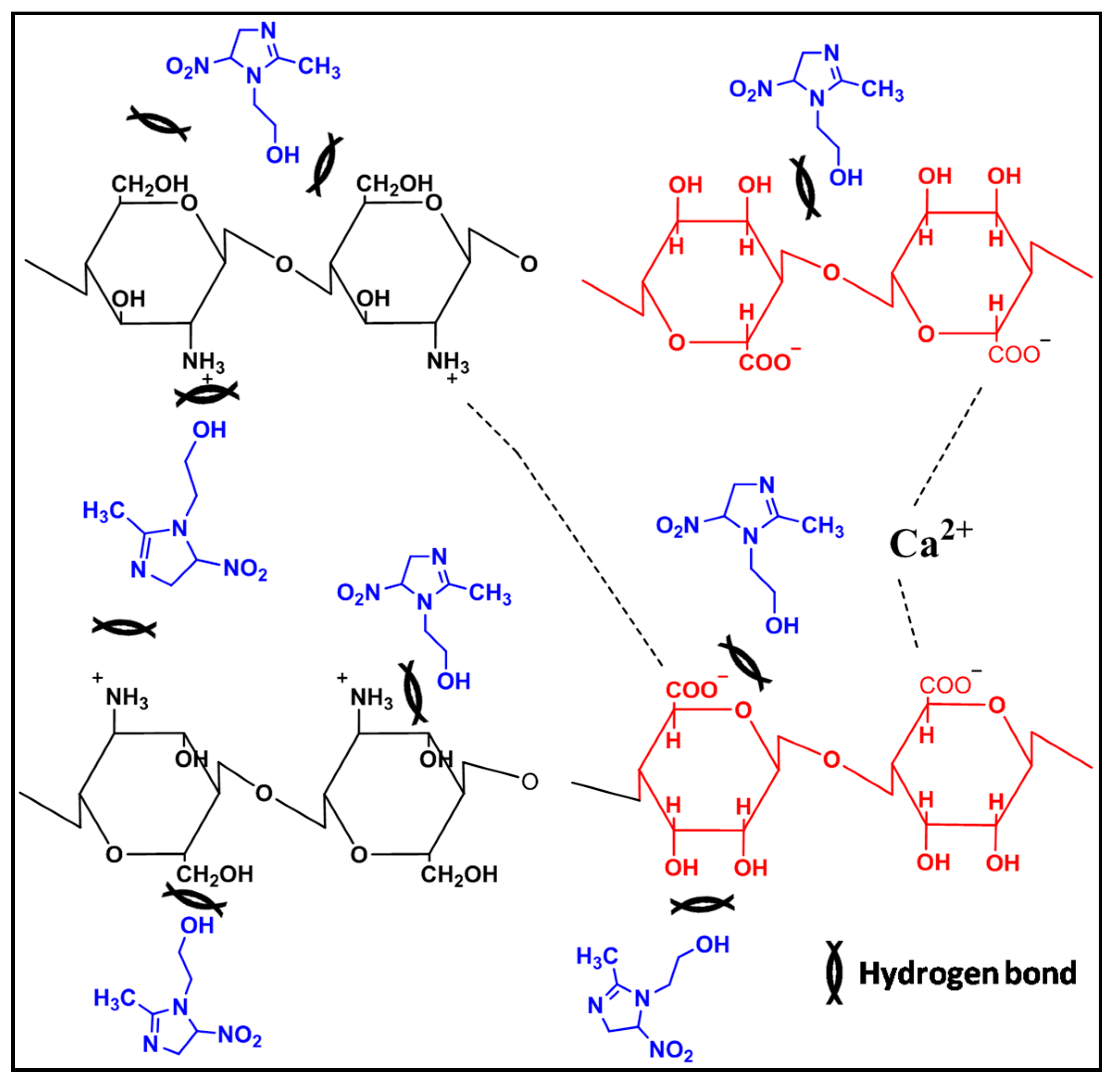
3.10. Interactions between Chemical Components of MET-CS-AlgNPs Nanocomposites
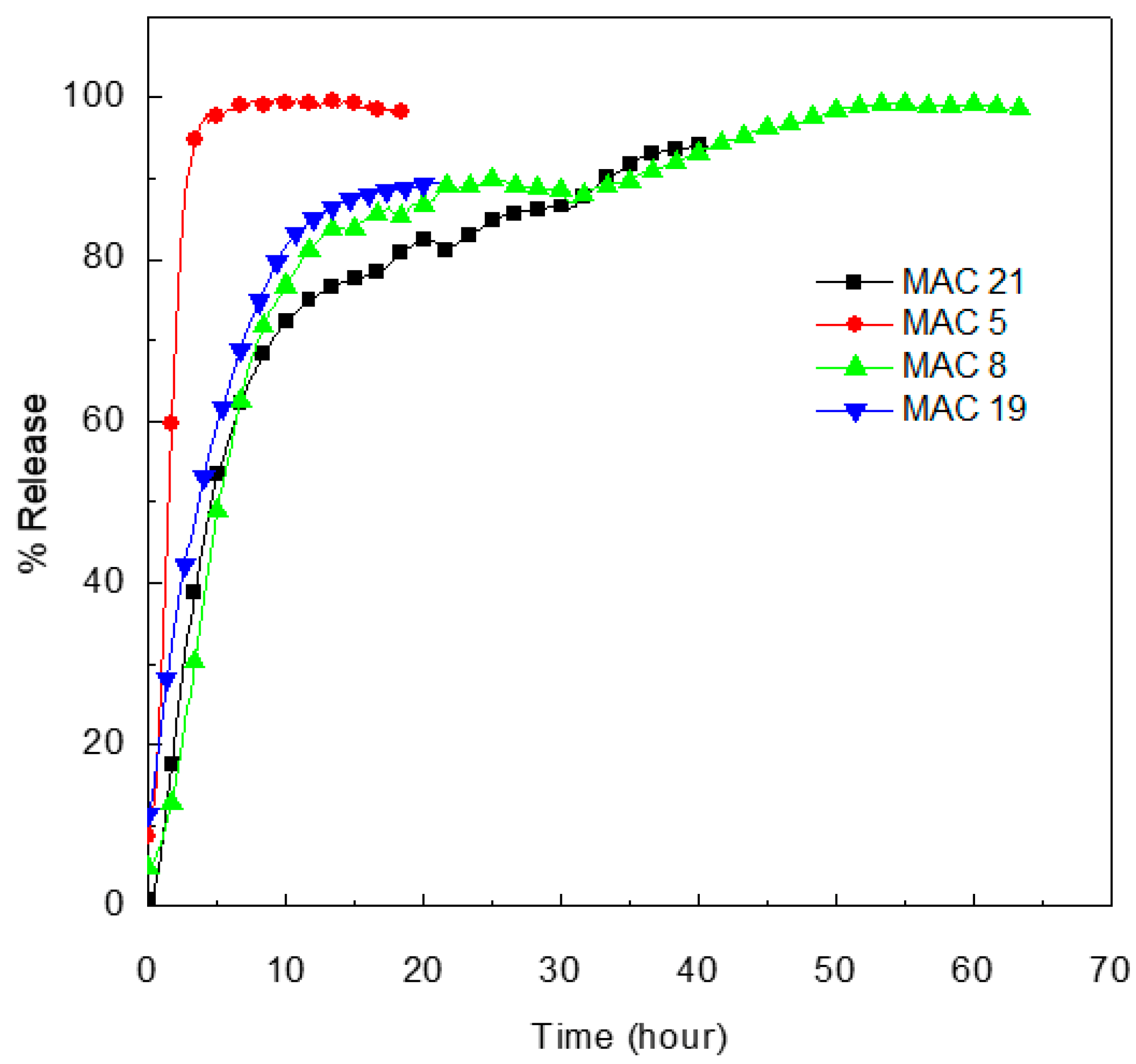
3.11. Release Properties of MET from MET-CS-AlgNPs Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdallah, I.; Ibrahim, A.; Ibrahim, N.; Rizk, M.; Tawakkol, S. Simultaneous determination of atenolol and nifedipine by using spectrophotometric method with multivariate calibration and HPLC method implementing “design of experiment”. Pharm. Anal. Acta 2015, 6, 2. [Google Scholar]
- Agrahari, V.; Meng, J.; Zhang, T.; Youan, B.-B.C. Application of design of experiment and simulation methods to liquid chromatography analysis of topical HIV microbicides stampidine and HI443. J. Anal. Bioanal. Tech. 2014, 5, 180. [Google Scholar] [PubMed]
- Lewis, G.A.; Mathieu, D.; Phan-Tan-Luu, R. Pharmaceutical Experimental Design; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Rani, A.P.; Hema, V. Full factorial design in formulation of lamotrigine suspension using locust bean gum. Int. J. Chem. Sci. 2013, 11, 751–760. [Google Scholar]
- Kumar, R.S.; Yagnesh, T.N.S.; Kumar, V.G. Optimisation of ibuprofen fast dissolving tablets employing starch xanthate using 23 factorial design. Int. J. Appl. Pharm. 2017, 9, 51–59. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, J.S. The 2 k—P fractional factorial designs. Technometrics 1961, 3, 311–351. [Google Scholar] [CrossRef]
- Gunst, R.F.; Mason, R.L. Fractional factorial design. Wiley Interdiscip. Rev. Comput. Stat. 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Shahabadi, S.M.S.; Reyhani, A. Optimization of operating conditions in ultrafiltration process for produced water treatment via the full factorial design methodology. Sep. Purif. Technol. 2014, 132, 50–61. [Google Scholar] [CrossRef]
- Salea, R.; Widjojokusumo, E.; Hartanti, A.W.; Veriansyah, B.; Tjandrawinata, R.R. Supercritical fluid carbon dioxide extraction of Nigella sativa (black cumin) seeds using taguchi method and full factorial design. Biochem. Compd. 2013, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Jacques, P.; Hbid, C.; Destain, J.; Razafindralambo, H.; Paquot, M.; De Pauw, E.; Thonart, P. Optimization of biosurfactant lipopeptide production from Bacillus subtilis S499 by Plackett-Burman design. Appl. Biothem. Biotechnol. 1999, 77, 223–233. [Google Scholar] [CrossRef]
- Ahuja, S.; Ferreira, G.; Moreira, A. Application of plackett-Burman design and response surface methodology to achieve exponential growth for aggregated shipworm bacterium. Biotechnol. Bioeng. 2004, 85, 666–675. [Google Scholar] [CrossRef]
- Sarlak, N.; Nejad, M.A.F.; Shakhesi, S.; Shabani, K. Effects of electrospinning parameters on titanium dioxide nanofibers diameter and morphology: An investigation by Box–Wilson central composite design (CCD). Chem. Eng. J. 2012, 210, 410–416. [Google Scholar] [CrossRef]
- Wsól, V.; Fell, A.F. Central composite design as a powerful optimisation technique for enantioresolution of the rac-11-dihydrooracin—The principal metabolite of the potential cytostatic drug oracin. J. Biochem. Biophys. Methods 2002, 54, 377–390. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.; Ferreira, H.; Matos, G.; David, J.; Brandao, G.; da Silva, E.P.; Portugal, L.; Dos Reis, P.; Souza, A. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tak, B.-Y.; Tak, B.-S.; Kim, Y.-J.; Park, Y.-j.; Yoon, Y.-H.; Min, G.-H. Optimization of color and COD removal from livestock wastewater by electrocoagulation process: Application of Box–Behnken design (BBD). J. Ind. Eng. Chem. 2015, 28, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Thirugnanasambandham, K.; Sivakumar, V.; Shine, K. Optimization of reverse osmosis treatment process to reuse the distillery wastewater using Taguchi design. Desalin. Water Treat. 2016, 57, 24222–24230. [Google Scholar] [CrossRef]
- Luo, W.; Pla-Roca, M.; Juncker, D. Taguchi design-based optimization of sandwich immunoassay microarrays for detecting breast cancer biomarkers. Anal. Chem. 2011, 83, 5767–5774. [Google Scholar] [CrossRef]
- O’shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef]
- Lesch, S. Sensor-directed response surface sampling designs for characterizing spatial variation in soil properties. Comput. Electron. Agric. 2005, 46, 153–179. [Google Scholar] [CrossRef]
- Chowdary, K.; Shankar, K.R. Optimization of pharmaceutical product formulation by factorial designs: Case studies. J. Pharm.Res. 2016, 15, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.J. Selection of subsets of regression variables. J. R. Stat. Soc. Ser. A Gen. 1984, 147, 389–410. [Google Scholar] [CrossRef]
- Wagner, J.M.; Shimshak, D.G. Stepwise selection of variables in data envelopment analysis: Procedures and managerial perspectives. Eur. J. Oper. Res. 2007, 180, 57–67. [Google Scholar] [CrossRef]
- Effroymson, M. Multiple regression analysis. In Mathematical Methods for Digital Computers; Ralson, A., Wilf, H.S., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 1960. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 326. [Google Scholar]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawahar, N.; Meyyanathan, S. Polymeric nanoparticles for drug delivery and targeting: A comprehensive review. Int. J. HealthAllied Sci. 2012, 1, 217. [Google Scholar] [CrossRef]
- Allemann, E.; Gurny, R.; Doelker, E. Drug-loaded nanoparticles: Preparation methods and drug targeting issues. Eur. J. Pharm. Biopharm. 1993, 39, 173–191. [Google Scholar]
- Yih, T.; Al-Fandi, M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef]
- Meyer, M. Bioavailability of drugs and bioequivalence. Encycl. Pharm. Technol. 1998, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Shi, X.W.; Du, Y.M.; Tang, Y.F. Quaternized chitosan/alginate nanoparticles for protein delivery. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 83, 383–390. [Google Scholar] [CrossRef]
- Azhar, F.F.; Olad, A. A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate–chitosan/montmorillonite nanocomposite systems. Appl. Clay Sci. 2014, 101, 288–296. [Google Scholar] [CrossRef]
- Dai, Y.N.; Li, P.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Swelling characteristics and drug delivery properties of nifedipine-loaded pH sensitive alginate–chitosan hydrogel beads. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 493–500. [Google Scholar] [CrossRef]
- Taleb, M.F.A.; Alkahtani, A.; Mohamed, S.K. Radiation synthesis and characterization of sodium alginate/chitosan/hydroxyapatite nanocomposite hydrogels: A drug delivery system for liver cancer. Polym. Bull. 2015, 72, 725–742. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Berzina-Cimdina, L. Functionalized hydroxyapatite scaffolds coated with sodium alginate and chitosan for controlled drug delivery. Proc. Est. Acad. Sci. 2012, 61, 193. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Borzacchiello, A.; Guarino, V.; Serri, C.; Biondi, M.; Ambrosio, L. Design of electrospayed non-spherical poly (l-lactide-co-glicolide) microdevices for sustained drug delivery. J. Mater. Sci. Mater. Med. 2014, 25, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, R.K.; Naik, J.B. Aceclofenac microspheres: Quality by design approach. Mater. Sci. Eng. C 2014, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, D.-P.; Ye, S.; Zhao, X. Strontium-substituted, luminescent and mesoporous hydroxyapatite microspheres for sustained drug release. J. Mater. Sci. Mater. Med. 2014, 25, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Parmar, S.J.; Easwaran, S. Metronidazole-loaded nanostructured lipid carriers to improve skin deposition and retention in the treatment of rosacea. Drug Dev. Ind. Pharm. 2019, 45, 1039–1051. [Google Scholar] [CrossRef]
- Vazini, H. Anti-Trichomonas vaginalis activity of nano Micana cordifolia and Metronidazole: An in vitro study. J. Parasit. Dis. 2017, 41, 1034–1039. [Google Scholar] [CrossRef]
- Nasongkla, N.; Tanesanukul, C.; Nilyok, S.; Wongsuwan, N.; Tancharoen, S.; Nilanont, S. Nano-coating of metronidazole on dental implants for antibacterial application. In Proceedings of the 2018 IEEE 12th International Conference on Nano/Molecular Medicine and Engineering (NANOMED), Waikiki Beach, HI, USA, 2–5 December 2018; pp. 59–62. [Google Scholar]
- Nasseh, N.; Barikbin, B.; Taghavi, L.; Nasseri, M.A. Adsorption of metronidazole antibiotic using a new magnetic nanocomposite from simulated wastewater (isotherm, kinetic and thermodynamic studies). Compos. Part B Eng. 2019, 159, 146–156. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R.G. Preparation of chitosan-alginate nanoparticles for trans-cinnamaldehyde entrapment. J. Food Sci. 2015, 80, N2305–N2315. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 52, pp. 218–286. [Google Scholar]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G. Characterization of physicochemical and thermal properties of chitosan and sodium alginate after biofield treatment. Pharm. Anal. Acta 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Nath, G.; Bansal, M.; Mishra, B. Development and evaluation of biodegradable chitosan films of metronidazole and levofloxacin for the management of periodontitis. AAPS Pharm. Sci. Tech. 2016, 17, 1312–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.D.C.; Tharani, C.; Narayanan, N.; Kumar, C.S. Formulation and characterization of Methotrexate loaded sodium alginate chitosan Nanoparticles. Indian J. Res. Pharm. Biotechnol. 2013, 1, 915. [Google Scholar]
- De Souza, N.; de Souza, F.; Basílio, I.; Medeiros, A.; Oliveira, E.; Santos, A.; Macwdo, R.; Macędo, R. Thermal stability of metronidazole drug and tablets. J. Therm. Anal. Calorim. 2003, 72, 535–538. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Neto, C.d.T.; Giacometti, J.; Job, A.; Ferreira, F.; Fonseca, J.; Pereira, M. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Paban Kundu, P. Chitosan-graft-PAMAM/alginate core-shell nanoparticles: A safe and promising oral insulin carrier in Animal Model. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Patel, B.K.; Parikh, R.H.; Aboti, P.S. Development of oral sustained release rifampicin loaded chitosan nanoparticles by design of experiment. J. Drug Deliv. 2013, 2013, 370938. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Bhuket, P.R.N.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yan, L.; Hou, W.-G.; Liu, S.-J. Synthesis and release behavior of composites of camptothecin and layered double hydroxide. J. Solid State Chem. 2010, 183, 1811–1816. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Ofomaja, A.E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater. 2006, 129, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sakore, S.; Chakraborty, B. Formulation and evaluation of enalapril maleate sustained release matrix tablets. Int. J. Pharm. 2013, 4, 21–26. [Google Scholar]







| Parameter | Levels (mg) | |||
|---|---|---|---|---|
| Low | Medium | High | ||
| A | Alg | 200 | - | 400 |
| B | CS | 50 | 100 | 200 |
| C | CaCl2 | 30 | - | 60 |
| Std Order | Run Order | Sample Code | Alg | CS | CaCl2 |
|---|---|---|---|---|---|
| 17 | 1 | MAC1 | 200 | 200 | 30 |
| 24 | 2 | MAC2 | 400 | 200 | 60 |
| 10 | 3 | MAC3 | 400 | 100 | 60 |
| 2 | 4 | MAC4 | 200 | 50 | 60 |
| 35 | 5 | MAC5 | 400 | 200 | 30 |
| 20 | 6 | MAC6 | 400 | 50 | 60 |
| 32 | 7 | MAC7 | 400 | 50 | 60 |
| 6 | 8 | MAC8 | 200 | 200 | 60 |
| 22 | 9 | MAC9 | 400 | 100 | 60 |
| 29 | 10 | MAC10 | 200 | 200 | 30 |
| 36 | 11 | MAC11 | 400 | 200 | 60 |
| 14 | 12 | MAC12 | 200 | 50 | 60 |
| 25 | 13 | MAC13 | 200 | 50 | 30 |
| 5 | 14 | MAC14 | 200 | 200 | 30 |
| 9 | 15 | MAC15 | 400 | 100 | 30 |
| 1 | 16 | MAC16 | 200 | 50 | 30 |
| 31 | 17 | MAC17 | 400 | 50 | 30 |
| 26 | 18 | MAC18 | 200 | 50 | 60 |
| 3 | 19 | MAC19 | 200 | 100 | 30 |
| 7 | 20 | MAC20 | 400 | 50 | 30 |
| 16 | 21 | MAC21 | 200 | 100 | 60 |
| 11 | 22 | MAC22 | 400 | 200 | 30 |
| 28 | 23 | MAC23 | 200 | 100 | 60 |
| 27 | 24 | MAC24 | 200 | 100 | 30 |
| 13 | 25 | MAC25 | 200 | 50 | 30 |
| 23 | 26 | MAC26 | 400 | 200 | 30 |
| 30 | 27 | MAC27 | 200 | 200 | 60 |
| 15 | 28 | MAC28 | 200 | 100 | 30 |
| 34 | 29 | MAC29 | 400 | 100 | 60 |
| 18 | 30 | MAC30 | 200 | 200 | 60 |
| 12 | 31 | MAC31 | 400 | 200 | 60 |
| 19 | 32 | MAC32 | 400 | 50 | 30 |
| 4 | 33 | MAC33 | 200 | 100 | 60 |
| 8 | 34 | MAC34 | 400 | 50 | 60 |
| 33 | 35 | MAC35 | 400 | 100 | 30 |
| 21 | 36 | MAC36 | 400 | 100 | 30 |
| LE model | ||||||||
| DF | Adj SS | Adj MS | F value | Coef | T Value | VIF | P value | |
| Model | 7 | 9585.39 | 1369.34 | 337.95 | 47.908 | 67.86 | - | 0.000 |
| Alg | 1 | 1771.00 | 1771.00 | 437.07 | −7.385 | −20.91 | 1.04 | 0.000 |
| CS | 1 | 431.85 | 431.85 | 106.58 | 4.361 | 10.32 | 1.04 | 0.000 |
| CaCl2 | 1 | 208.24 | 208.24 | 51.39 | −2.532 | −7.17 | 1.04 | 0.000 |
| CS*CS | 1 | 2.09 | 2.09 | 0.51 | −0.605 | −0.72 | 1.03 | 0.480 |
| Alg*CS | 1 | 236.52 | 136.52 | 9.01 | 1.252 | 3.00 | 1.04 | 0.006 |
| Alg*CaCl2 | 1 | 6545.51 | 6545.51 | 1615.40 | −13.998 | −40.19 | 1.01 | 0.000 |
| CS*CaCl2 | 1 | 22.71 | 22.71 | 5.60 | 0.987 | 2.37 | 1.04 | 0.026 |
| Lack-of-fit | 4 | 20.77 | 5.19 | 1.35 | - | - | - | 0.283 |
| Particle size model | ||||||||
| Model | 7 | 141548 | 20221.1 | 202.86 | 185.00 | 51.90 | - | 0.000 |
| Alg | 1 | 45889 | 45889.3 | 460.35 | −43.50 | −21.46 | 1.10 | 0.000 |
| CS | 1 | 30270 | 30270 | 303.67 | 44.42 | 17.43 | 1.12 | 0.000 |
| CaCl2 | 1 | 19575 | 19574.9 | 196.37 | −28.53 | −14.01 | 1.12 | 0.000 |
| CS*CS | 1 | 6104 | 6103.7 | 61.23 | −36.54 | −7.83 | 1.13 | 0.000 |
| Alg*CS | 1 | 1963 | 1962.6 | 19.69 | 11.64 | 4.44 | 1.19 | 0.000 |
| Alg*CaCl2 | 1 | 700 | 700.2 | 7.02 | −5.43 | −2.65 | 1.06 | 0.016 |
| CS*CaCl2 | 1 | 11146 | 11145.6 | 111.81 | −27.64 | −10.57 | 1.17 | 0.000 |
| Lack-of-fit | 4 | 173 | 43.4 | 0.38 | - | - | - | 0.821 |
| Zeta potential model | ||||||||
| Model | 7 | 399.875 | 57.125 | 303.51 | −10.501 | −44.19 | - | 0.000 |
| Alg | 1 | 85.093 | 85.093 | 452.11 | 2.119 | 21.26 | 1.07 | 0.000 |
| CS | 1 | 0.256 | 0.256 | 1.36 | 0.133 | 1.17 | 1.19 | 0.263 |
| CaCl2 | 1 | 191.991 | 191.991 | 1020.07 | 3.308 | 31.94 | 1.25 | 0.000 |
| CS*CS | 1 | 30.172 | 30.172 | 160.31 | 3.347 | 12.66 | 1.13 | 0.000 |
| Alg*CS | 1 | 0.404 | 0.404 | 2.15 | 0.164 | 1.47 | 1.17 | 0.165 |
| Alg*CaCl2 | 1 | 181.922 | 181.922 | 966.57 | -3.182 | -31.09 | 1.22 | 0.000 |
| CS*CaCl2 | 1 | 1.855 | 1.855 | 9.85 | 0.344 | 3.14 | 1.05 | 0.007 |
| Lack-of-fit | 3 | 0.562 | 0.187 | 0.99 | - | - | - | 0.432 |
| Regression Model | R-sq (%) | R-sq (adj)% |
|---|---|---|
| LE= −46.07 + 0.3252Alg − 0.0045 CS +2.5210CaCl2 0.000108 CS*CS+0.000167Alg*CS − 0.009332Alg*CaCl2+ 0.000878 CS*CaCl2 | 98.91 | 98.62 |
| Size= 96.7 − 0.4660Alg + 2.856CS + 2.256CaCl2 − 0.006495CS*CS + 0.001552Alg*CS − 0.00362 Alg*CaCl2 − 0.02457CS*CaCl2 | 98.68 | 98.19 |
| Potential= −43.80 + 0.11391Alg − 0.1673CS + 0.8187CaCl2 + 0.000595CS*CS + 0.000022Alg*CS − 0.002121Alg*CaCl2 + 0.000305CS*CaCl2 | 99.35 | 99.02 |
| Value | Alg (350 mg) | CS (150 mg) | CaCl2 (40 mg) |
|---|---|---|---|
| Optimization Responses | |||
| LE | 46.0 ± 2.1% | ||
| Minimum Size | 164.71 ± 20.03 nm | ||
| Zeta potential | −9.25 ± 0.51 mV | ||
| No. | Alg | CS | CaCl2 | %LE | Particle Size (nm) | Zeta Potential (mV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Theo | Error % | Exp | Theo | Error % | Exp | Theo | Error % | ||||
| 1 | 300 | 100 | 50 | 45.0 | 43.0 | 4.7 | 115 | 126 | 8.7 | −9.5 | −8.9 | 6.7 |
| 2 | 200 | 200 | 30 | 43.3 | 45.5 | 4.8 | 285 | 277 | 2.9 | −14.5 | −16.2 | 10.5 |
| 3 | 350 | 150 | 40 | 48.8 | 46.0 | 6.1 | 150 | 165 | 9.1 | −10.8 | −11.5 | 6.1 |
| Samples | R2 | |||
|---|---|---|---|---|
| Pseudo-First Order | Pseudo-Second Order | Hixson-Crowell Model | Korsmeyer-Peppas Model | |
| MAC 8 | 0.917 | 0.988 | 0.781 | 0.877 |
| MAC 21 | 0.903 | 0.956 | 0.734 | 0.882 |
| MAC 19 | 0.930 | 0.990 | 0.822 | 0.891 |
| MAC 5 | 0.664 | 0.977 | 0.787 | 0.856 |
| Equation | ln(qe − qt) = lnqe − k1t | t/qt = 1/k2qe2 + t/qe | ||
| qe is the quantity released at equilibrium, qt is the quantity released at the time (t), Mo is the initial quantity of drug in the nanocomposite, q∞ is the release at the infinite time and k is the rate constant of the release kinetics | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbagh, H.A.K.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Abudayeh, Z.; Ayoub, R.; Abudoleh, S.M. A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers 2020, 12, 772. https://doi.org/10.3390/polym12040772
Sabbagh HAK, Hussein-Al-Ali SH, Hussein MZ, Abudayeh Z, Ayoub R, Abudoleh SM. A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers. 2020; 12(4):772. https://doi.org/10.3390/polym12040772
Chicago/Turabian StyleSabbagh, Hazem Abdul Kader, Samer Hasan Hussein-Al-Ali, Mohd Zobir Hussein, Zead Abudayeh, Rami Ayoub, and Suha Mujahed Abudoleh. 2020. "A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design" Polymers 12, no. 4: 772. https://doi.org/10.3390/polym12040772
APA StyleSabbagh, H. A. K., Hussein-Al-Ali, S. H., Hussein, M. Z., Abudayeh, Z., Ayoub, R., & Abudoleh, S. M. (2020). A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers, 12(4), 772. https://doi.org/10.3390/polym12040772