Evaluation of Repellent Effectiveness of Polyvinyl Alcohol/Eucalyptus globules Nanofibrous Membranes against Forcipomyia taiwana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
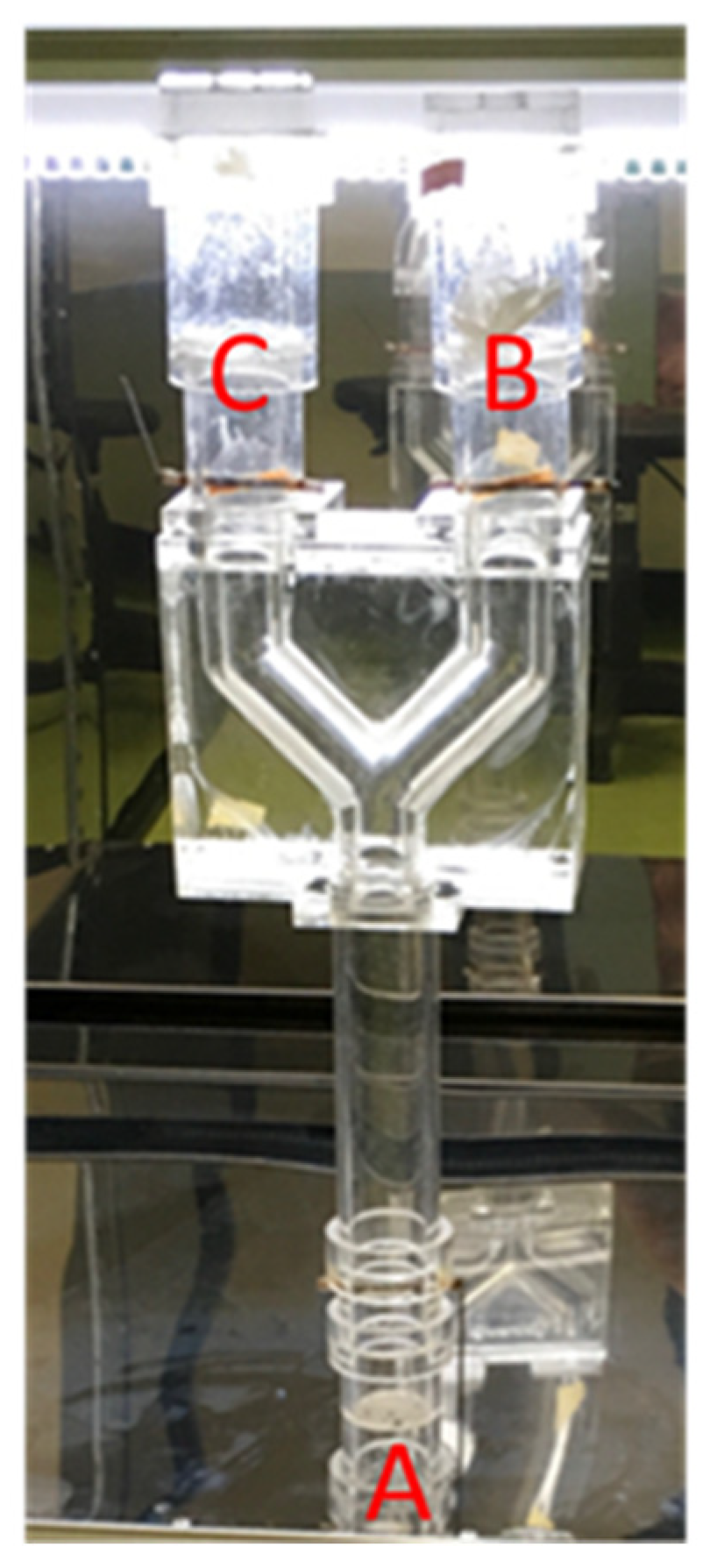
2.2.1. PVA Nanofibrous Membranes
2.2.2. PVA/EGO Nanofibrous Membranes
2.3. Tests
2.3.1. Mechanical Properties
2.3.2. Infrared Moisture Determination Balance
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. MTT Assay
2.3.5. Repellent Timeliness Measurement
3. Results and Discussion
3.1. Effects of Viscosity and Electrical Conductivity of PVA Solutions on PVA Nanofibrous Membranes
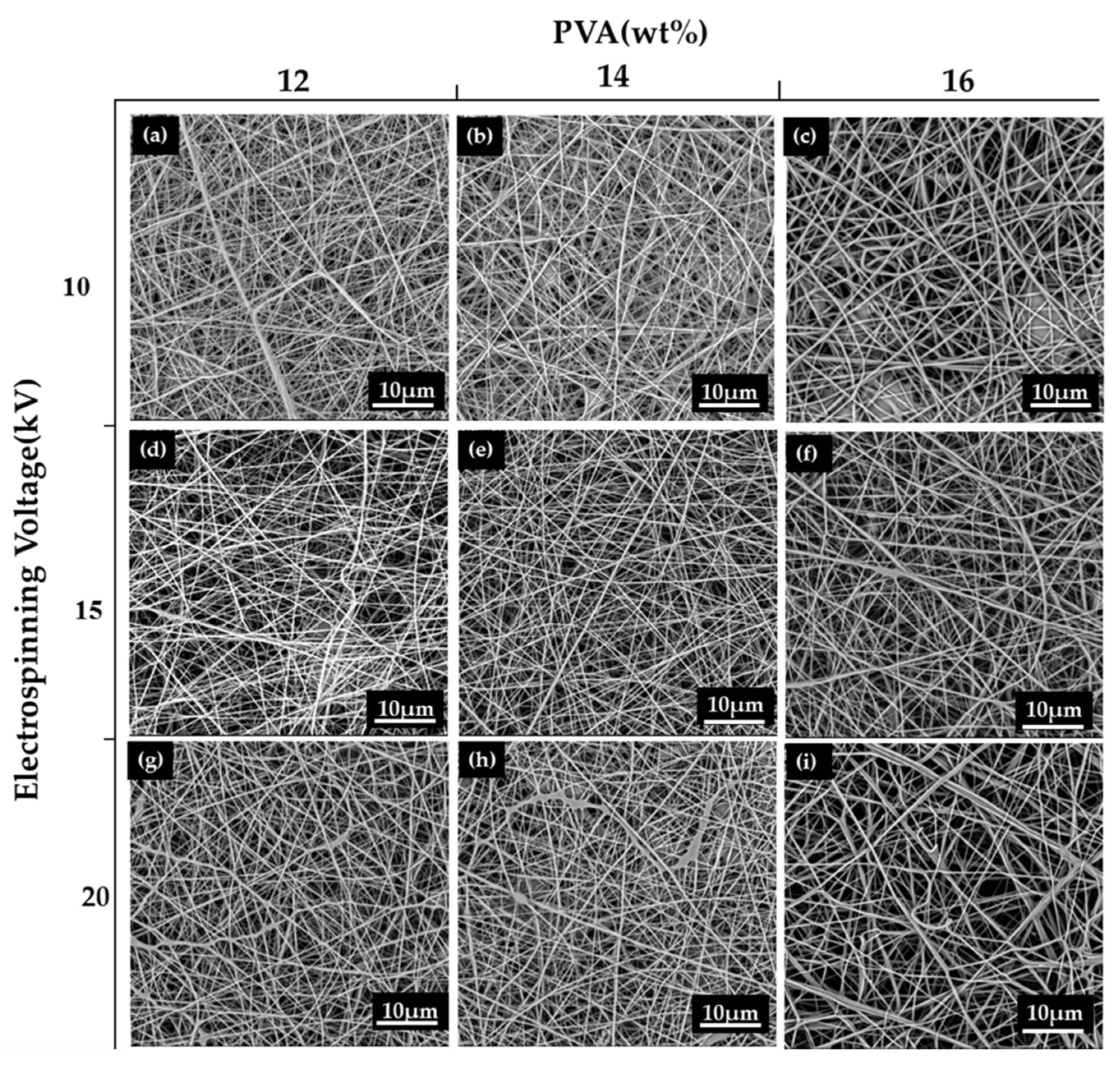
3.2. Effect of Electrospinning Voltage on Morphology of PVA Nanofibrous Membranes
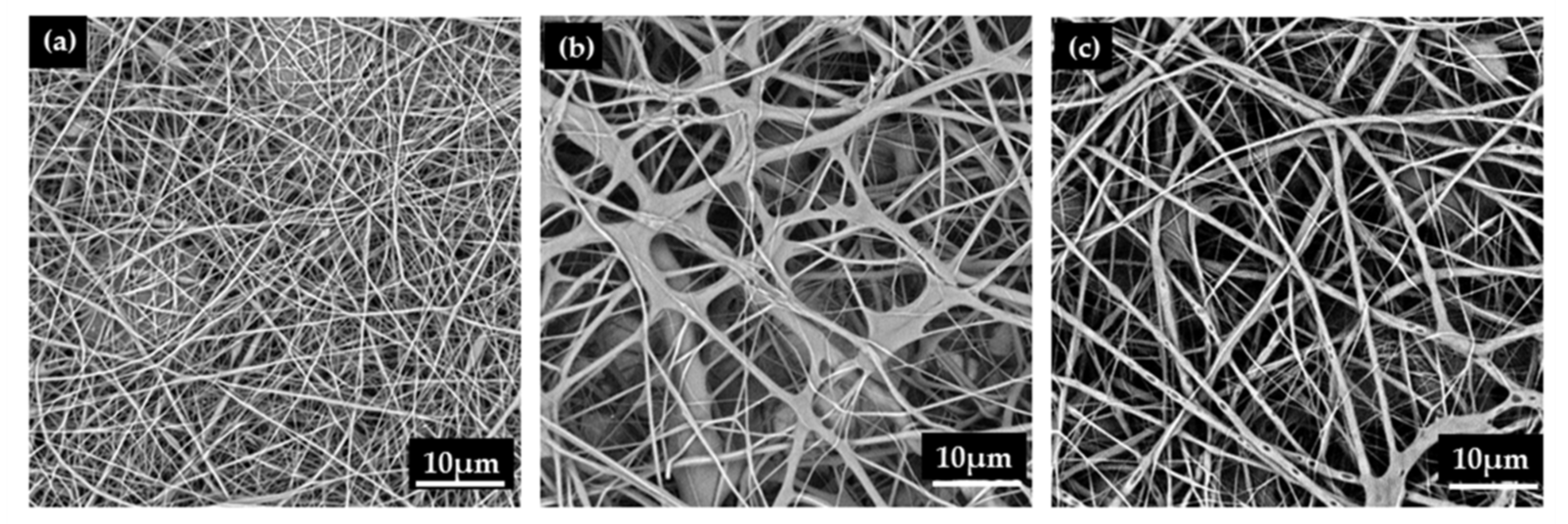
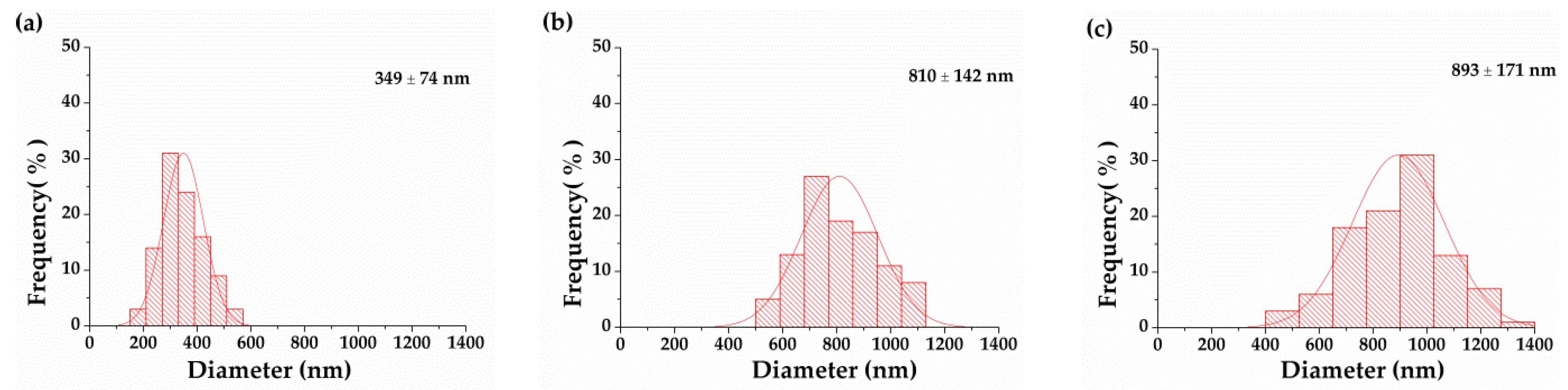
3.3. Effect of PVA/EGO Ratio on Morphology of PVA/EGO Nanofibrous Membranes
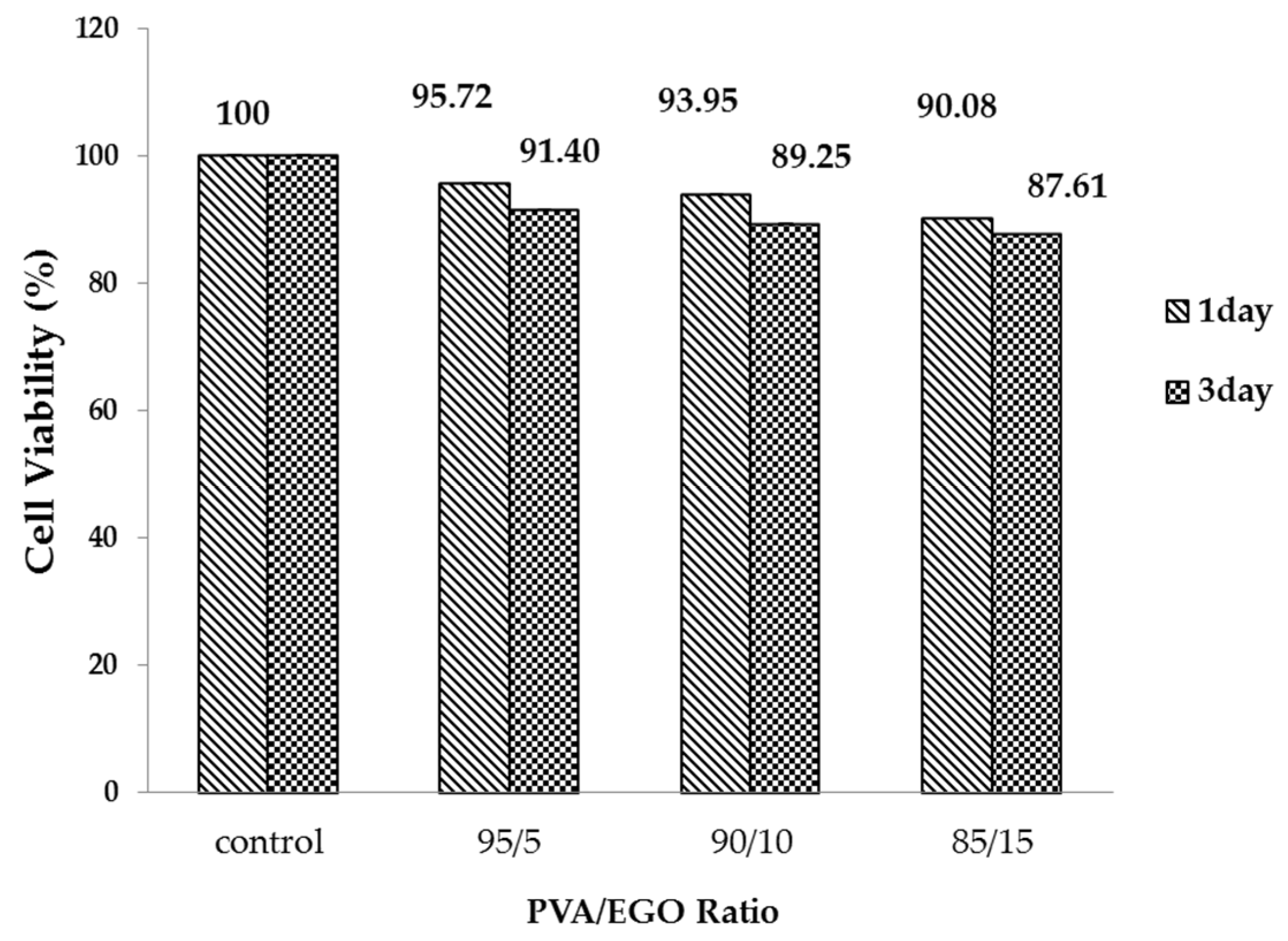
3.4. Effect of Culture Time on Cell Viability of PVA/EGO Nanofibrous Membranes
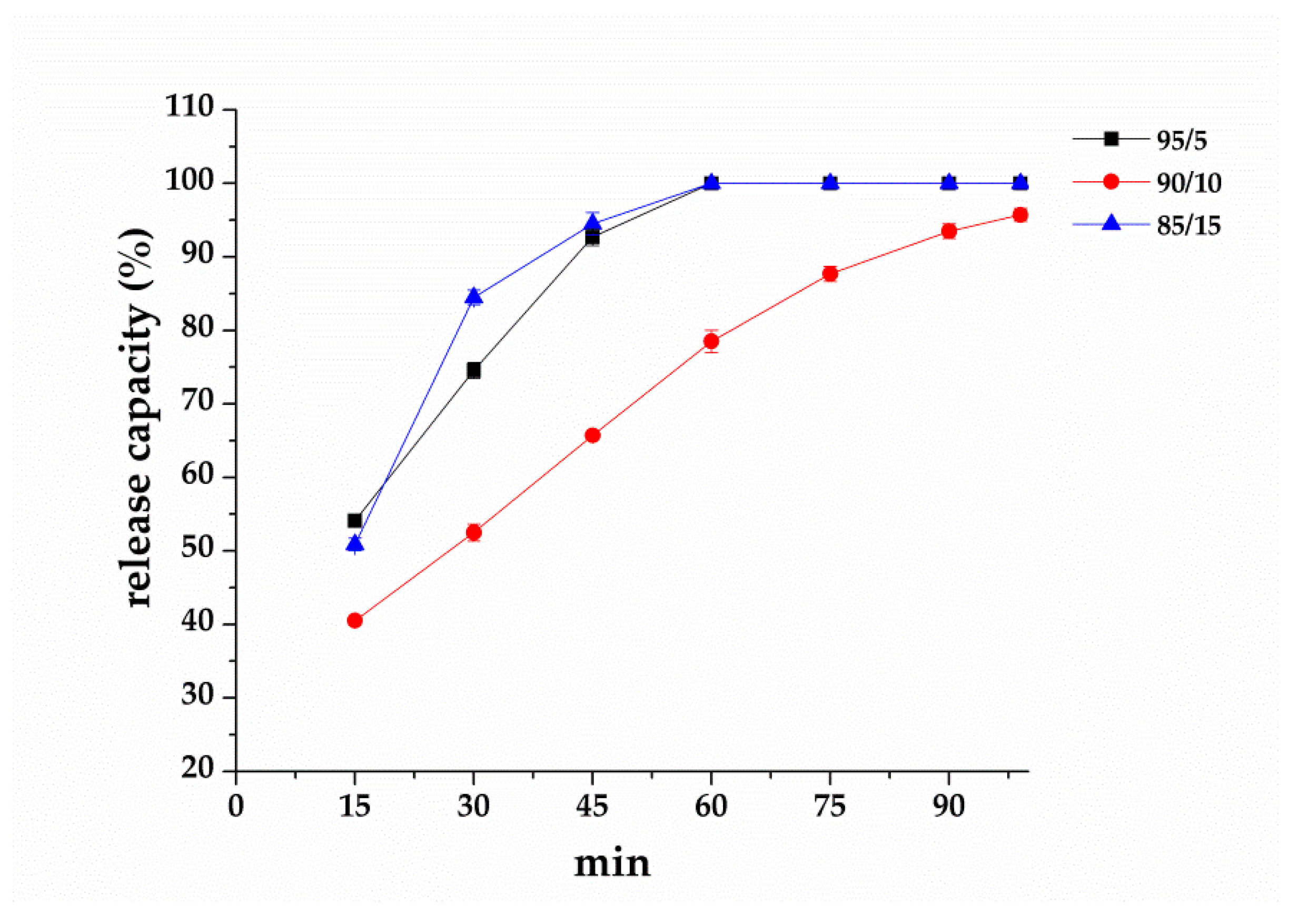
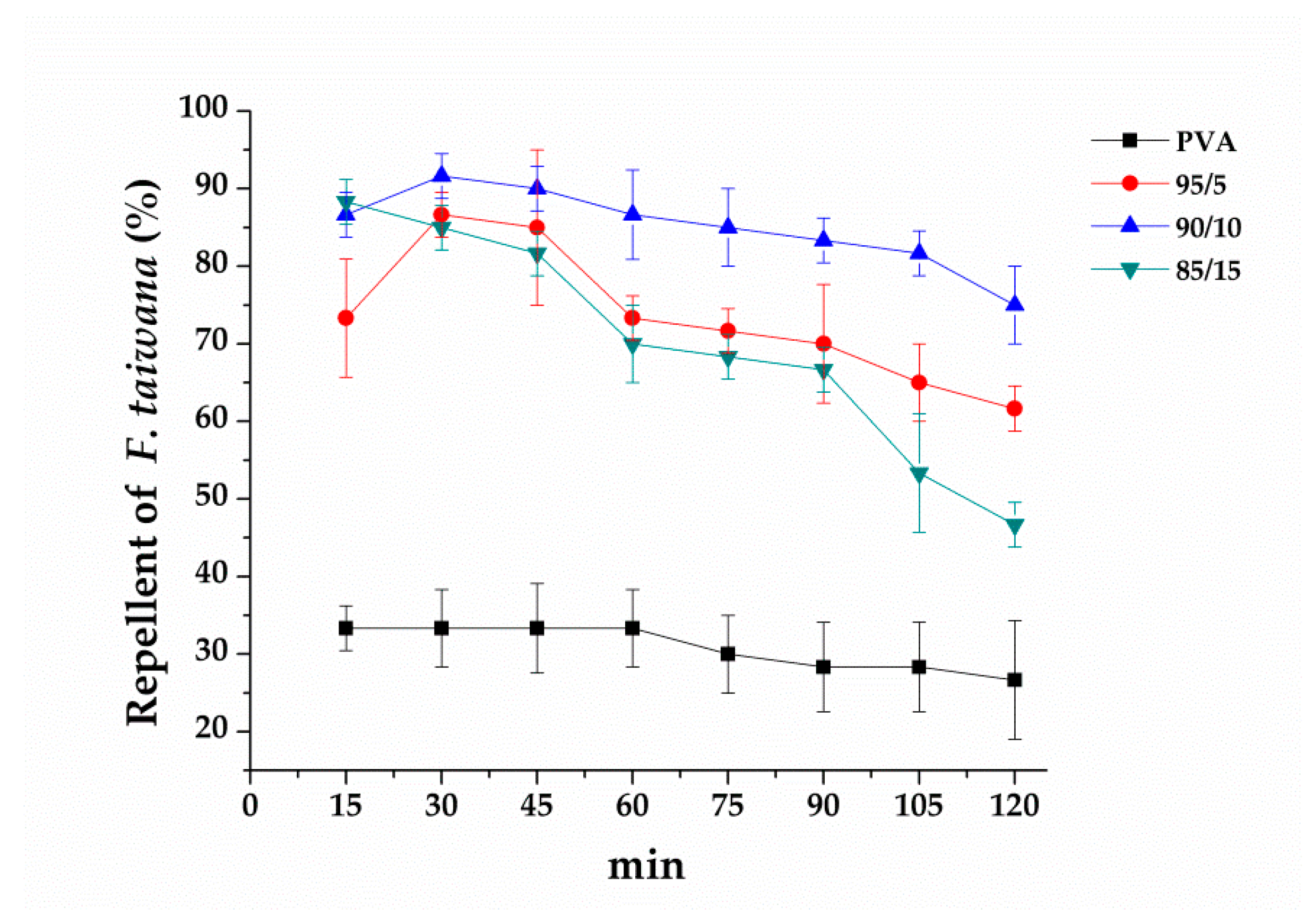
3.5. Effect of PVA/EGO Ratios on Repellent Effectiveness of PVA/EGO Nanofibrous Membranes
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Jun, H.W.; McCall, J.W. Pharmacokinetics, formulation, and safety of insect repellent n, n-diethyl-3-methylbenzamide (deet): A review. J. Am. Mosq. Control Assoc. 1998, 14, 12–27. [Google Scholar] [PubMed]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techadamrongsin, Y. Repellency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 2001, 26, 76–82. [Google Scholar] [PubMed]
- Antwi, F.B.; Shama, L.M.; Peterson, R.K. Risk assessments for the insect repellents deet and picaridin. Regul. Toxicol. Pharm. 2008, 51, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.D.; Lin, C.C.; Liou, W.M.; Wang, W.L. Wolbachia in field populations of Forcipomyia taiwana (diptera: Ceratopogonidae) in Taiwan. J. Asia Pac. Entomol. 2011, 14, 341–348. [Google Scholar] [CrossRef]
- Chen, H.W.; Chou, J.Y.; Lin, C.C.; Wen, Y.-D.; Wang, W.L. Seasonal yeast compositions in Forcipomyia taiwana (diptera: Ceratopogonidae). J. Asia Pac. Entomol. 2016, 19, 509–514. [Google Scholar] [CrossRef]
- Shih, C.-L.; Liao, Q.-M.; Wang, Y.-Y.; Tu, W.-C. Abundance and host-seeking activity of the biting midge, Forcipomyia taiwana (diptera: Ceratopogonidae). J. Asia Pac. Entomol. 2019, 22, 1053–1059. [Google Scholar] [CrossRef]
- Specos, M.M.; Garcia, J.; Tornesello, J.; Marino, P.; Vecchia, M.D.; Tesoriero, M.D.; Hermida, L. Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans. Roy. Soc. Trop. Med. Hyg. 2010, 104, 653–658. [Google Scholar] [CrossRef]
- Ishnava, K.B.; Chauhan, J.B.; Barad, M.B. Anticariogenic and phytochemical evaluation of eucalyptus globules labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against aedes, anopheles, and culex mosquitoes. Parasitol. Res. 2006, 99, 478. [Google Scholar] [CrossRef]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.L.; Silva, R.A.; Barros, R.S.; Sousa, R.N.; Sousa, L.C.; Brito, E.S.; Souza-Neto, M.A. Chemical composition of Eucalyptus spp. Essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet. Parasitol. 2010, 167, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.S.; Salem, M.Z.M.; Soliman, A.M. Repellent, attractive, and insecticidal effects of essential oils from Schinus terebinthifolius fruits and Corymbia citriodora leaves on two whitefly species, Bemisia tabaci, and Trialeurodes ricini. Sci. Hortic. 2017, 216, 111–119. [Google Scholar] [CrossRef]
- Wali, A.; Zhang, Y.; Sengupta, P.; Higaki, Y.; Takahara, A.; Badiger, M.V. Electrospinning of non-ionic cellulose ethers/polyvinyl alcohol nanofibers: Characterization and applications. Carbohydr. Polym. 2018, 181, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Shaohua, J.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Wang, X.; Yue, T.; Lee, T.C. Development of pleurocidin-poly(vinyl alcohol) electrospun antimicrobial nanofibers to retain antimicrobial activity in food system application. Food Control 2015, 54, 150–157. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Influence of solvent characteristics in triaxial electrospun fiber formation. React. Funct. Polym. 2015, 90, 36–46. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Kim, H.Y.; Bang, H.J.; Jung, Y.H.; Lee, S.G. The change of bead morphology formed on electrospun polystyrene fibers. Polymer 2003, 44, 4029–4034. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. A Phys. Eng. Asp. 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Lou, C.W.; Wu, Z.H.; Lee, M.C.; Chen, Y.-S.; Lin, J.-H. Polyvinyl alcohol/lithospermum erythrorhizon nanofibrous membrane: Characterizations, in vitro drug release, and cell viability. Appl. Sci. Basel 2017, 7, 1143. [Google Scholar] [CrossRef] [Green Version]
- Feng, J. The stretching of an electrified non-newtonian jet: A model for electrospinning. Phys. Fluids 2002, 14, 3912–3926. [Google Scholar] [CrossRef] [Green Version]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and electrically forced jets. I. Stability theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef] [Green Version]
- Ojha, S.S.; Afshari, M.; Kotek, R.; Gorga, R.E. Morphology of electrospun nylon-6 nanofibers as a function of molecular weight and processing parameters. J. Appl. Polym. Sci. 2008, 108, 308–319. [Google Scholar] [CrossRef]
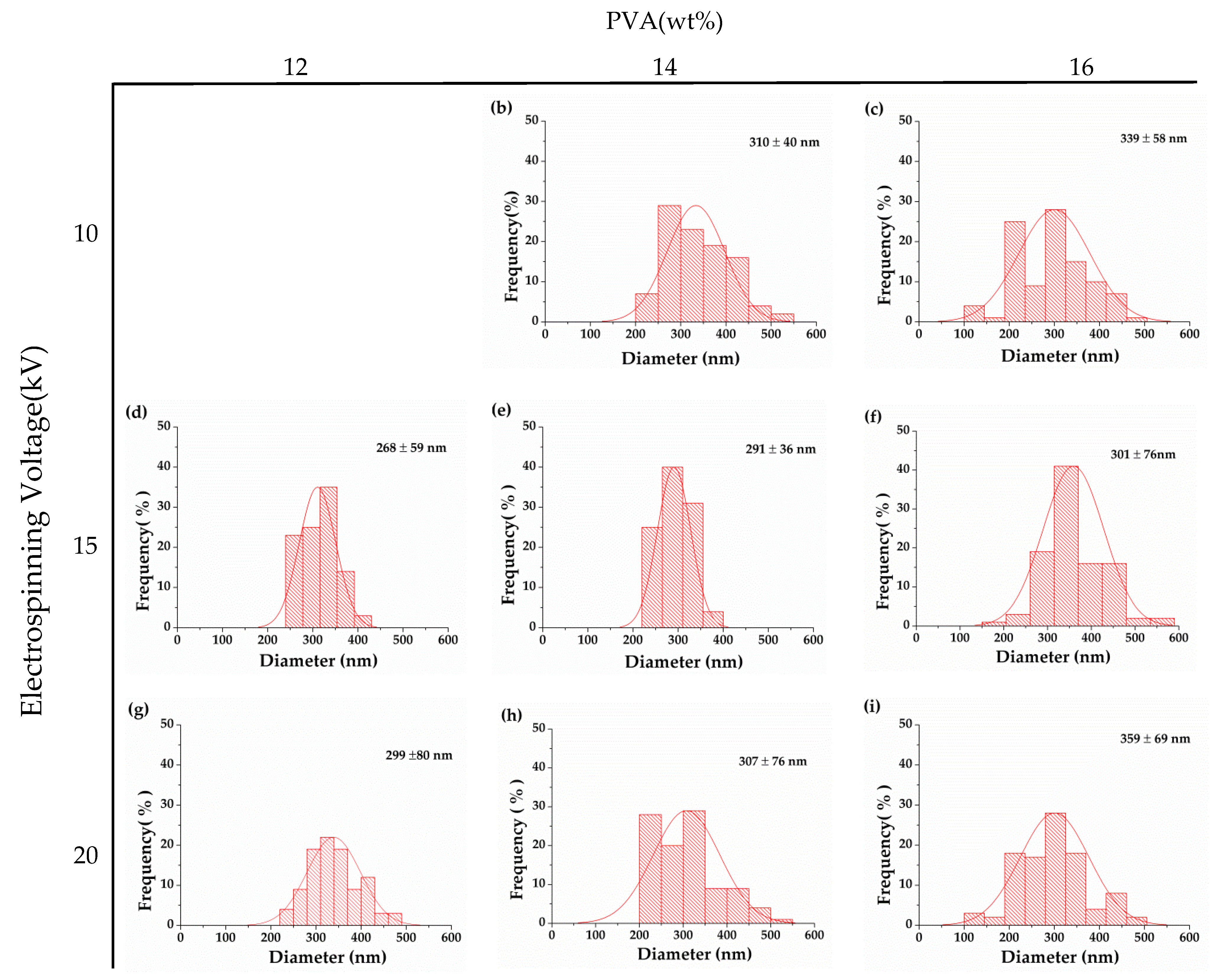
| PVA (wt%) | Viscosity (cP) | Electrical Conductivity (µS/cm) | Voltage (kV) | Diameter Distribution (nm) | Average Diameter (nm) | Stress (N) |
|---|---|---|---|---|---|---|
| 12 | 438.6 | 527 | 10 | 223–506 | 333 ± 63 | 0.22 ± 0.10 |
| 15 | 141–482 | 268 ± 59 | 0.23 ± 0.05 | |||
| 20 | 141–463 | 299 ± 80 | 0.26 ± 0.05 | |||
| 14 | 676 | 554 | 10 | 250–397 | 310 ± 40 | 0.23 ± 0.05 |
| 15 | 235–375 | 291 ± 36 | 0.26 ± 0.10 | |||
| 20 | 210–538 | 307 ± 76 | 0.30 ± 0.10 | |||
| 16 | 1054 | 651 | 10 | 223–82 | 339 ± 58 | 0.23 ± 0.05 |
| 15 | 141–482 | 301 ± 76 | 0.23 ± 0.05 | |||
| 20 | 197–559 | 359 ± 69 | 0.25 ± 0.10 |
| PVA (14 wt%) + Essential Oil | Viscosity (cP) | Electrical Conductivity (µS/cm) | Voltage (kV) | Diameter Distribution (nm) | Average Diameter (nm) | Stress (N) |
|---|---|---|---|---|---|---|
| 95/5 | 1405 | 465 | 15 | 197–516 | 349 ± 74 | 0.26 ± 0.05 |
| 90/10 | 1586 | 319 | 15 | 530–1080 | 810 ±142 | 0.23 ± 0.05 |
| 85/15 | 1847 | 210 | 15 | 442–1353 | 893 ±171 | 0.20 ± 0.05 |
| 80/20 | 2108 | 196 | 15 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.-W.; Hsieh, M.-C.; Lu, C.-T.; Lai, M.-F.; Lee, M.-C.; Shiu, B.-C.; Lin, J.-H. Evaluation of Repellent Effectiveness of Polyvinyl Alcohol/Eucalyptus globules Nanofibrous Membranes against Forcipomyia taiwana. Polymers 2020, 12, 870. https://doi.org/10.3390/polym12040870
Lou C-W, Hsieh M-C, Lu C-T, Lai M-F, Lee M-C, Shiu B-C, Lin J-H. Evaluation of Repellent Effectiveness of Polyvinyl Alcohol/Eucalyptus globules Nanofibrous Membranes against Forcipomyia taiwana. Polymers. 2020; 12(4):870. https://doi.org/10.3390/polym12040870
Chicago/Turabian StyleLou, Ching-Wen, Ming-Chun Hsieh, Chao-Tsang Lu, Mei-Feng Lai, Mong-Chuan Lee, Bing-Chiuan Shiu, and Jia-Horng Lin. 2020. "Evaluation of Repellent Effectiveness of Polyvinyl Alcohol/Eucalyptus globules Nanofibrous Membranes against Forcipomyia taiwana" Polymers 12, no. 4: 870. https://doi.org/10.3390/polym12040870







