Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion and Low Water Absorption (V). Effects of Ester-linked Diamines with Different Lengths and Substituents
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Monomer Synthesis
2.1.2. Common Monomers
2.1.3. Polymerization and Thermal Imidization for PEsI Film Preparation
2.2. Measurements
2.2.1. Inherent Viscosities
2.2.2. Linear Coefficients of Thermal Expansion (CTE)
2.2.3. Linear Coefficients of Hygroscopic (Humidity) Expansion (CHE)
2.2.4. Glass Transition Temperatures
2.2.5. Water Absorption
2.2.6. Birefringence
2.2.7. Mechanical Properties
2.2.8. Flame Retardancy
2.2.9. Dielectric Constants and Dissipation Factors
3. Results and Discussion
3.1. PEsIs Derived from AB-HQ without Substituents
3.1.1. Polymerizability of AB-HQ
3.1.2. Film Properties
3.1.3. Isomer Effects
3.2. PEsIs Derived from AB-HQ Analogs with Small Substituents
3.3. PEsIs Derived from AB-HQ Analogs with Bulky Substituents
3.4. PEsIs Derived from Ester-linked Diamines with Longitudinally further Extended Structures
3.5. Data Analysis
3.5.1. Influence of Side Group Bulkiness on Tg and CTE
3.5.2. Influence of the Position of Ester-linked Aromatic unit on Properties
3.5.3. Correlation of WA and Imide Group Content
3.5.4. Correlation of CHE and WA
3.5.5. Correlation of Tensile Modulus and CTE
3.6. Flame Retardancy
3.7. Dielectric Properties
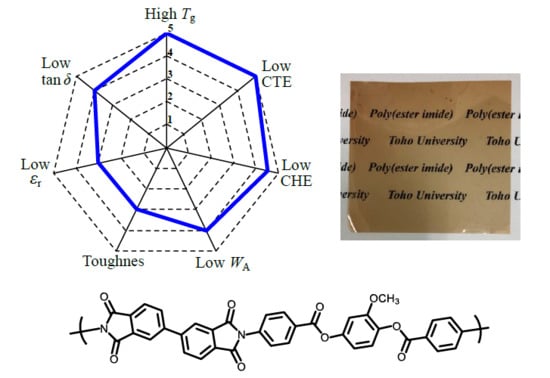
3.8. Performance Balance of the PEsIs for Use in High-performance FPCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides: Thermally Stable Polymers; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Bessonov, M.I.; Zubkov, V.A. Polyamic Acid and Polyimides: Synthesis, Transformation and Structure; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Sachdev, H.S.; Khojasteh, M.M.; Feger, C. Advances in Polyimides and Low Dielectric Polymer; Society of Plastic Engineers: New York, NY, USA, 1997. [Google Scholar]
- Ando, S.; Ueda, M.; Kakimoto, M.; Kochi, M.; Takeichi, T.; Hasegawa, M.; Yokota, R. The Latest Polyimides: Fundamentals and Applications, 2nd ed.; NTS: Tokyo, Japan, 2010. [Google Scholar]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Yang, S.Y. Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Chemical Industry Press: Beijing, China; Elsevier: Amsterdam, The Netherland, 2018. [Google Scholar]
- Hergenrother, P.M. The use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koseki, K. Poly (ester imide) s possessing low coefficient of thermal expansion and low water absorption. High Perform. Polym. 2006, 18, 697–717. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tsujimura, Y.; Koseki, K.; Miyazaki, T. Poly (ester imide) s possessing low CTE and low water absorption (II). Effect of substituents. Polym. J. 2008, 40, 56–67. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sakamoto, Y.; Tanaka, Y.; Kobayashi, Y. Poly(ester imide)s possessing low coefficients of thermal expansion (CTE) and low water absorption (III). Use of bis(4-aminophenyl)terephthalate and effect of substituents. Eur. Polym. J. 2010, 46, 1510–1524. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saito, T.; Tsujimura, Y. Poly(ester imide)s possessing low coefficients of thermal expansion and low water absorption (IV): Effects of ester-linked tetracarboxylic dianhydrides with longitudinally extended structures. Polym. Adv. Technol. 2020, 31, 389–406. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J. Optically transparent aromatic poly(ester imide)s with low coefficients of thermal expansion (1). Self-orientation behavior during solution casting process and substituent effect. Polymer 2015, 74, 1–15. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirai, T.; Ishigami, T.; Takahashi, S.; Ishii, J. Optically transparent aromatic poly(ester imide)s with low coefficients of thermal expansion. 2: Effect of the introduction of alkyl-substituted p-biphenylene units. Polym. Int. 2018, 67, 431–444. [Google Scholar] [CrossRef]
- Hishiki, T.; Hasegawa, M. Low-CTE and low-water absorption poly(ester imide)s (14). Use of novel ester-containing diamines with various substituents. Polym. Prepr. Jpn. 2008, 57, 4178. [Google Scholar]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and properties of novel asymmetric biphenyl type polyimides. Homo-and copolymers and blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Sensui, N.; Ishii, J.; Takata, A.; Oami, Y.; Hasegawa, M.; Yokota, R. Ultra-low CTE and improved toughness of PMDA/PDA polyimide-based molecular composites containing asymmetric BPDA-type polyimides. High Perform. Polym. 2009, 21, 709–728. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Numata, S.; Oohara, S.; Fujisaki, K.; Imaizumi, J.; Kinjyo, N. Thermal expansion behavior of various aromatic polyimides. J. Appl. Polym. Sci. 1986, 31, 101–110. [Google Scholar] [CrossRef]
- Numata, S.; Fujisaki, K.; Kinjyo, N. Re-examination of the relationship between packing coefficient and thermal expansion coefficient for aromatic polyimides. Polymer 1987, 28, 2282–2288. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization. 2. In-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Coburn, J.C.; Pottiger, M.T. Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 207–247. [Google Scholar]
- KAPTON® H Film Data Sheet. Available online: https://www.td-net.co.jp/kapton/data/download/documents/kapton2007.pdf (accessed on 24 March 2020).
- Hasegawa, M.; Kaneki, T.; Tsukui, M.; Okubo, N.; Ishii, J. High-temperature polymers overcoming the trade-off between excellent thermoplasticity and low thermal expansion properties. Polymer 2016, 99, 292–306. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Wada, Y. Approaches to improve the film ductility of colorless cycloaliphatic polyimides. Polym. Adv. Technol. 2018, 29, 921–933. [Google Scholar] [CrossRef]
- Korshak, V.V.; Vinogradova, S.V.; Vygodskii, Y.S. Cardo polymers. J. Macromol. Sci. Rev. Macromol. Chem. Part C 1974, 11, 45–142. [Google Scholar] [CrossRef]
- Yang, C.P.; Lin, J.H. Syntheses and properties of aromatic polyamides and polyimides derived from 9,9-bis[4-(p-aminophenoxy)phenyl]fluorene. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2153–2163. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tominaga, A. Fluorene-Containing Poly (ester imide) s and their Application to Positive-Type Photosensitive Heat-Resistant Materials. Macromol. Mater. Eng. 2011, 296, 1002–1017. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, S.; Tsukuda, S.; Hirai, T.; Ishii, J.; Yamashina, Y.; Kawamura, Y. Symmetric and asymmetric spiro-type colorless poly(ester imide)s with low coefficients of thermal expansion, high glass transition temperatures, and excellent solution-processability. Polymer 2019, 169, 167–184. [Google Scholar] [CrossRef]
- Ishii, J.; Shimizu, N.; Ishihara, N.; Ikeda, Y.; Sensui, N.; Matano, T.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (4): Casting-and melt-induced in-plane orientation. Eur. Polym. J. 2010, 46, 69–80. [Google Scholar] [CrossRef]
- Hasegawa, M.; Honda, T.; Kurokawa, S.; Shindo, K.; Ishii, J. Low-CTE and low-water absorption poly(ester imide)s (40). Structural factors influencing sub-glass transitions. Polym. Prepr. Jpn. 2012, 61, 4120. [Google Scholar]
- Baklagina, Y.G.; Milevskaya, I.S.; Efanova, N.V.; Sidorovich, A.V.; Zubkov, V.A. Structure of rigid-chain polyimides from pyromellitic dianhydride. Vysokomol. Soedin. 1976, A18, 1235–1242. [Google Scholar]
- Obata, Y.; Okuyama, K.; Kurihara, S.; Kitano, Y.; Jinda, T. X-ray structure analysis of a wholly aromatic polyimide with a rigid-rod chain. Macromolecules 1995, 28, 1547–1551. [Google Scholar] [CrossRef]
- UPILEX® S Film Data Sheet. Available online: http://www.upilex.jp/en/upilex_grade.html#01 (accessed on 24 March 2020).
- U-Polymer (U-100) Film Data Sheet. Available online: https://www.unitika.co.jp/plastics/e/products/par/upolymer/u-100/ (accessed on 24 March 2020).
- Shi, Z.; Fujita, A. Extremely enhanced hydrolytic stability of poly (ethylene terephthalate) films. Polym. Eng. Sci. 2018, 58, 261–271. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Hori, A.; Ishii, J.; Hasegawa, M. Low-CTE and low-water absorption poly(ester imide)s (39). Strategies for further decreasing tensile modulus. Polym. Prepr. Jpn. 2012, 61, 4116. [Google Scholar]
- Lu, S.Y.; Hamerton, L. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Hong, X.; Wang, C. Synthesis and properties of a novel phosphorous-containing flame-retardant hardener for epoxy resin. J. Appl. Polym. Sci. 2013, 128, 2759–2765. [Google Scholar] [CrossRef]
- Kraray Co. URL. Available online: https://www.kuraray.com/products/liquid_crystalline (accessed on 24 March 2020).












| No. | Diamine (mol %) | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AB-HQ | PMDA | 0.57 | ND a | 3.6 | 6.52 | 7.7/9.8 | 0.227 | 526 | 483 | 0.86 | ---- |
| 2 | ibid | s-BPDA | 1.72 | ND | 0.7 | 6.41 | 5.8/7.4 | 0.211 | 530 | 489 | 0.52 | 0.5 |
| 3 | ibid | TA-HQ | 1.21 | ND | 13.2 | 5.61 | 15.3/20.1 | 0.207 | 498 | 478 | 0.42 | ---- |
| 4 | AB-HQ (70) 4,4′-ODA (30) | s-BPDA | 1.85 | ND | 8.0 | 5.11 | 23.1/38.4 | 0.258 | 528 | 507 | 0.82 | 4.9 |
| 5 | AB-HQ (60) 4,4′-ODA (40) | s-BPDA | 1.16 | ND | 11.7 | 4.99 | 35.8/47.1 | 0.296 | 524 | 503 | 0.64 | 4.5 |
| No. | Diamine | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | AB-MHQ | PMDA | 1.21 | ND a | –0.6 | 8.00 | 9.2/12.8 | 0.327 | 492 | 464 | 0.88 | ---- |
| 7 | ibid | s-BPDA | 1.16 | ND | 9.5 | 6.91 | 3.4/4.6 | 0.181 | 498 | 473 | 0.58 | 2.3 |
| 8 | ibid | TA-HQ | 1.53 | 342 | 15.5 | 5.46 | 26.4/34.4 | 0.242 | 476 | 442 | 0.23 | ---- |
| No. | Diamine (mol %) | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | AB-MeOHQ | PMDA | 1.57 | ND a | –0.6 | 7.68 | 4.7/6.2 | 0.213 | 467 | 451 | 1.20 | ---- |
| 10 | ibid | s-BPDA | 1.36 | 424 | 5.6 | 6.01 | 15.1/23.0 | 0.236 | 473 | 458 | 0.41 | 3.1 |
| 11 | ibid | TA-HQ | 2.05 | 338 | 16.3 | 5.85 | 2.5/3.1 | 0.129 | 444 | 427 | 0.73 | ---- |
| 12 | AB-MeOHQ (70)4,4′-ODA (30) | s-BPDA | 1.58 | 430 | 13.5 | 4.01 | 16.5/27.7 | 0.197 | 470 | 461 | 0.78 | 2.5 |
| No. | Diamine (mol %) | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | AB-PhHQ | PMDA | 1.12 | ND a | 3.7 | 5.87 | 6.9/9.3 | 0.239 | 491 | 446 | 1.10 | ---- |
| 14 | ibid | s-BPDA | 0.92 | 371 | 7.3 | 4.89 | 2.0/2.5 | 0.091 | 488 | 456 | 0.46 | 1.0 |
| 15 | ibid | TA-HQ | 1.25 | 352 | 25.8 | 5.01 | 13.5/22.2 | 0.215 | 478 | 445 | 0.34 | ---- |
| 16 | AB-PhHQ (70) 4,4′-ODA (30) | s-BPDA | 1.15 | 326 | 29.7 | 4.71 | 7.7/10.3 | 0.206 | 484 | 463 | 0.53 | 2.2 |
| No. | Diamine (mol %) | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | AB-14DHN | PMDA | 1.51 | ND a | 7.2 | 5.58 | 2.4/2.7 | 0.154 | 492 | 1.09 | ---- | |
| 18 | ibid | s-BPDA | 1.24 | ND | 10.7 | 4.09 | 1.8/2.2 | 0.103 | 488 | 422 | 0.58 | ---- |
| 19 | ibid | TA-HQ | 1.12 | >405 | 16.1 | 5.41 | 5.5/8.6 | 0.194 | 469 | 451 | 0.38 | ---- |
| 20 | AB-14DHN (70) 4,4′-ODA (30) | s-BPDA | 1.28 | ND | 16.0 | 4.51 | 6.9/10.9 | 0.199 | 471 | 456 | 0.65 | 2.7 |
| No. | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 21 | PMDA | 0.51 | ND a | 26.3 | 4.75 | 3.5/4.4 | 0.121 | 518 | 477 | 0.48 |
| 22 | s-BPDA | 0.89 | ND | 18.8 | 5.44 | 4.4/6.3 | 0.151 | 512 | 491 | 0.41 |
| 23 | TA-HQ | 0.59 | ND | 20.6 | 5.28 | 5.5/7.3 | 0.153 | 483 | 464 | 0.56 |
| No. | Diamine (mol %) | TCDA | ηinh PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb Ave/Max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/RH%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | AB-DP44BP | PMDA | 2.05 | ND a | 11.2 | 4.83 | 6.6/8.7 | 0.187 | 499 | 415 | 0.49 | ---- |
| 25 | ibid | s-BPDA | 1.07 | 433 | 12.8 | 5.29 | 3.7/4.4 | 0.169 | 501 | 461 | 0.36 | 5.2 |
| 26 | ibid | TA-HQ | 1.28 | 322 | 24.0 | 4.99 | 9.5/16.6 | 0.204 | 479 | 433 | 0.35 | ---- |
| 27 | AB-DP44BP (80) 4,4′-ODA (20) | s-BPDA | 1.02 | 372 | 31.0 | 2.58 | 6.9/8.5 | 0.116 | 508 | 417 | 0.71 | 7.7 |
| No. | Diamines (mol %) | Tetracarboxylic Dianhydrides | UL-94, V-0 (d) |
|---|---|---|---|
| 4 | AB-HQ (70) 4,4′-ODA (30) | s-BPDA | Failed a (20 μm) |
| 5 | AB-HQ (60) 4,4′-ODA (40) | s-BPDA | Failed a (24 μm) |
| 9 | AB-MeOHQ | PMDA | Passed (19 μm) |
| 10 | AB-MeOHQ | s-BPDA | Failed b (26 μm) |
| 11 | AB-MeOHQ | TA-HQ | Failed a (31 μm) |
| 12 | AB-MeOHQ (70) 4,4′-ODA (30) | s-BPDA | Passed (24 μm) |
| 20 | AB-14DHN (70) 4,4′-ODA (30) | s-BPDA | Failed a (25 μm) |
| 27 | AB-DP44BP (80) 4,4′-ODA (20) | s-BPDA | Passed (29 μm) |
| Properties | Parameters | Relative Rank | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Heat resistance | Tg (°C) | <210 | 220–250 | 260–290 | 300–330 | >350 or ND a |
| Low thermal expansion property | CTE (ppm K−1) | >70 | 60–50 | 45–35 | 30–20 | <10 |
| Low hygroscopic expansion property | CHE (ppm/RH%) | >30 | 25–20 | 15–10 | 8–4 | <2 |
| Low water absorption | WA (%) | >3.0 | 2.5–2.0 | 1.5–1.0 | 0.8–0.4 | <0.2 |
| Toughness | εbmax (%) | No film-forming ability or <2 | 5–10 | 20–30 | 40–60 | >80 |
| Low dielectric constant b in GHz range | εr | >3.7 | 3.6–3.4 | 3.3–3.1 | 3.0–2.8 | <2.7 |
| Low dissipation factor in GHz range b | tan δ | >0.02 | 0.01–0.008 | 0.007–0.005 | 0.004–0.002 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Hishiki, T. Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion and Low Water Absorption (V). Effects of Ester-linked Diamines with Different Lengths and Substituents. Polymers 2020, 12, 859. https://doi.org/10.3390/polym12040859
Hasegawa M, Hishiki T. Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion and Low Water Absorption (V). Effects of Ester-linked Diamines with Different Lengths and Substituents. Polymers. 2020; 12(4):859. https://doi.org/10.3390/polym12040859
Chicago/Turabian StyleHasegawa, Masatoshi, and Tomoaki Hishiki. 2020. "Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion and Low Water Absorption (V). Effects of Ester-linked Diamines with Different Lengths and Substituents" Polymers 12, no. 4: 859. https://doi.org/10.3390/polym12040859
APA StyleHasegawa, M., & Hishiki, T. (2020). Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion and Low Water Absorption (V). Effects of Ester-linked Diamines with Different Lengths and Substituents. Polymers, 12(4), 859. https://doi.org/10.3390/polym12040859