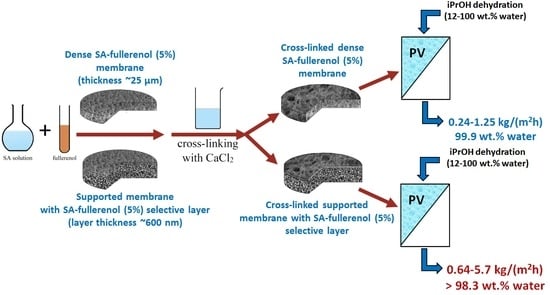
Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Dense Membranes
2.2.2. Supported Membranes
2.3. Fourier Transforms Infrared Spectroscopy (FTIR)
2.4. Nuclear Magnetic Resonance (NMR)
2.5. Scanning Electron Microscopy (SEM)
2.6. Atomic Force Microscopy (AFM)
2.7. Thermogravimetric Analysis (TGA)
2.8. Swelling Measurement
2.9. Pervaporation Experiment
3. Results
3.1. The Development and Investigation of Dense Membranes
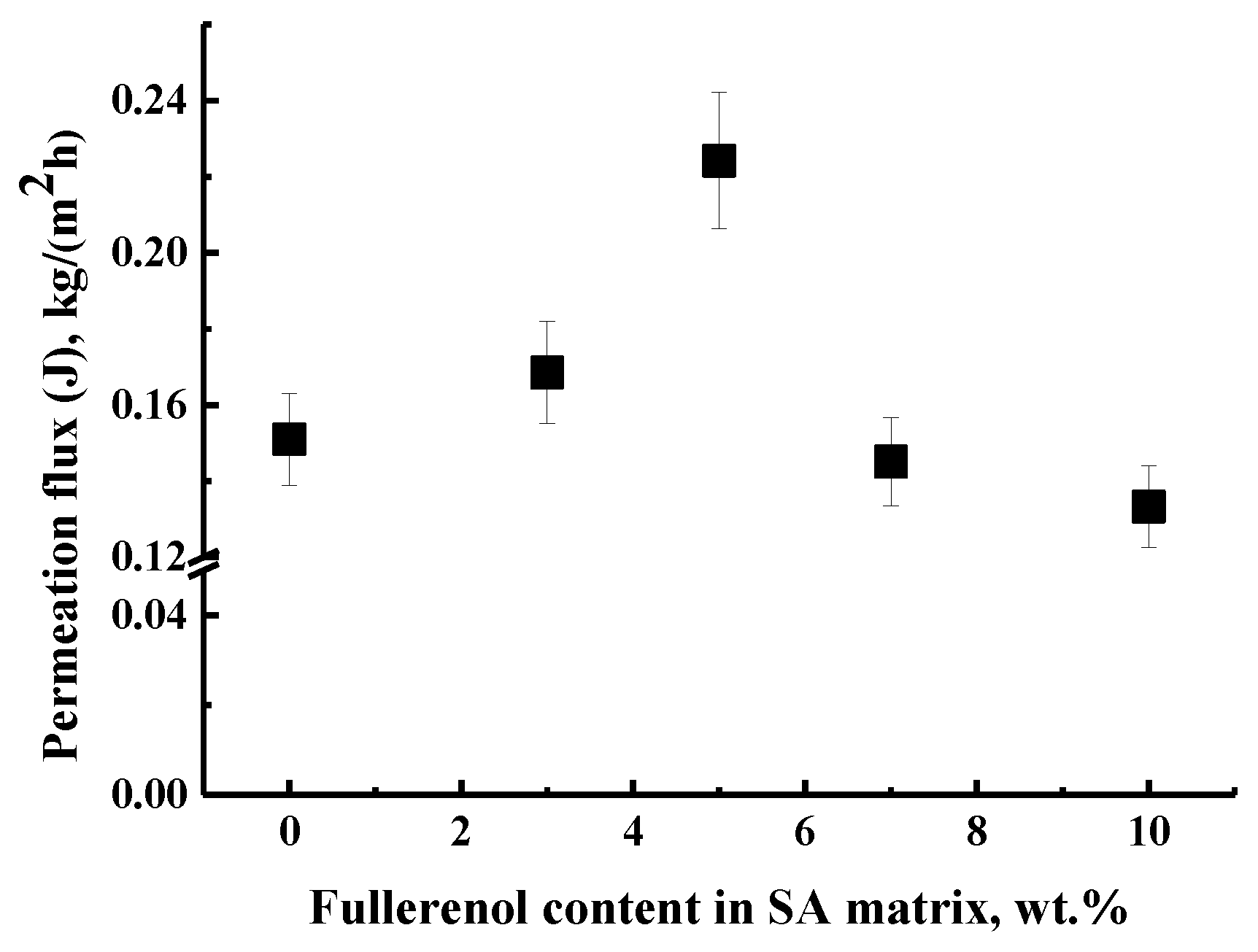
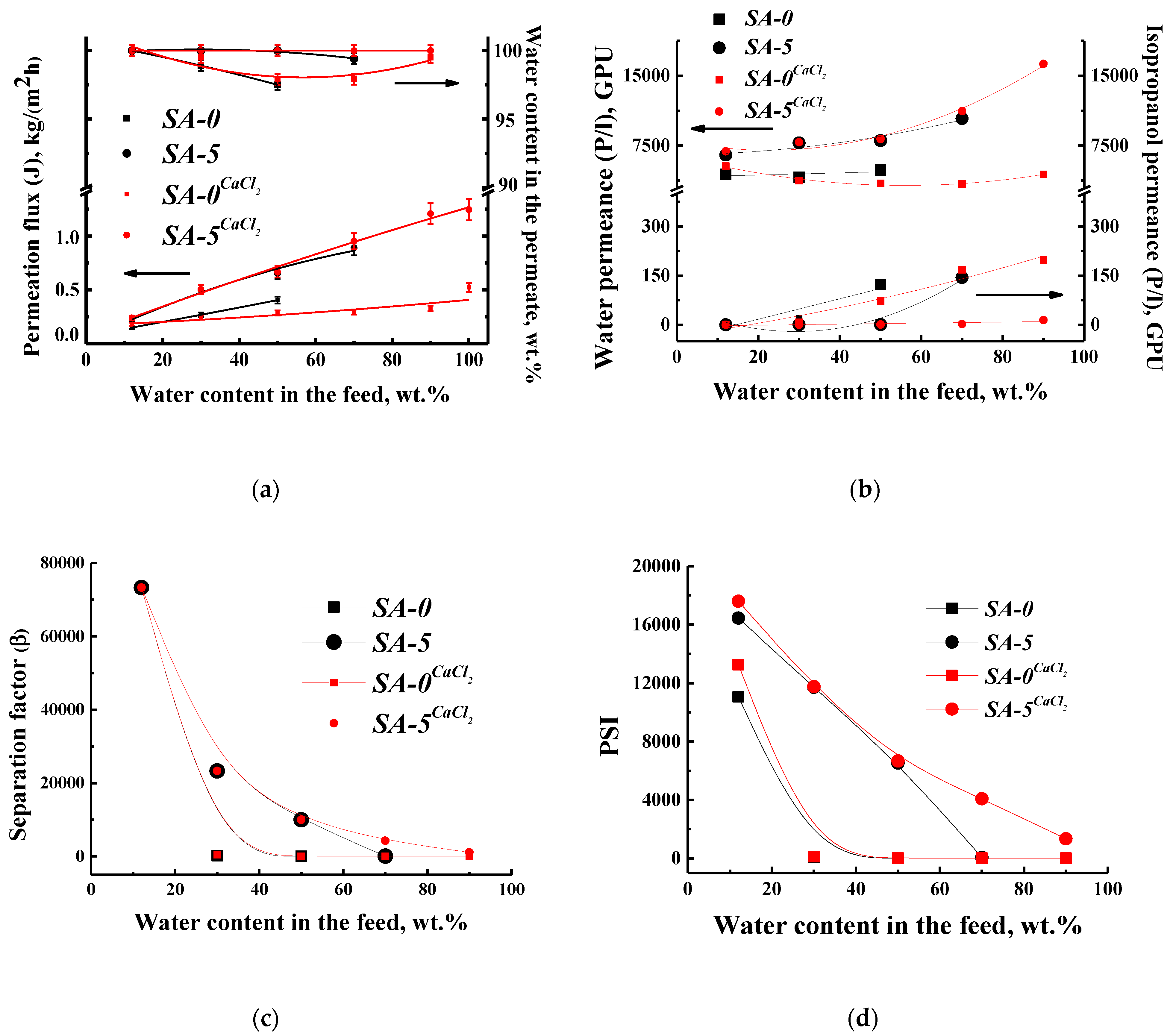
3.1.1. Pervaporation Performance of Dense SA Membranes
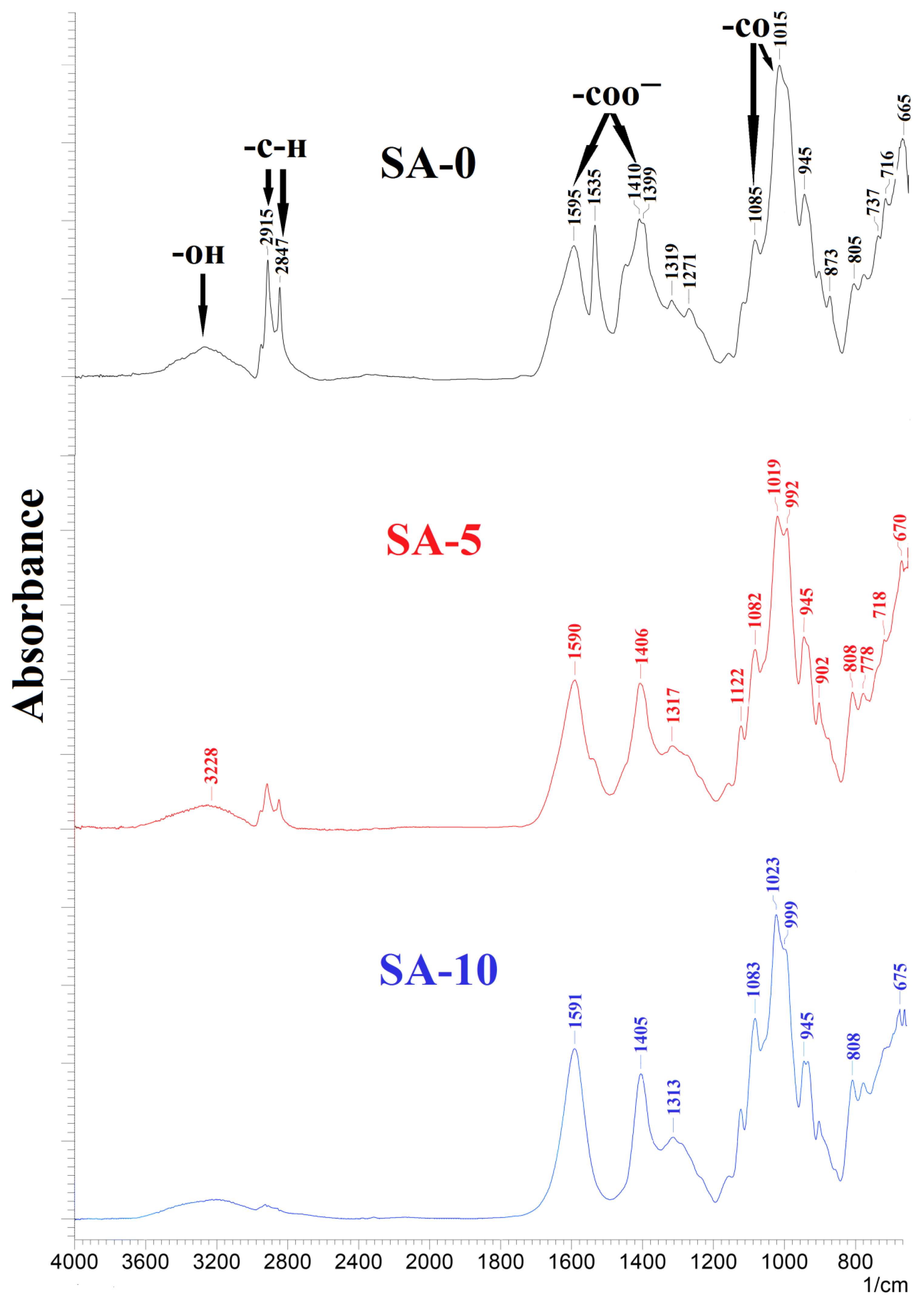
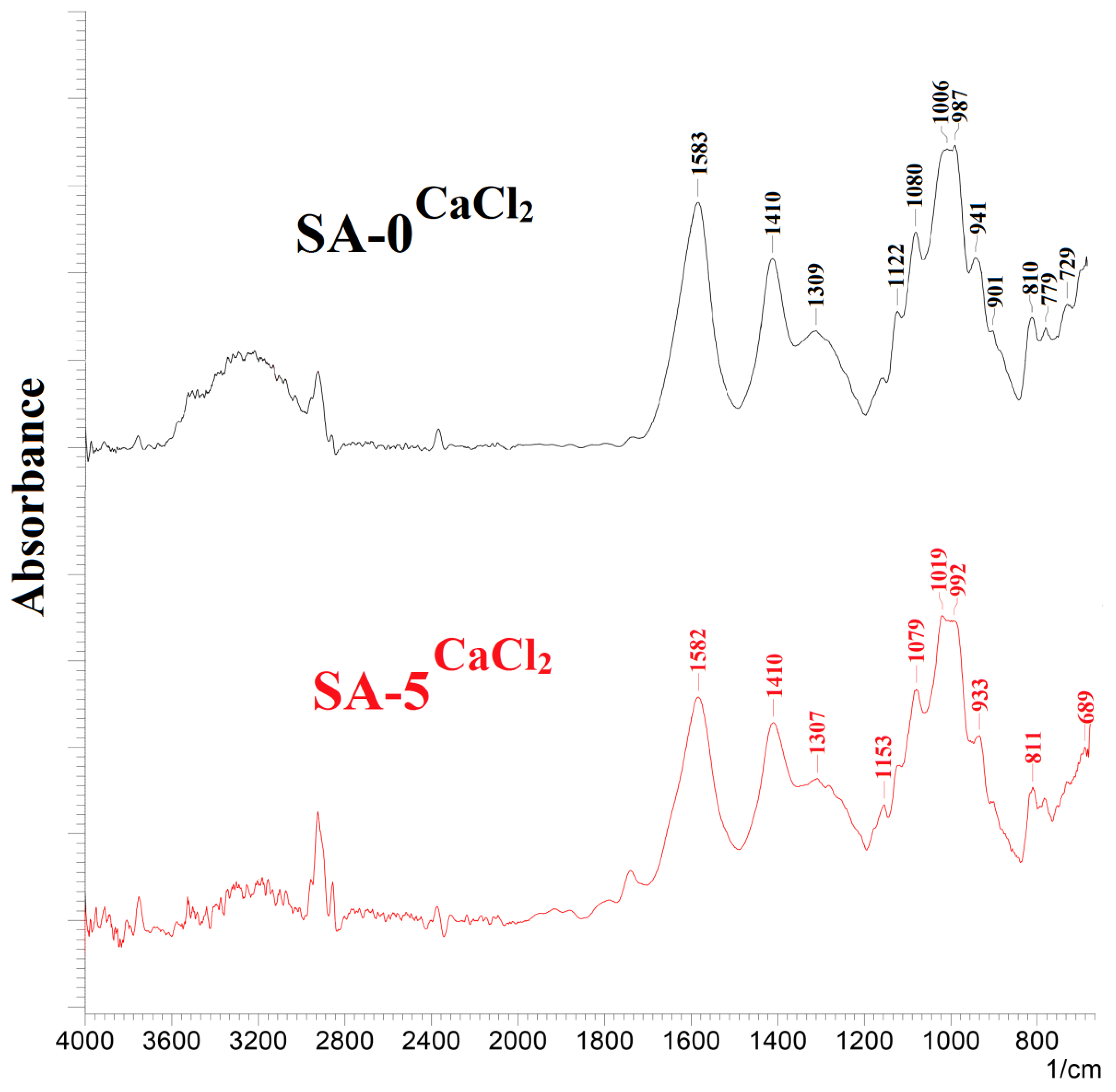
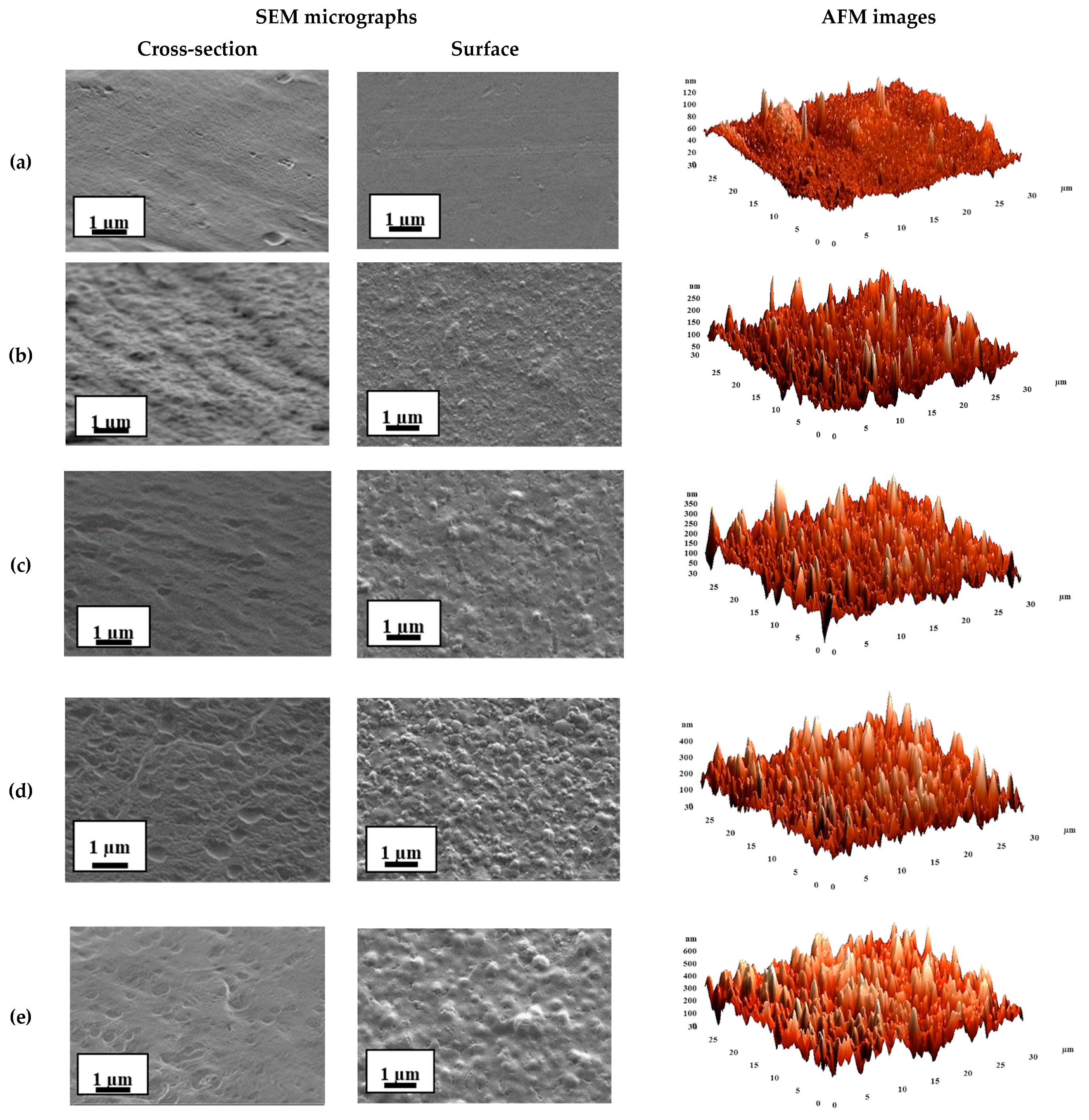
3.1.2. Structure and Physicochemical Properties Investigation
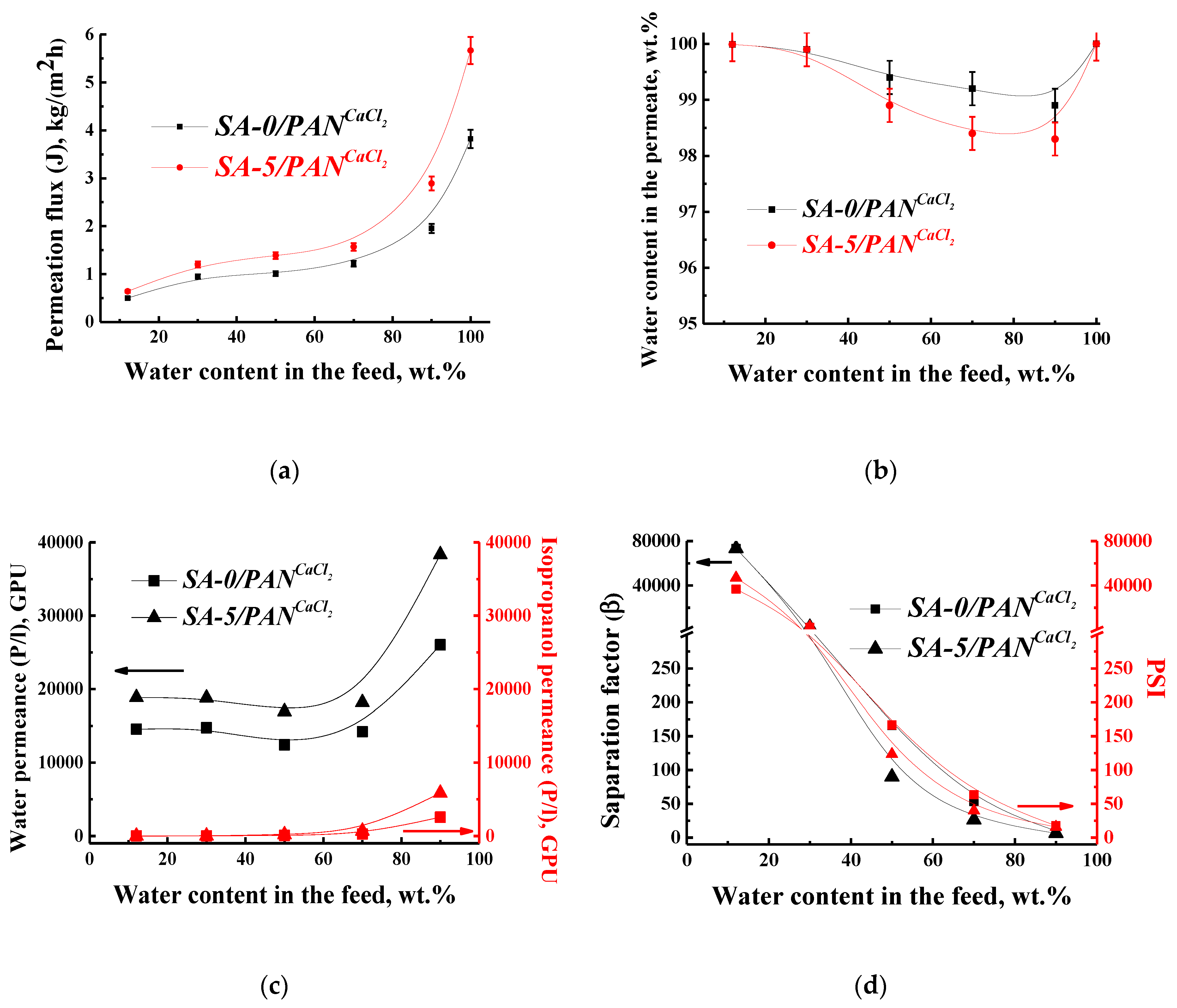
3.2. The Development and Investigation of Supported Membranes
3.3. Comparison of Performance with SA-Based Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jyothi, M.S.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.P.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef]
- Horsley, L.H. Azeotropic Data-III. In Advances in Chemistry Series 116; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1973; p. 18. [Google Scholar]
- Thu, H.E.; Ng, S.F. Gelatine enhances drug dispersion in alginate bilayer film via the formation of crystalline microaggregates. Int. J. Pharm. 2013, 454, 99–106. [Google Scholar] [CrossRef]
- Kosik, A.; Luchowska, U.; Święszkowski, W. Electrolyte alginate/poly-l-lysine membranes for connective tissue development. Mater. Lett. 2016, 184, 104–107. [Google Scholar] [CrossRef]
- By, E.; Venkatesan, J.; Anil, S.; Kim, S.; Corporation, X.; States, U. Chemical Modification of Alginate—Seaweed Polysaccharides; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128098165. [Google Scholar]
- Zhao, X.; Qin, A.; Liu, D.; He, C. Tuning the antifouling property of PVDF ultrafiltration membrane with surface anchored polyelectrolyte complexes for sewage treatment. RSC Adv. 2015, 5, 63580–63587. [Google Scholar] [CrossRef]
- Li, J.; Si, X.; Li, X.; Wang, N.; An, Q.; Ji, S. Preparation of acid-resistant PEI/SA composite membranes for the pervaporation dehydration of ethanol at low pH. Sep. Purif. Technol. 2018, 192, 205–212. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Z.; Cheng, X.; Chen, C.; Yang, H.; Wu, H.; Pan, F.; Zhang, P.; Cao, X. Elevating the selectivity of layer-by-layer membranes by in situ bioinspired mineralization. J. Memb. Sci. 2016, 520, 364–373. [Google Scholar] [CrossRef]
- Munavalli, B.; Torvi, A.; Kariduraganavar, M. A facile route for the preparation of proton exchange membranes using sulfonated side chain graphite oxides and crosslinked sodium alginate for fuel cell. Polymer 2018, 142, 293–309. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Lee, K.H. Vapor permeation separation of methanol-water mixtures: Effect of experimental conditions. Ind. Eng. Chem. Res. 2013, 52, 10450–10459. [Google Scholar] [CrossRef]
- Kahya, S.; Şanli, O. Separation of dimethylformamide/water mixtures through sodium alginate and sodium alginate/clinoptilolite composite membranes by vapor permeation with and without feed-membrane temperature difference. Desalin. Water Treat. 2014, 52, 3517–3525. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Turczyn, R.; Konieczny, K. The study of ethanol and water vapour permeation process through alginate membranes modified by magnetic powders. Desalin. Water Treat. 2017, 64, 339–344. [Google Scholar] [CrossRef]
- Zhao, F.Y.; An, Q.F.; Ji, Y.L.; Gao, C.J. A novel type of polyelectrolyte complex/MWCNT hybrid nanofiltration membranes for water softening. J. Memb. Sci. 2015, 492, 412–421. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.J.; Lee, K.H. Chlorine resistant binary complexed NaAlg/PVA composite membrane for nanofiltration. Sep. Purif. Technol. 2014, 137, 21–27. [Google Scholar] [CrossRef]
- Yakoumis, I.; Theodorakopoulos, G.; Papageorgiou, S.K.; Romanos, G.; Veziri, C.; Panias, D. Tubular C/Cu decorated γ-alumina membranes for NO abatement. J. Memb. Sci. 2016, 515, 134–143. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K. Dehydration of dioxane by pervaporation using filled blend membranes of polyvinyl alcohol and sodium alginate. Carbohydr. Polym. 2014, 101, 1154–1165. [Google Scholar] [CrossRef]
- Moulik, S.; Nazia, S.; Vani, B.; Sridhar, S. Pervaporation separation of acetic acid/water mixtures through sodium alginate/polyaniline polyion complex membrane. Sep. Purif. Technol. 2016, 170, 30–39. [Google Scholar] [CrossRef]
- Hosseini, S.; Charkhi, A.; Minuchehr, A.; Ahmadi, S.J. Dehydration of acetonitrile using cross-linked sodium alginate membrane containing nano-sized NaA zeolite. Chem. Pap. 2017, 71, 1143–1153. [Google Scholar] [CrossRef]
- Xing, R.; Pan, F.; Zhao, J.; Cao, K.; Gao, C.; Yang, S.; Liu, G.; Wu, H.; Jiang, Z. Enhancing the permeation selectivity of sodium alginate membrane by incorporating attapulgite nanorods for ethanol dehydration. RSC Adv. 2016, 6, 14381–14392. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Cheng, X.; Yang, H.; Tang, L.; Liu, G.; Wang, M.; Wu, H.; Pan, F.; Cao, X. Water-selective permeation in hybrid membrane incorporating multi-functional hollow ZIF-8 nanospheres. J. Memb. Sci. 2018, 555, 146–156. [Google Scholar] [CrossRef]
- Dudek, G.; Krasowska, M.; Turczyn, R.; Gnus, M.; Strzelewicz, A. Structure, morphology and separation efficiency of hybrid Alg/Fe3O4 membranes in pervaporative dehydration of ethanol. Sep. Purif. Technol. 2017, 182, 101–109. [Google Scholar] [CrossRef]
- Ji, C.H.; Xue, S.M.; Xu, Z.L. Novel Swelling-Resistant Sodium Alginate Membrane Branching Modified by Glycogen for Highly Aqueous Ethanol Solution Pervaporation. ACS Appl. Mater. Interfaces 2016, 8, 27243–27253. [Google Scholar] [CrossRef]
- Uragami, T.; Banno, M.; Miyata, T. Dehydration of an ethanol/water azeotrope through alginate-DNA membranes cross-linked with metal ions by pervaporation. Carbohydr. Polym. 2015, 134, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Magalad, V.T.; Gokavi, G.S.; Ranganathaiah, C.; Burshe, M.H.; Han, C.; Dionysiou, D.D.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric blend nanocomposite membranes for ethanol dehydration-effect of morphology and membrane-solvent interactions. J. Memb. Sci. 2013, 430, 321–329. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Bai, S.; Zhang, L.; Li, W.; Xing, W. Development of stable and active PVA-PSSA/SA-PVA catalytic composite membrane for esterification enhancement. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R.; Gnus, M.; Konieczny, K. Pervaporative dehydration of ethanol/water mixture through hybrid alginate membranes with ferroferic oxide nanoparticles. Sep. Purif. Technol. 2018, 193, 398–407. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Z.; Zhao, J.; Cao, K.; Zhang, Q.; Pan, F. High pervaporation dehydration performance of the composite membrane with an ultrathin alginate/poly(acrylic acid)-Fe3O4 active layer. Ind. Eng. Chem. Res. 2014, 53, 1606–1616. [Google Scholar] [CrossRef]
- Dudek, G.; Krasowska, M.; Turczyn, R.; Strzelewicz, A.; Djurado, D.; Pouget, S. Clustering method performance assessment of alginate hybrid membranes for pervaporation dehydration of ethanol. Chem. Eng. Res. Des. 2019, 144, 483–493. [Google Scholar] [CrossRef]
- Yang, H.; Wu, H.; Pan, F.; Li, Z.; Ding, H.; Liu, G.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Highly water-permeable and stable hybrid membrane with asymmetric covalent organic framework distribution. J. Memb. Sci. 2016, 520, 583–595. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, X.; Cheng, X.; Pan, F.; Wu, H.; Liu, G.; Song, Y.; Cao, X.; Jiang, Z. Highly water-selective membranes based on hollow covalent organic frameworks with fast transport pathways. J. Memb. Sci. 2018, 565, 331–341. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Hilmioglu, N.D. Pervaporation of ethanol/water mixtures by zeolite filled sodium alginate membrane. Desalin. Water Treat. 2013, 51, 637–643. [Google Scholar] [CrossRef]
- Su, Z.; Chen, J.H.; Sun, X.; Huang, Y.; Dong, X. Amine-functionalized metal organic framework (NH2-MIL-125(Ti)) incorporated sodium alginate mixed matrix membranes for dehydration of acetic acid by pervaporation. RSC Adv. 2015, 5, 99008–99017. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, Z.; Zhao, M.; Wu, H.; Pan, F.; Mayta, J.Q.; Chang, Z.; Bu, X. Enhanced dehydration performance of hybrid membranes by incorporating lanthanide-based MOFs. J. Memb. Sci. 2018, 546, 31–40. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Z.; Cao, K.; Nair, S.; Cheng, X.; Zhao, J.; Gomaa, H.; Wu, H.; Pan, F. Pervaporation performance comparison of hybrid membranes filled with two-dimensional ZIF-L nanosheets and zero-dimensional ZIF-8 nanoparticles. J. Memb. Sci. 2017, 523, 185–196. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Cheng, X.; Guo, S.; Tang, L.; Yang, H.; Wu, H.; Pan, F.; Zhang, P.; Cao, X.; et al. Bimetallic metal-organic frameworks nanocages as multi-functional fillers for water-selective membranes. J. Memb. Sci. 2018, 545, 19–28. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Gladchenko, S.V.; Pen’kova, A.V.; Kuznetsov, V.M.; Toikka, A.M. Synthesis of fullerene-polyphenylene oxide membranes for separating aqueous-organic mixtures. Russ. J. Appl. Chem. 2005, 78, 1468–1473. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Pientka, Z.; Toikka, A.M. Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. Desalin. Water Treat. 2010, 14, 83–88. [Google Scholar] [CrossRef]
- Penkova, A.V.; Polotskaya, G.A.; Gavrilova, V.A.; Toikka, A.M.; Liu, J.-C.; Trchová, M.; Šlouf, M.; Pientka, Z. Polyamide membranes modified by carbon nanotubes: Application for pervaporation. Sep. Sci. Technol. 2010, 45, 35–41. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Atta, R.R.; Zolotarev, A.A.; Mazur, A.S.; Vezo, O.S.; Lahderanta, E.; Markelov, D.A.; Ermakov, S.S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Savon, N.A.; Missyul, A.B.; Mazur, A.S.; Kuzminova, A.I.; Zolotarev, A.A.; Mikhailovskii, V.; Lahderanta, E.; Markelov, D.A.; et al. Novel mixed-matrix membranes based on polyvinyl alcohol modified by carboxyfullerene for pervaporation dehydration. Sep. Purif. Technol. 2018, 204, 1–12. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers (Basel) 2018, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Chen, B.; Semenov, K.N.; Kroto, H.W. Transport properties of cross-linked fullerenol–PVA membranes. Carbon N. Y. 2014, 76, 446–450. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, Z.; Zhao, C.; Gomaa, H.; Pan, F. Enhanced pervaporative performance of hybrid membranes containing Fe3O4@CNT nanofillers. J. Memb. Sci. 2015, 492, 230–241. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Memb. Sci. 2013, 425–426, 77–88. [Google Scholar] [CrossRef]
- Suhas, D.P.; Raghu, A.V.; Jeong, H.M.; Aminabhavi, T.M. Graphene-loaded sodium alginate nanocomposite membranes with enhanced isopropanol dehydration performance via a pervaporation technique. RSC Adv. 2013, 3, 17120. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhao, J.; Zhao, C.; Gao, C.; Pan, F.; Wang, B.; Cao, X.; Yang, J. Enhanced water permeation through sodium alginate membranes by incorporating graphene oxides. J. Memb. Sci. 2014, 469, 272–283. [Google Scholar] [CrossRef]
- Wang, M.; Pan, F.; Yang, L.; Song, Y.; Wu, H.; Cheng, X.; Liu, G.; Yang, H.; Wang, H.; Jiang, Z.; et al. Graphene oxide quantum dots incorporated nanocomposite membranes with high water flux for pervaporative dehydration. J. Memb. Sci. 2018, 563, 903–913. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; He, G.; Xing, R.; Pan, F.; Jiang, Z.; Zhang, P.; Cao, X.; Wang, B. Incorporating Zwitterionic Graphene Oxides into Sodium Alginate Membrane for Efficient Water/Alcohol Separation. ACS Appl. Mater. Interfaces 2016, 8, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Sokolova, M.P.; Mikhailova, M.T.; Polyakov, E.S.; Ermakov, S.S.; Markelov, D.A.; Roizard, D. Improvement of pervaporation PVA membranes by the controlled incorporation of fullerenol nanoparticles. Mater. Des. 2016, 96, 416–423. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.A.; Sokolova, M.P.; Dmitrenko, M.E.; Toikka, A.M. Polyvinyl alcohol membranes modified by low-hydroxylated fullerenol. J. Memb. Sci. 2015, 491. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Strzelewicz, A.; Turczyn, R.; Krasowska, M. The influence of metal oxides on the separation properties of hybrid alginate membranes. Sep. Sci. Technol. 2018, 53, 1178–1190. [Google Scholar] [CrossRef]
- Liu, Y.; Olewski, T.; Vechot, L. Modelling of a cryogenic liquid pool boiling using CFD simulation. J. Loss. Prev. Process Ind. 2011, 35, 125–134. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Memb. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Roizard, D.; Favre, E. CHAPTER 12 Trends in design and preparation of polymeric membranes for pervaporation. In Advanced Materials for Membrane Preparation; Bentham Science Publishers Ltd.: Shaka, UAE, 2011; pp. 154–182. [Google Scholar]
- Subrahmanyam, R.; Gurikov, P.; Dieringer, P.; Sun, M.; Smirnova, I. On the Road to Biopolymer Aerogels—Dealing with the Solvent. Gels 2015, 1, 291–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, C.M. Hansen Solubility Parameters. A User’s Book, 2nd ed.; CRC Press: London, UK, 2007; ISBN 9780849372483. [Google Scholar]
- Rynkowska, E.; Dzieszkowski, K.; Lancien, A.; Fatyeyeva, K.; Szymczyk, A.; Kujawa, J.; Marais, S.; Wolan, A. Physicochemical properties and pervaporation performance of dense membranes based on cellulose acetate propionate ( CAP ) and containing polymerizable ionic liquid (PIL). J. Memb. Sci. 2017, 544, 243–251. [Google Scholar] [CrossRef]
- Rychlewska, K.; Kujawski, W.; Konieczny, K. Pervaporative performance of PEBA and PDMS based commercial membranes in thiophene removal from its binary mixtures with hydrocarbons. Fuel Process. Technol. 2017, 165, 9–18. [Google Scholar] [CrossRef]
- Xie, H.R.; Ji, C.H.; Xue, S.M.; Xu, Z.L.; Yang, H.; Ma, X.H. Enhanced pervaporation performance of SA-PFSA/ceramic hybrid membranes for ethanol dehydration. Sep. Purif. Technol. 2018, 206, 218–225. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Podolsky, N.E.; Marcos, M.A.; Cabaleiro, D.; Semenov, K.N.; Lugo, L.; Petrov, A.V.; Charykov, N.A.; Sharoyko, V.V.; Vlasov, T.D.; Murin, I.V. Physico-chemical properties of C60(OH)22–24 water solutions: Density, viscosity, refraction index, isobaric heat capacity and antioxidant activity. J. Mol. Liq. 2019, 278, 342–355. [Google Scholar] [CrossRef]
- Cao, K.; Jiang, Z.; Zhang, X.; Zhang, Y.; Zhao, J.; Xing, R.; Yang, S.; Gao, C.; Pan, F. Highly water-selective hybrid membrane by incorporating g-C3N4 nanosheets into polymer matrix. J. Memb. Sci. 2015, 490, 72–83. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Deramos, C.M.; Irwin, A.E.; Nauss, J.L.; Stout, B.E. 13C NMR and molecular modeling studies of alginic acid binding with alkaline earth and lanthanide metal ions. Inorganica Chim. Acta 1997, 256, 69–75. [Google Scholar] [CrossRef]
- Fischer, F.G.; Dörfel, H. Die Polyuronsäuren der Braunalgen (Kohlenhydrate der Algen I). Hoppe-Seyler’s Z. für Physiol. Chem. 1955, 302, 186–203. [Google Scholar] [CrossRef]
- Wang, H.; Wen, X.; Zhang, X.; Liu, C. Acetylation of Microcrystalline Cellulose by Transesterification in AmimCl/DMSO Cosolvent System. Molecules 2017, 22, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Hu, W.; Russell, T.P. Measuring the degree of crystallinity in semicrystalline regioregular poly(3-hexylthiophene). Macromolecules 2016, 49, 4501–4509. [Google Scholar] [CrossRef]
- Simstich, B.; Oeller, H.J. Membrane technology for the future treatment of paper mill effluents: Chances and challenges of further system closure. Water Sci. Technol. 2010, 62, 2190–2197. [Google Scholar] [CrossRef]
- RajiniKanth, V.; Ravindra, S.; Madalageri, P.M.; Kajjari, P.B.; Mulaba-Bafubiandi, A.F. Study of enhanced physical and pervaporation properties in composite membrane. Membr. Water Treat. 2017, 8, 483–498. [Google Scholar]
- Adoor, S.G.; Rajineekanth, V.; Nadagouda, M.N.; Chowdoji Rao, K.; Dionysiou, D.D.; Aminabhavi, T.M. Exploration of nanocomposite membranes composed of phosphotungstic acid in sodium alginate for separation of aqueous-organic mixtures by pervaporation. Sep. Purif. Technol. 2013, 113, 64–74. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Premakshi, H.G.; Kariduraganavar, M.Y. Development of novel alginate–silica hybrid membranes for pervaporation dehydration of isopropanol. Polym. Bull. 2016, 73, 743–762. [Google Scholar] [CrossRef] [Green Version]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of polyelectrolyte complex membranes for the pervaporation separation of water–isopropanol mixtures using sodium alginate and gelatin. Polym. Bull. 2018, 75, 851–875. [Google Scholar] [CrossRef]
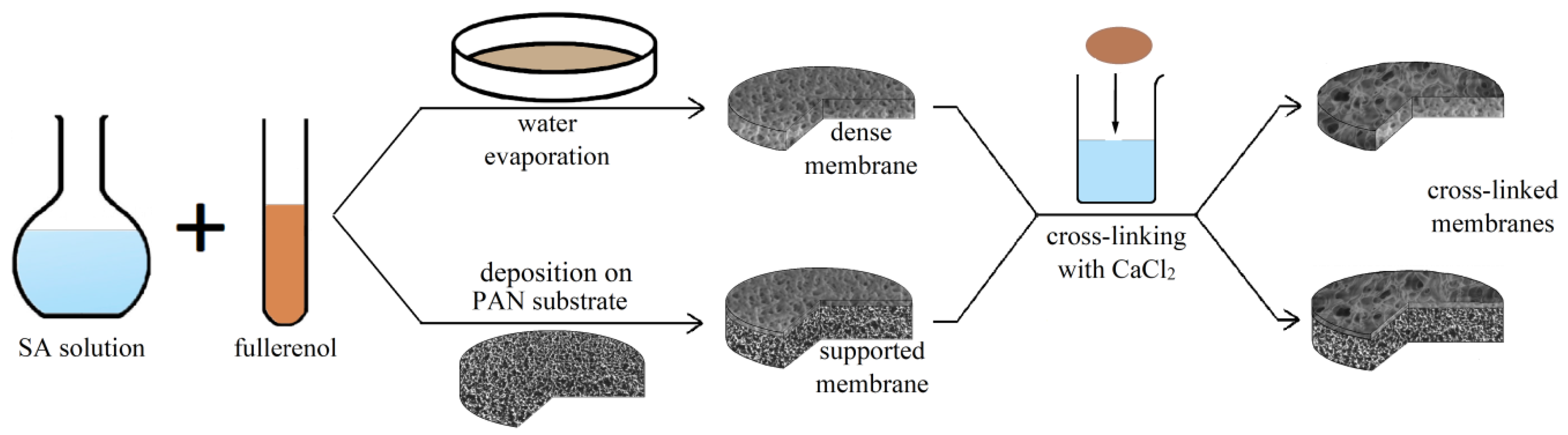
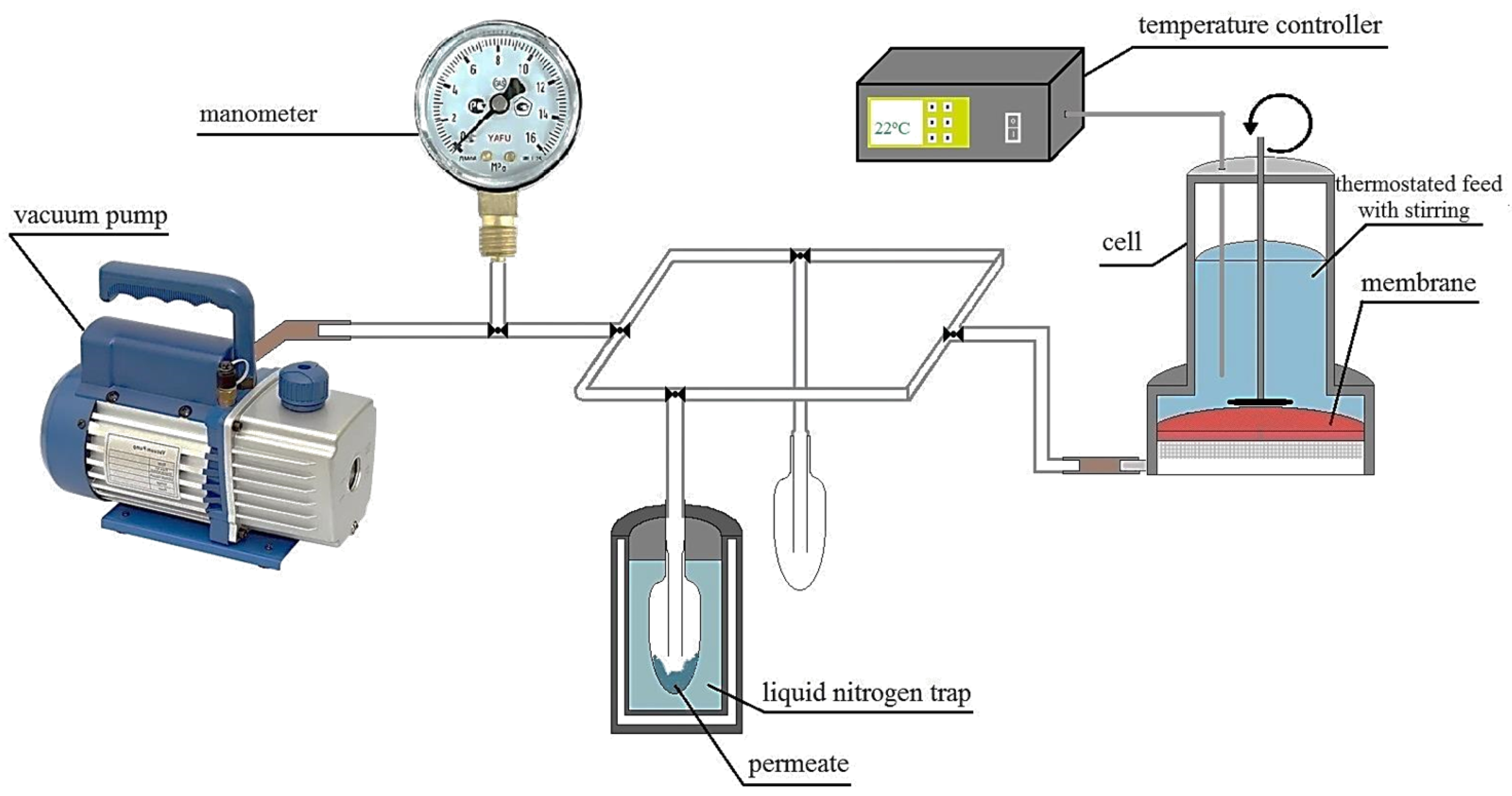
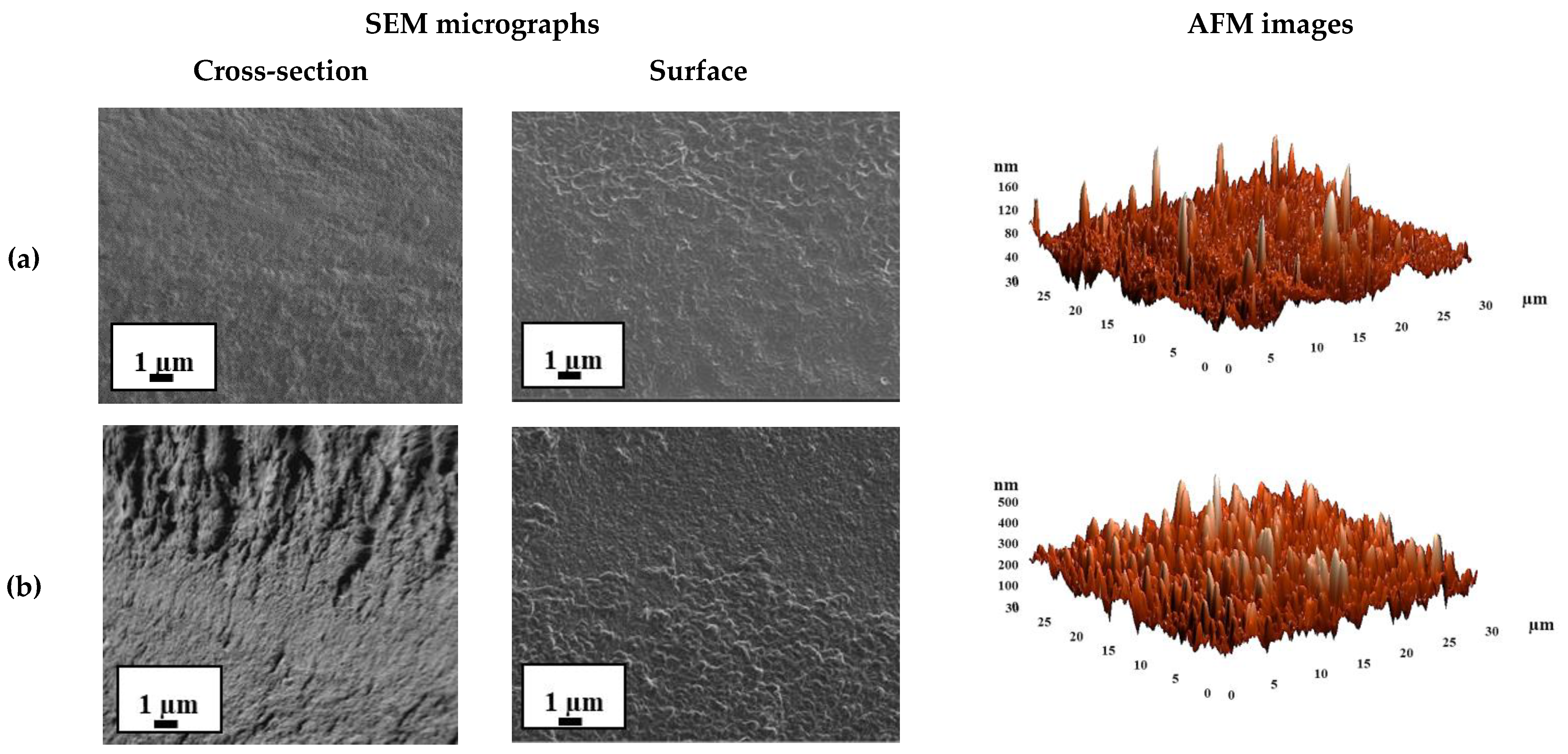

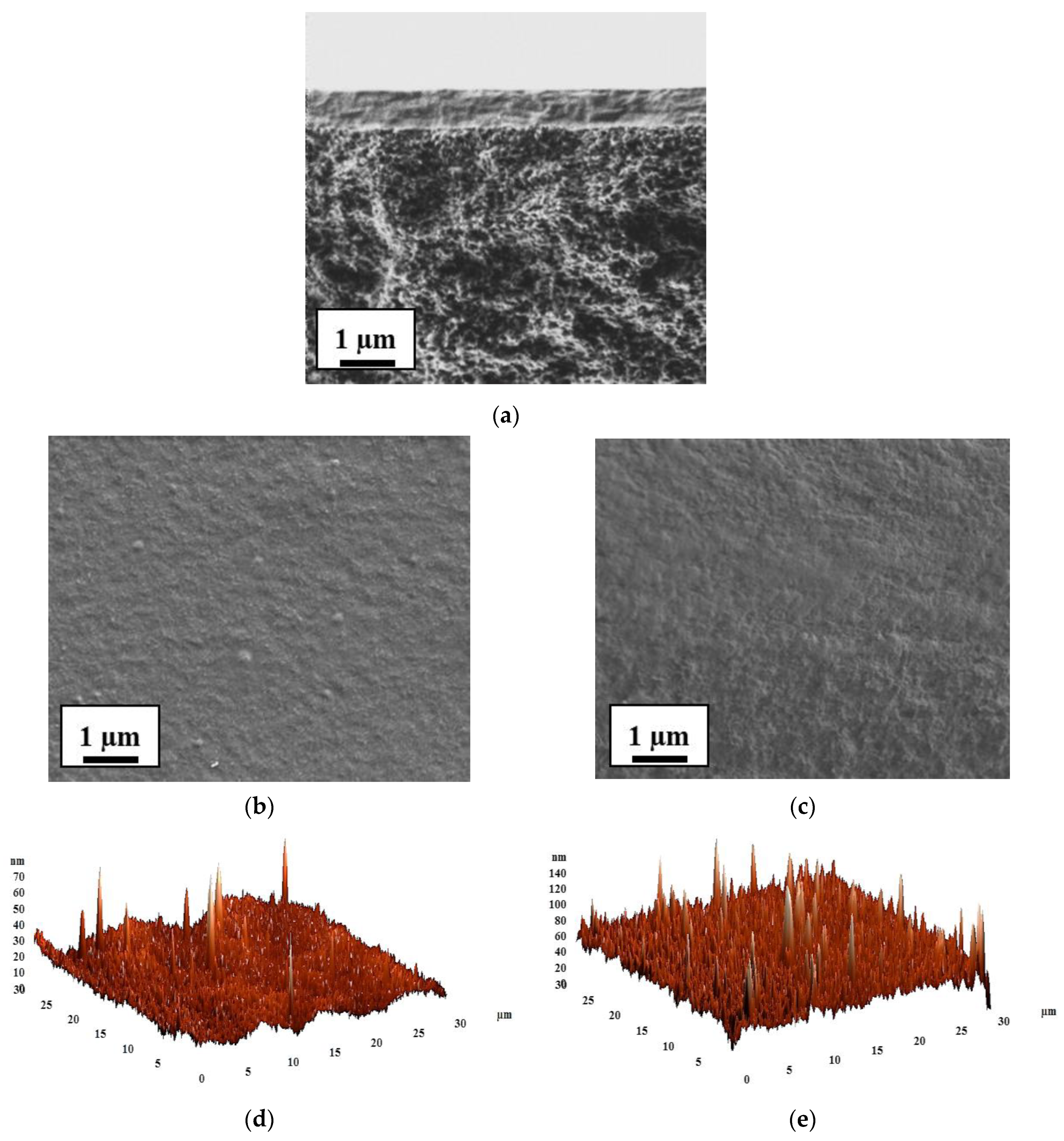
| Membrane | Type | Thickness, μm | Content of Fullerenol, wt % | Cross-Linking Method |
|---|---|---|---|---|
| SA-0 | dense | 25 | 0 | - |
| SA-3 | dense | 25 | 3 | - |
| SA-5 | dense | 25 | 5 | - |
| SA-7 | dense | 25 | 7 | - |
| SA-10 | dense | 25 | 10 | - |
| SA-0CaCl2 | dense | 25 | 0 | 1.25 wt % calcium chloride (CaCl2) |
| SA-5CaCl2 | dense | 25 | 5 | 1.25 wt % calcium chloride (CaCl2) |
| SA-0/PANCaCl2 | supported | 0.6 | 0 | 1.25 wt % calcium chloride (CaCl2) |
| SA-5/PANCaCl2 | supported | 0.6 | 5 | 1.25 wt % calcium chloride (CaCl2) |
| Polymer and Solvents | Hansen Solubility Parameters [MPa1/2] | References | |||
|---|---|---|---|---|---|
| δd | δp | δh | δt | ||
| Sodium alginate | - | - | - | 37 | [58] |
| Water | 15.5 | 16 | 42.3 | 47.8 | [59] |
| Isopropanol | 15.8 | 6.1 | 16.4 | 23.6 | |
| Membranes | Crystalline Phase, % |
|---|---|
| SA-0 | 40 |
| SA-5 | 53 |
| SA-10 | 41 |
| SA-0CaCl2 | 58 |
| SA-5CaCl2 | 41 |
| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| SA-0 | 7.2 ± 2 | 10.2 ± 3 |
| SA-3 | 25.2 ± 3 | 33.8 ± 4 |
| SA-5 | 32.8 ± 3 | 42.6 ± 4 |
| SA-7 | 46.9 ± 5 | 59.7 ± 5 |
| SA-10 | 67.3 ± 5 | 84.8 ± 6 |
| SA-0CaCl2 | 11.2 ± 3 | 15.9 ± 4 |
| SA-5CaCl2 | 45.7 ± 5 | 59.1 ± 5 |
| Membranes | Swelling Degree in Water (S), % | Swelling Degree in the Azeotropic Mixture (S), % |
|---|---|---|
| SA-0 | - | 15 |
| SA-3 | - | 13 |
| SA-5 | - | 12 |
| SA-7 | - | 14 |
| SA-10 | - | 19 |
| SA-0CaCl2 | 83 | 48 |
| SA-5CaCl2 | 77 | 24 |
| Membranes | Ra, nm | Rq, nm |
|---|---|---|
| SA-0/PANCaCl2 | 3.8 ± 2 | 5.1 ± 2 |
| SA-5/PANCaCl2 | 7.6 ± 3 | 10.8 ± 4 |
| Membranes | Thickness of Selective Layer, µm | Water Content in the Feed, wt % | Temperature, °C | Permeation Flux, kg/(m2 h) | Separation Factor (β) | Reference |
|---|---|---|---|---|---|---|
| SA-5CaCl2 | 25 | 12 | 22 | 0.240 | 73,326 | This study |
| SA-5/PANCaCl2 | 0.6 | 12 | 22 | 0.641 | 73,326 | This study |
| PERVAP™ 1201 | - | 12 | 22 | 0.028 | 73,326 | This study |
| Alg-chitosan-wrapped MWCNT (2%) | 50 | 10 | 30 | 0.218 | 6419 | [45] |
| Alg-phosphomolybdic acid (10%) | 50 | 10 | 30 | 0.282 | 9028 | [72] |
| Alg-phosphotungstic acid modified by ammonium carbonate (10%) | 50 | 10 | 30 | 0.316 | 8991 | [73] |
| Alg-3-aminopropyl triethoxysilane (APTEOS)/TEOS (30%) | 50 | 5 | 30 | 0.044 | 17,253 | [74] |
| Alg-gelatin (10%) | 45 | 10 | 30 | 0.085 | 4277 | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. https://doi.org/10.3390/polym12040864
Dmitrenko M, Liamin V, Kuzminova A, Mazur A, Lahderanta E, Ermakov S, Penkova A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers. 2020; 12(4):864. https://doi.org/10.3390/polym12040864
Chicago/Turabian StyleDmitrenko, Mariia, Vladislav Liamin, Anna Kuzminova, Anton Mazur, Erkki Lahderanta, Sergey Ermakov, and Anastasia Penkova. 2020. "Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol" Polymers 12, no. 4: 864. https://doi.org/10.3390/polym12040864
APA StyleDmitrenko, M., Liamin, V., Kuzminova, A., Mazur, A., Lahderanta, E., Ermakov, S., & Penkova, A. (2020). Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers, 12(4), 864. https://doi.org/10.3390/polym12040864