In Vivo Stability of Polyurethane-Based Electrospun Vascular Grafts in Terms of Chemistry and Mechanics
Abstract
1. Introduction
2. Materials and Methods
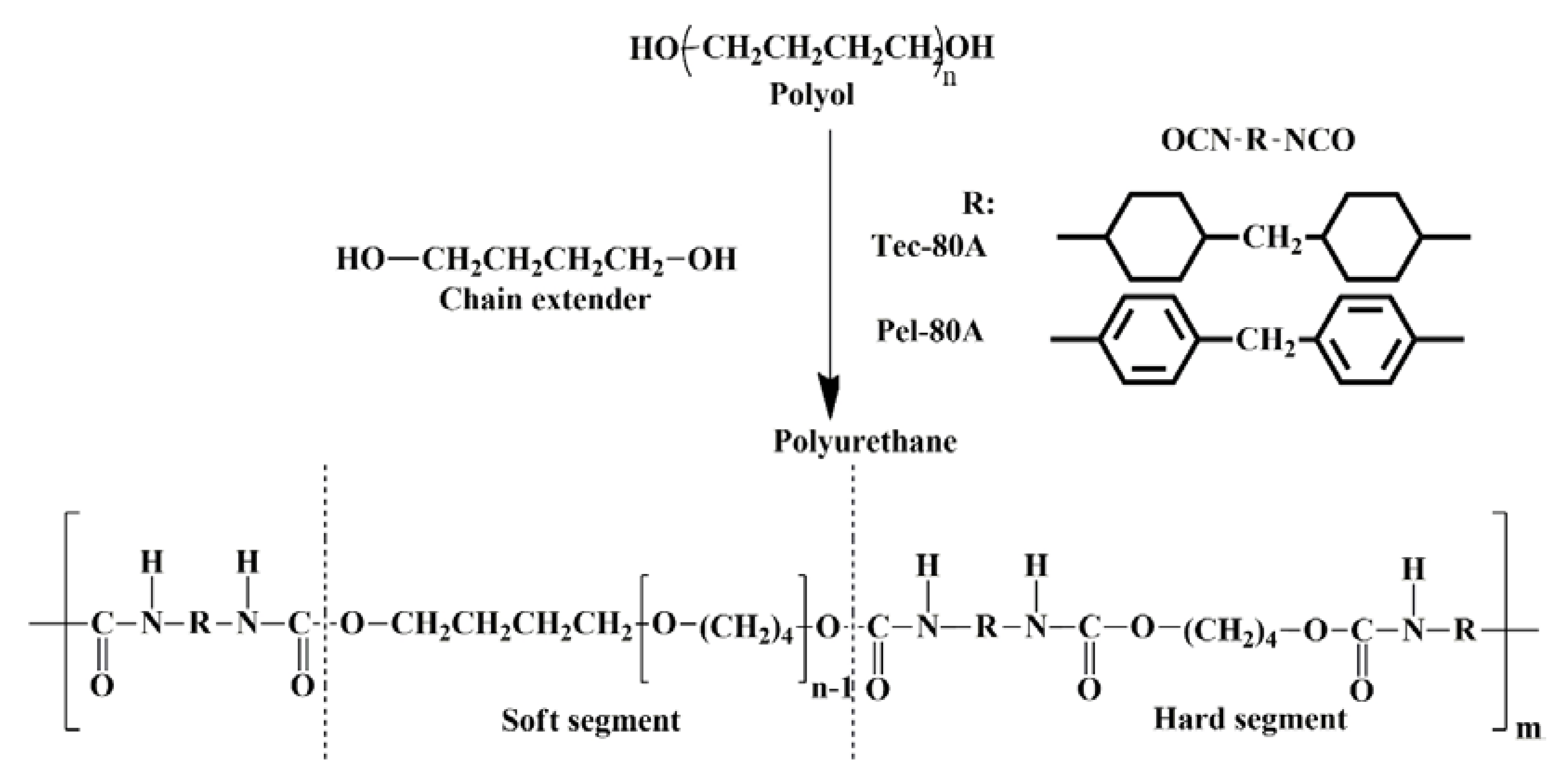
2.1. Electrospinning of Vascular Grafts
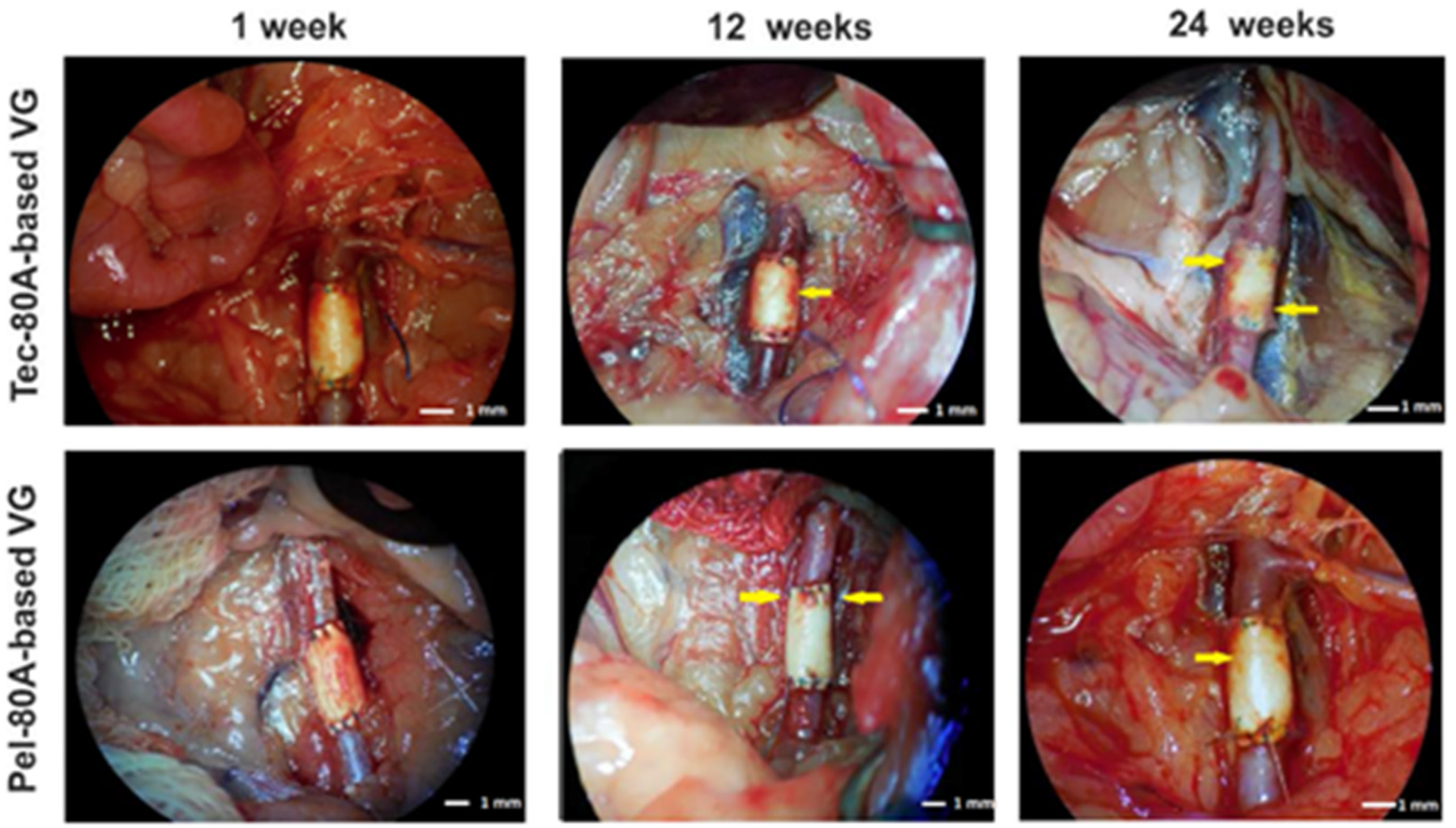
2.2. Implanting VGs and Examining Their In Vivo Functioning
2.3. VG Treatment after Explanting
2.4. Gel Permeation Chromatography (GPC)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
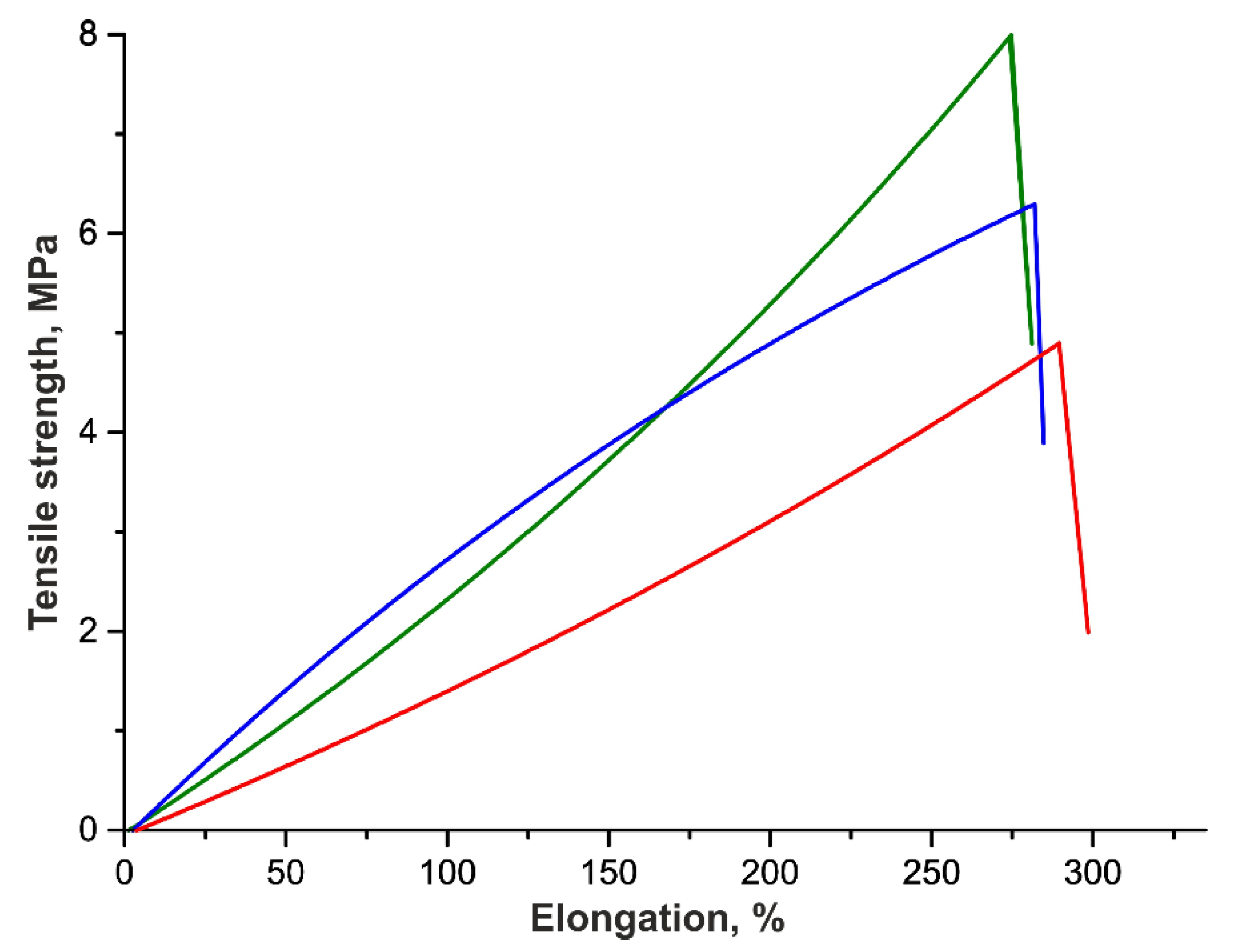
2.6. Testing Tensile Strength
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- LaPorte, R.J. Hydrophilic Polymer Coatings for Medical Devices; Routledge: New York, NY, USA, 2017; pp. 156–159. [Google Scholar]
- Kheradvar, A.; Groves, E.M.; Dasi, L.P.; Alavi, S.H.; Tranquillo, R.; Grande-Allen, K.J.; Simmons, C.A.; Griffith, B.; Falahatpisheh, A.; Goergen, C.J.; et al. Emerging trends in heart valve engineering: Part I. Solutions for future. Ann. Biomed. Eng. 2015, 43, 833–843. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Hergenrother, R.W.; Wabers, H.D.; Cooper, S.L. Effect of hard segment chemistry and strain on the stability of polyurethanes: In vivo biostability. Biomaterials 1993, 14, 449–458. [Google Scholar] [CrossRef]
- Szycher, M. Biostability of polyurethane elastomers: A critical review. J. Biomater. Appl. 1988, 3, 297–402. [Google Scholar] [CrossRef]
- Simmons, A.; Hyvarinen, J.; Odell, R.A.; Martin, D.J.; Gunatillake, P.A.; Noble, K.R.; Poole-Warren, L.A. Long-term in vivo biostability of poly (dimethylsiloxane)/poly (hexamethylene oxide) mixed macrodiol-based polyurethane elastomers. Biomaterials 2004, 25, 4887–4900. [Google Scholar] [CrossRef]
- Grasl, C.; Bergmeister, H.; Stoiber, M.; Schima, H.; Weigel, G. Electrospun polyurethane vascular grafts: In vitro mechanical behavior and endothelial adhesion molecule expression. J. Biomed. Mater. Res. A 2010, 93, 716–723. [Google Scholar]
- Bergmeister, H.; Seyidova, N.; Schreiber, C.; Strobl, M.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Fröhlich, S.; Marchetti-Deschmann, M.; et al. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater. 2015, 11, 104–113. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fiber surface. Polym. Adv. Technol. 2017, 28, 819–827. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Salick, M.R.; Cordie, T.M.; Peng, X.F.; Turng, L.S. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mat. Sci. Eng. C Mater. 2015, 49, 40–50. [Google Scholar] [CrossRef]
- Firoozi, S.; Derakhshan, M.A.; Karimi, R.; Rashti, A.; Negahdari, B.; Faridi Majidi, R.; Mashaghi, S.; Ghanbari, H. Fabrication and characterization of nanofibrous tricuspid valve scaffold based on polyurethane for heart valve tissue engineering. Nanomed. Res. J. 2017, 2, 131–141. [Google Scholar]
- Puperi, D.S.; Kishan, A.; Punske, Z.E.; Wu, Y.; Cosgriff-Hernandez, E.; West, J.L.; Grande-Allen, K.J. Electrospun polyurethane and hydrogel composite scaffolds as biomechanical mimics for aortic valve tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 1546–1558. [Google Scholar] [CrossRef]
- D’Amore, A.; Luketich, S.K.; Raffa, G.M.; Olia, S.; Menallo, G.; Mazzola, A.; D’Accardi, F.; Grunberg, T.; Gu, X.; Pilato, M.; et al. Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. Biomaterials 2018, 150, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun polyurethane/keratin/AgNP biocomposite mats for biocompatible and antibacterial wound dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef]
- Masaeli, E.; Karamali, F.; Loghmani, S.; Eslaminejad, M.B.; Nasr-Esfahani, M.H. Bio-engineered electrospun nanofibrous membranes using cartilage extracellular matrix particles. J. Mater. Chem. B 2017, 5, 765–776. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Kvon, R.I.; Kiseleva, E.V.; Stepanova, A.O.; Laktionov, P.P. The study of the surface layer of 3D-matrices for tissue engineering. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2017, 11, 139–145. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Gostev, A.A.; Gao, Y.; Chesalov, Y.A.; Shutov, A.V.; Pokushalov, E.A.; Karpenko, A.A.; Laktionov, P.P. Mechanical properties and biological behavior of 3D matrices produced by electrospinning from protein-enriched polyurethane. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Gostev, A.A.; Chesalov, Y.A.; Karpenko, A.A.; Karaskov, A.M.; Laktionov, P.P. Study of hemocompatibility and endothelial cell interaction of Tecoflex-based electrospun vascular grafts. Int. J. Polym. Mater. PO 2018, 68, 34–43. [Google Scholar] [CrossRef]
- Farah, S.; Kunduru, K.R.; Basu, A.; Domb, A.J. Molecular weight determination of polyethylene terephthalate. In Poly (Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; William Andrew Publishing: Waltham, MA, USA, 2015; pp. 143–165. [Google Scholar]
- Kolesov, S.V.; Zaidullin, I.S.; Spirikhin, L.V.; Volodina, V.P.; Kukovinets, O.S.; Sigaeva, N.N.; Vildanova, R.R. Modification of chitosan and hyaluronic acid to obtain sustainable hydrogels. In Physical Chemistry for the Chemical and Biochemical Sciences; Apple Academic Press: Oakville, ON, Canada, 2016; pp. 31–48. [Google Scholar]
- Popova, I.V.; Stepanova, A.O.; Plotnikova, T.A.; Sergeevichev, D.S.; Akulov, A.E.; Pokushalov, A.A.; Laktionov, P.P.; Karpenko, A.A. Study of patency of vascular grafts manufactured by means of electrospinning. Angiol. Sosud. Khir. 2015, 21, 136–138. [Google Scholar]
- ISO 7198:1998 Cardiovascular Implants—Tubular Vascular Prostheses; ISO: Washington, DC, USA, 1998.
- Kucińska-Lipka, J.; Gubańska, I.; Janik, H. Gelatin-modified polyurethanes for soft tissue scaffold. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Gregory, E.K.; Webb, A.; Vercammen, J.M.; Kelly, M.E.; Akar, B.; van Lith, R.; Bahnson, E.M.; Jiang, W.; Ameer, G.A.; Kibbe, M.R. Inhibiting intimal hyperplasia in prosthetic vascular grafts via immobilized all-trans retinoic acid. J. Control. Release 2018, 274, 69–80. [Google Scholar] [CrossRef]
- Kohler, T.R.; Kirkman, T.R.; Kraiss, L.W.; Zierler, B.K.; Clowes, A.W. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circ. Res. 1991, 69, 1557–1565. [Google Scholar] [CrossRef]
- Martins, M.C.; Wang, D.; Ji, J.; Feng, L.; Barbosa, M.A. Albumin and fibrinogen adsorption on PU–PHEMA surfaces. Biomaterials 2003, 24, 2067–2076. [Google Scholar] [CrossRef]
- Stokes, K.B. Polyether polyurethanes: Biostable or not? J. Biomater. Appl. 1988, 3, 228–259. [Google Scholar] [CrossRef]
- Gruendling, T.; Junkers, T.; Guilhaus, M.; Barner-Kowollik, C. Mark–Houwink parameters for the universal calibration of acrylate, methacrylate and vinyl acetate polymers determined by online size-exclusion chromatography—Mass spectrometry. Macromol. Chem. Phys. 2010, 211, 520–528. [Google Scholar] [CrossRef]
- Ma, J.; Liang, B.; Cui, P.; Dai, H.; Huang, R. Dilute solution properties of hydrophobically associating polyacrylamide: Fitted by different equations. Polymer 2003, 44, 1281–1286. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Martin, D.J.; Meijs, G.F.; McCarthy, S.J.; Adhikari, R. Designing biostable polyurethane elastomers for biomedical implants. Aust. J. Chem. 2003, 56, 545–557. [Google Scholar] [CrossRef]
- Pinchuk, L. A review of the biostability and carcinogenicity of polyurethanes in medicine and the new generation of ‘biostable’ polyurethanes. J. Biomater. Sci. Polym. Ed. 1995, 6, 225–267. [Google Scholar] [CrossRef]
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polym. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Mathur, A.B.; Collier, T.O.; Kao, W.J.; Wiggins, M.; Schubert, M.A.; Hiltner, A.; Anderson, J.M. In vivo biocompatibility and biostability of modified polyurethanes. J. Biomed. Mater. Res. 1997, 36, 246–257. [Google Scholar] [CrossRef]
- Schubert, M.A.; Wiggins, M.J.; Anderson, J.M.; Hiltner, A. Comparison of two antioxidants for poly (etherurethane urea) in an accelerated in vitro biodegradation system. J. Biomed. Mater. Res. 1997, 34, 493–505. [Google Scholar] [CrossRef]
- Thomas, V.; Jayabalan, M. A new generation of high flex life polyurethane urea for polymer heart valve—Studies on in vivo biocompatibility and biodurability. J. Biomed. Mater. Res. A 2009, 89, 192–205. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.J.; Meijs, G.F.; Mitchell, N.; Gunatillake, P.A.; Schindhelm, K. In-vivo degradation of polyurethanes: Transmission-FTIR microscopic characterization of polyurethanes sectioned by cryomicrotomy. Biomaterials 1997, 18, 1387–1409. [Google Scholar] [CrossRef]
- Therona, J.P.; Knoetzeb, J.H.; Sandersonc, R.D.; Hunterd, R.; Mequaninte, K.; Franza, T.; Zillaa, P.; Bezuidenhouta, D. Modification, crosslinking and reactive electrospinning of a thermoplastic medical polyurethane for vascular graft applications. Acta Biomater. 2010, 6, 2434–2447. [Google Scholar] [CrossRef]
- Bergmeister, H.; Schreiber, C.; Grasl, C.; Walter, I.; Plasenzotti, R.; Stoiber, M.; Bernhard, D.; Schima, H. Healing characteristics of electrospun polyurethane grafts with various porosities. Acta Biomater. 2013, 9, 6032–6040. [Google Scholar] [CrossRef]
| No. | Composition of Matrix | Mn, kDa | Mw, kDa | n |
|---|---|---|---|---|
| 1 | Tec-80A control | 102 ± 2 | 152 ± 4 | 1.49 ± 0.02 |
| 2 | Tec-80A (1 week after implantation) | 90 ± 2 | 137 ± 3 | 1.52 ± 0.04 |
| 3 | Tec-80A (12 weeks after implantation) | 81 ± 3 | 130 ± 5 | 1.60 ± 0.02 |
| 4 | Tec-80A (24 weeks after implantation) | 91 ± 3 | 138 ± 5 | 2.13 ± 0.01 |
| p | 0.02 | 0.04 | 0.24 | |
| 5 | Pel-80A control | 105 ± 4 | 190 ± 6 | 1.80 ± 0.04 |
| 6 | Pel-80A (1 week after implantation) | 105 ± 6 | 190 ± 7 | 1.80 ± 0.05 |
| 7 | Pel-80A (12 weeks after implantation) | 115 ± 5 | 190 ± 3 | 1.65 ± 0.04 |
| 9 | Pel-80A (24 weeks after implantation) | 110 ± 3 | 194 ± 5 | 1.76 ± 0.01 |
| p | 0.11 | 0.79 | 0.06 |
| Polyurethane in Matrix | Time after Explantation | I1703/I1730 | I1110/I1220 | I1075/I1220 | I1110/I1075 |
|---|---|---|---|---|---|
| Tec-80A | Control | 0.84 ± 0.03 | 2.86 ± 0.21 | 0.75 ± 0.06 | 3.81 ± 0.12 |
| 1 week | 0.87 ± 0.05 | 2.38 ± 0.19 | 0.88 ± 0.07 | 2.70 ± 0.16 | |
| 12 weeks | 0.77 ± 0.03 | 2.10 ± 0.17 | 0.80 ± 0.07 | 2.63 ± 0.14 | |
| 24 weeks | 0.85 ± 0.04 | 2.28 ± 0.17 | 0.80 ± 0.05 | 2.90 ± 0.10 | |
| p | 0.07 | 0.04 | 0.19 | 0.03 | |
| Pel-80A | Control | 0.94 ± 0.05 | 0.90 ± 0.07 | 0.88 ± 0.06 | 1.03 ± 0.08 |
| 1 week | 0.90 ± 0.05 | 0.91 ± 0.08 | 0.89 ± 0.07 | 1.03 ± 0.09 | |
| 12 weeks | 0.88 ± 0.06 | 0.89 ± 0.08 | 0.87 ± 0.06 | 1.03 ± 0.08 | |
| 24 weeks | 0.78 ± 0.05 | 0.87 ± 0.06 | 0.88 ± 0.06 | 0.99 ± 0.07 | |
| p | 0.06 | 0.81 | 0.83 | 0.90 |
| Tensile Strength, MPa | Elongation, % | Thickness of the VG, µm | |
|---|---|---|---|
| Initial | 6.6 ± 0.6 | 308 ± 9 | 138 ± 9 |
| 3 months | 4.8 ± 0.7 | 304 ± 10 | 170 ± 17 |
| 6 months | 7.7 ± 0.7 | 290 ± 13 | 198 ± 16 |
| p | 0.03 | 0.39 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostev, A.A.; Shundrina, I.K.; Pastukhov, V.I.; Shutov, A.V.; Chernonosova, V.S.; Karpenko, A.A.; Laktionov, P.P. In Vivo Stability of Polyurethane-Based Electrospun Vascular Grafts in Terms of Chemistry and Mechanics. Polymers 2020, 12, 845. https://doi.org/10.3390/polym12040845
Gostev AA, Shundrina IK, Pastukhov VI, Shutov AV, Chernonosova VS, Karpenko AA, Laktionov PP. In Vivo Stability of Polyurethane-Based Electrospun Vascular Grafts in Terms of Chemistry and Mechanics. Polymers. 2020; 12(4):845. https://doi.org/10.3390/polym12040845
Chicago/Turabian StyleGostev, Alexander A., Inna K. Shundrina, Vitaliy I. Pastukhov, Alexey V. Shutov, Vera S. Chernonosova, Andrey A. Karpenko, and Pavel P. Laktionov. 2020. "In Vivo Stability of Polyurethane-Based Electrospun Vascular Grafts in Terms of Chemistry and Mechanics" Polymers 12, no. 4: 845. https://doi.org/10.3390/polym12040845
APA StyleGostev, A. A., Shundrina, I. K., Pastukhov, V. I., Shutov, A. V., Chernonosova, V. S., Karpenko, A. A., & Laktionov, P. P. (2020). In Vivo Stability of Polyurethane-Based Electrospun Vascular Grafts in Terms of Chemistry and Mechanics. Polymers, 12(4), 845. https://doi.org/10.3390/polym12040845





