Fabrication of PCL Scaffolds by Supercritical CO2 Foaming Based on the Combined Effects of Rheological and Crystallization Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. GPC Measurements
2.2. Foaming Process
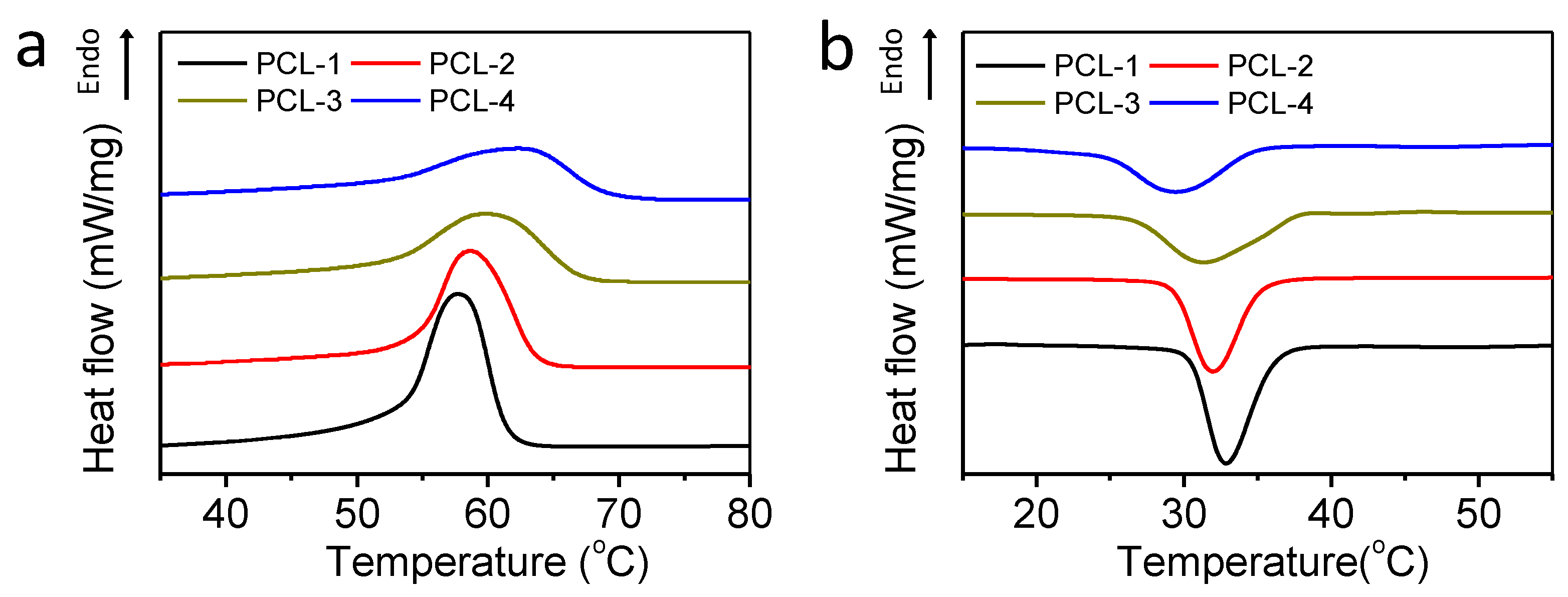
2.3. Thermal Analyses
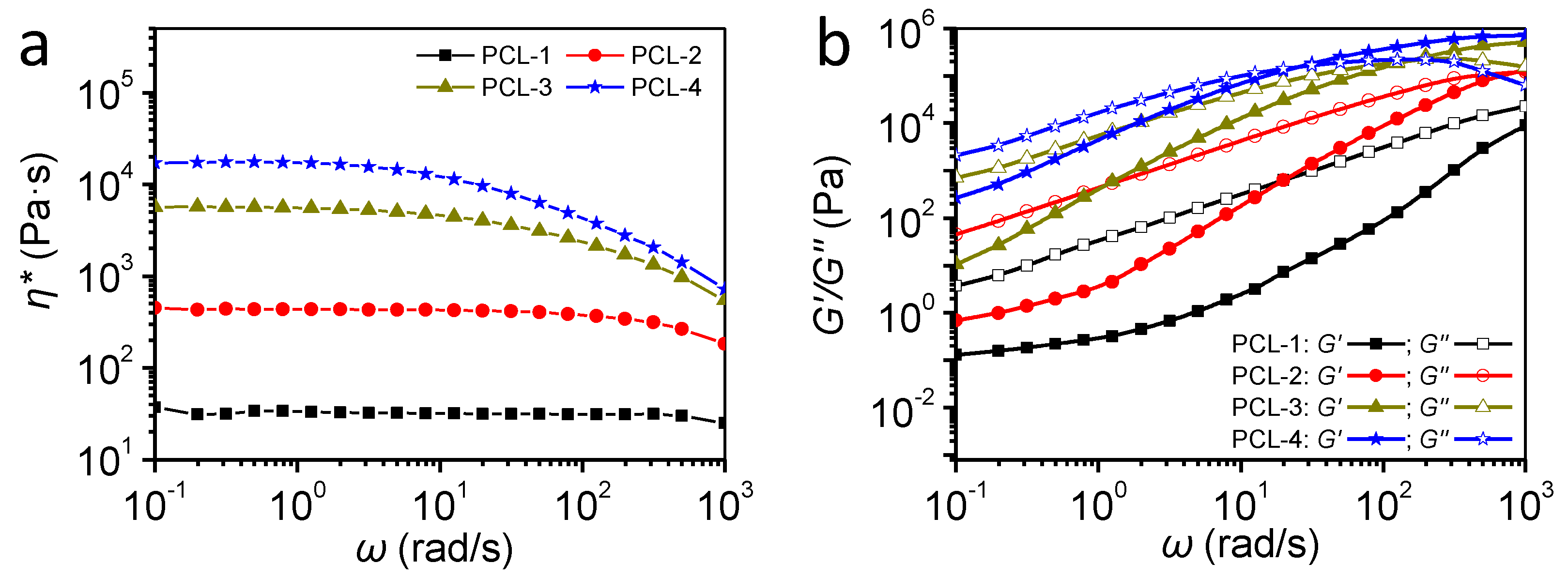
2.4. Rheological Measurements
2.5. Scanning Electron Microscopy (SEM)
2.6. Measurement of Porosity
2.7. Measurement of Interconnectivity
2.8. Measurements of Compressive Properties
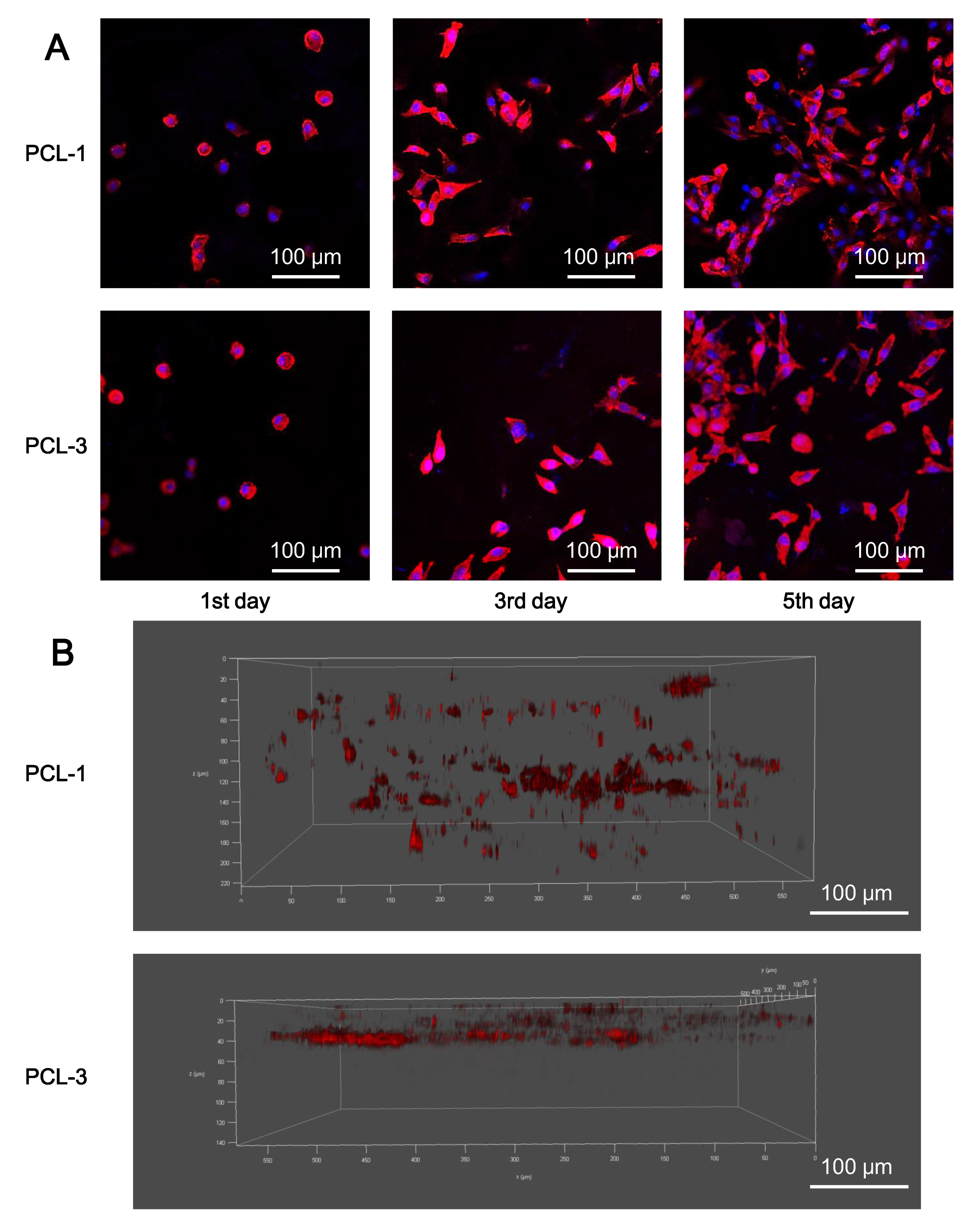
2.9. In Vitro Cell Tests
3. Results and Discussion
3.1. Thermal Analyses
3.2. Rheological Behaviors of PCL Samples
3.3. Porous Morphology of PCL Scaffolds
3.4. Compressive Properties of Porous PCL Scaffolds
3.5. Biocompatibility of Porous PCL Scaffolds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Li, D.; Zhang, K.; Jiang, L.; Shi, C.; Fangteng, J.; Zheng, C.; Yang, B.; Sun, H. Novel synthesized nanofibrous scaffold efficiently delivered hBMP-2 encoded in adenoviral vector to promote bone regeneration. J. Biomed. Nanotechnol. 2017, 13, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv. Healthc. Mater. 2015, 4, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Luetzow, K.; Klein, F.; Weigel, T.; Apostel, R.; Weiss, A.; Lendlein, A. Formation of poly (ε-caprolactone) scaffolds loaded with small molecules by integrated processes. J. Biomech. 2007, 40, S80–S88. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly (α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Prep. Morphol. 1999, 44, 446–455. [Google Scholar]
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102. [Google Scholar] [CrossRef]
- Quirk, R.A.; France, R.M.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. Sci. 2004, 8, 313–321. [Google Scholar] [CrossRef]
- Ismail, H.M.; Zamani, S.; Elrayess, M.A.; Kafienah, W.; Younes, H.M. New three-dimensional poly(decanediol-co-tricarballylate) elastomeric fibrous mesh fabricated by photoreactive electrospinning for cardiac tissue engineering applications. Polymers 2018, 10, 455. [Google Scholar] [CrossRef] [Green Version]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, J.P.; Langer, R. Novel approach to fabricate porous sponges of poly (D, L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Salerno, A.; Ambrosio, L.; Netti, P.A. Design and manufacture of microporous polymeric materials with hierarchal complex structure for biomedical application. Mater. Sci. Technol. 2013, 24, 1111–1117. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. CO2-assisted melting of semicrystalline polymers. Macromolecules 1997, 30, 8505–8507. [Google Scholar] [CrossRef] [Green Version]
- Riddle, K.W.; Mooney, D.J. Role of poly (lactide-co-glycolide) particle size on gas-foamed scaffolds. J. Biomater. Sci. Polym. Ed. 2004, 15, 1561–1570. [Google Scholar] [CrossRef]
- Lemon, G.; Reinwald, Y.; White, L.J.; Howdle, S.M.; Shakesheff, K.M.; King, J.R. Interconnectivity analysis of supercritical CO2-foamed scaffolds. Comput. Methods Programs Biomed. 2012, 106, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The effect of processing variables on morphological and mechanical properties of supercritical CO2 foamed scaffolds for tissue engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, J.J.A.; Silva, M.M.C.G.; Cartmell, S.H.; Guldberg, R.E.; Scotchford, C.A.; Howdle, S.M. Porous methacrylate tissue engineering scaffolds: Using carbon dioxide to control porosity and interconnectivity. J. Mater. Sci. 2006, 41, 4197. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Jiang, J.; Wang, X.; Hou, J.; Sun, S.; Li, Q. Fabrication of highly interconnected porous poly(ε-caprolactone) scaffolds with supercritical CO2 foaming and polymer leaching. J. Mater. Sci. 2019, 54, 5112–5126. [Google Scholar] [CrossRef]
- Salerno, A.; Iannace, S.; Netti, P.A. Graded biomimetic osteochondral scaffold prepared via CO2 foaming and micronized NaCl leaching. Mater. Lett. 2012, 82, 137–140. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, C.; Liu, T.; Chen, Z.; Qiao, Y.; Lu, J.; Yan, J.; Zhao, L.; Esseghir, M. Evaluation of LLDPE/LDPE blend foamability by in situ rheological measurements and bubble growth simulations. Chem. Eng. Sci. 2018, 192, 488–498. [Google Scholar] [CrossRef]
- Karimi, M.; Heuchel, M.; Weigel, T.; Schossig, M.; Hofmann, D.; Lendlein, A. Formation and size distribution of pores in poly(ε-caprolactone) foams prepared by pressure quenching using supercritical CO2. J. Supercrit. Fluids 2012, 61, 175–190. [Google Scholar] [CrossRef]
- Chen, C.X.; Liu, Q.Q.; Xin, X.; Guan, Y.X.; Yao, S.J. Pore formation of poly(ε-caprolactone) scaffolds with melting point reduction in supercritical CO2 foaming. J. Supercrit. Fluids 2016, 117, 279–288. [Google Scholar] [CrossRef]
- Cotugno, S.; Di Maio, E.; Mensitieri, G.; Iannace, S.; Roberts, G.W.; Carbonell, R.G.; Hopfenberg, H.B. Characterization of microcellular biodegradable polymeric foams produced from supercritical carbon dioxide solutions. Ind. Eng. Chem. Res. 2005, 44, 1795–1803. [Google Scholar] [CrossRef]
- Markočič, E.; Škerget, M.; Knez, Z. Effect of temperature and pressure on the behavior of poly(ε-caprolactone) in the presence of supercritical carbon dioxide. Ind. Eng. Chem. Res. 2013, 52, 15594–15601. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Autoclave foaming of poly(ε-Caprolactone) using carbon dioxide: Impact of crystallization on cell structure. J. Cell. Plast. 2007, 43, 459–489. [Google Scholar] [CrossRef] [Green Version]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Song, C.; Li, S.; Zhang, J.; Xi, Z.; Lu, E.; Zhao, L.; Cen, L. Controllable fabrication of porous PLGA/PCL bilayer membrane for GTR using supercritical carbon dioxide foaming. Appl. Surf. Sci. 2019, 472, 82–92. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Y.; Liu, T.; Zhao, L.; Park, C.B. Determination of modified polyamide 6’s foaming windows by bubble growth simulations based on rheological measurements. J. Appl. Polym. Sci. 2019, 136, 48138. [Google Scholar] [CrossRef]
- Reinwald, Y.; Johal, R.K.; Ghaemmaghami, A.M.; Rose, F.R.A.J.; Howdle, S.M.; Shakesheff, K.M. Interconnectivity and permeability of supercritical fluid-foamed scaffolds and the effect of their structural properties on cell distribution. Polymer (UK) 2014, 55, 435–444. [Google Scholar] [CrossRef]
- Baldwin, D.F.; Shimbo, M.; Suh, N.P. The Role of Gas Dissolution and Induced Crystallization During Microcellular Polymer Processing: A Study of Poly (Ethylene Terephthalate) and Carbon Dioxide Systems. J. Eng. Mater. Technol. 1995, 117, 62–74. [Google Scholar] [CrossRef]
- Ameli, A.; Nofar, M.; Jahani, D.; Rizvi, G.; Park, C.B. Development of high void fraction polylactide composite foams using injection molding: Crystallization and foaming behaviors. Chem. Eng. J. 2015, 262, 78–87. [Google Scholar] [CrossRef]
- Ji, G.; Zhai, W.; Lin, D.; Qian, R.; Zheng, W.; Dong, W.J. Microcellular Foaming of Poly(lactic acid)/Silica Nanocomposites in Compressed CO2: Critical Influence of Crystallite Size on Cell Morphology and Foam Expansion. Ind. Eng. Chem. Res. 2013, 52, 6390–6398. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Hu, W. Molecular Segregation in Polymer Melt Crystallization: Simulation Evidence and Unified-Scheme Interpretation. Macromolecules 2005, 38, 8712–8718. [Google Scholar] [CrossRef]
- Gupta, B.; Ray, A.R. Preparation of poly (ε-caprolactone)/poly (ε-caprolactone-co-lactide)(PCL/PLCL) blend filament by melt spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Baldenegro-Perez, L.A.; Navarro-Rodriguez, D.; Medellin-Rodriguez, F.J.; Hsiao, B.; Avila-Orta, C.A.; Sics, I. Molecular weight and crystallization temperature effects on poly (ethylene terephthalate)(PET) homopolymers, an isothermal crystallization analysis. Polymers 2014, 6, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Tung, L.; Buckser, S. The Effects of Molecular Weight on the Crystallinity of Polyethylene. J. Phys. Chem. 1958, 62, 1530–1534. [Google Scholar] [CrossRef]
- Colby, R.H.; Fetters, L.J.; Graessley, W.W. The melt viscosity-molecular weight relationship for linear polymers. Macromolecules 1987, 20, 2226–2237. [Google Scholar] [CrossRef]
- Xu, M.; Yan, H.; He, Q.; Chen, W.; Tao, L.; Ling, Z.; Park, C.B. Chain extension of polyamide 6 using multifunctional chain extenders and reactive extrusion for melt foaming. Eur. Polym. J. 2017, 96, 210–220. [Google Scholar] [CrossRef]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon dioxide-induced melting point depression of biodegradable semicrystalline polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Ding, W.; Kuboki, T.; Wong, A.; Park, C.B.; Sain, M. Rheology, thermal properties, and foaming behavior of high d-content polylactic acid/cellulose nanofiber composites. RSC Adv. 2015, 5, 91544–91557. [Google Scholar] [CrossRef]
- Breuls, R.G.M.; Jiya, T.U.; Smit, T.H. Scaffold stiffness influences cell behavior: Opportunities for skeletal tissue engineering. Open Orthop. J. 2008, 2, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [Green Version]
- Ghavidel Mehr, N.; Li, X.; Ariganello, M.B.; Hoemann, C.D.; Favis, B.D. Poly(ε-caprolactone) scaffolds of highly controlled porosity and interconnectivity derived from co-continuous polymer blends: Model bead and cell infiltration behavior. J. Mater. Sci. Mater. Med. 2014, 25, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
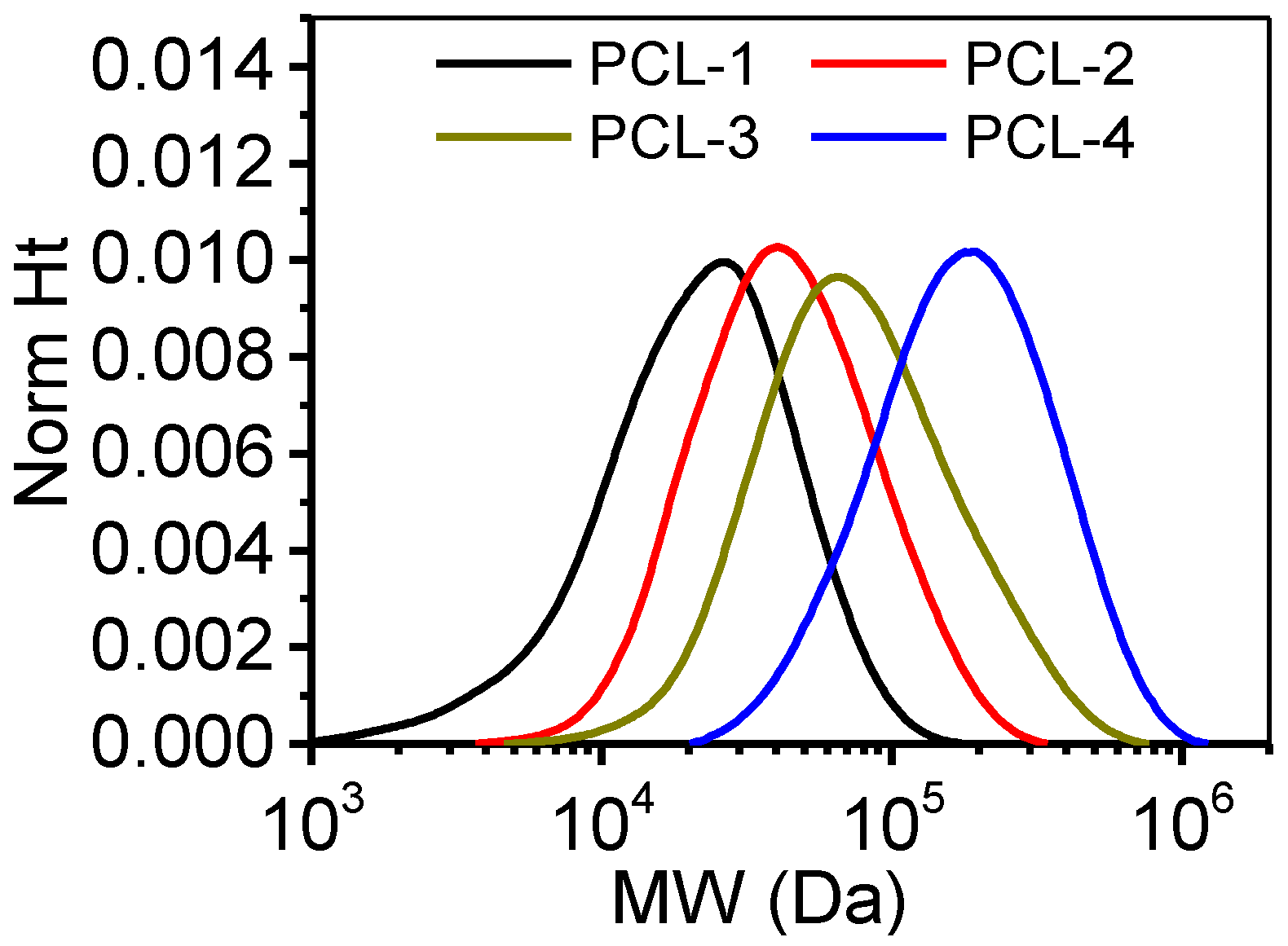



| Samples | Mw | Mn | PDI |
|---|---|---|---|
| PCL-1 | 27 kDa | 14 kDa | 1.876 |
| PCL-2 | 54 kDa | 32 kDa | 1.688 |
| PCL-3 | 100 kDa | 56 kDa | 1.781 |
| PCL-4 | 219 kDa | 137 kDa | 1.604 |
| Tm (°C) | Tc (°C) | Xc (%) | t1/2 (min) | |
|---|---|---|---|---|
| PCL-1 | 57.8 | 33.1 | 53 | 0.39 |
| PCL-2 | 58.9 | 32.2 | 44 | 0.49 |
| PCL-3 | 60.2 | 31.4 | 40 | 0.70 |
| PCL-4 | 63.0 | 30 | 38 | 0.78 |
| Samples | Complex Viscosity | Crystallization | Porous Morphology | |||
|---|---|---|---|---|---|---|
| Rate | Temperature | Average Pore Size (µm) | Interconnectivity (%) | Porosity (%) | ||
| PCL-1 | Lowest | Rapid | High | 125 | 96 | 91 |
| PCL-2 | low | Medium | medium | 73 | 91 | 91 |
| PCL-3 | Medium | Slow | Low | 43 | 82 | 92 |
| PCL-4 | High | Slowest | Lowest | 82 | 84 | 94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Luo, Y.; Liu, Y.; Li, S.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E. Fabrication of PCL Scaffolds by Supercritical CO2 Foaming Based on the Combined Effects of Rheological and Crystallization Properties. Polymers 2020, 12, 780. https://doi.org/10.3390/polym12040780
Song C, Luo Y, Liu Y, Li S, Xi Z, Zhao L, Cen L, Lu E. Fabrication of PCL Scaffolds by Supercritical CO2 Foaming Based on the Combined Effects of Rheological and Crystallization Properties. Polymers. 2020; 12(4):780. https://doi.org/10.3390/polym12040780
Chicago/Turabian StyleSong, Chaobo, Yunhan Luo, Yankai Liu, Shuang Li, Zhenhao Xi, Ling Zhao, Lian Cen, and Eryi Lu. 2020. "Fabrication of PCL Scaffolds by Supercritical CO2 Foaming Based on the Combined Effects of Rheological and Crystallization Properties" Polymers 12, no. 4: 780. https://doi.org/10.3390/polym12040780





