Bio-Compatible Ca-BDC/Polymer Monolithic Composites Templated from Bio-Active Ca-BDC Co-Stabilized CO2-in-Water High Internal Phase Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ca-BDC MOF
2.3. Ca-BDC MOF Emulsification Test
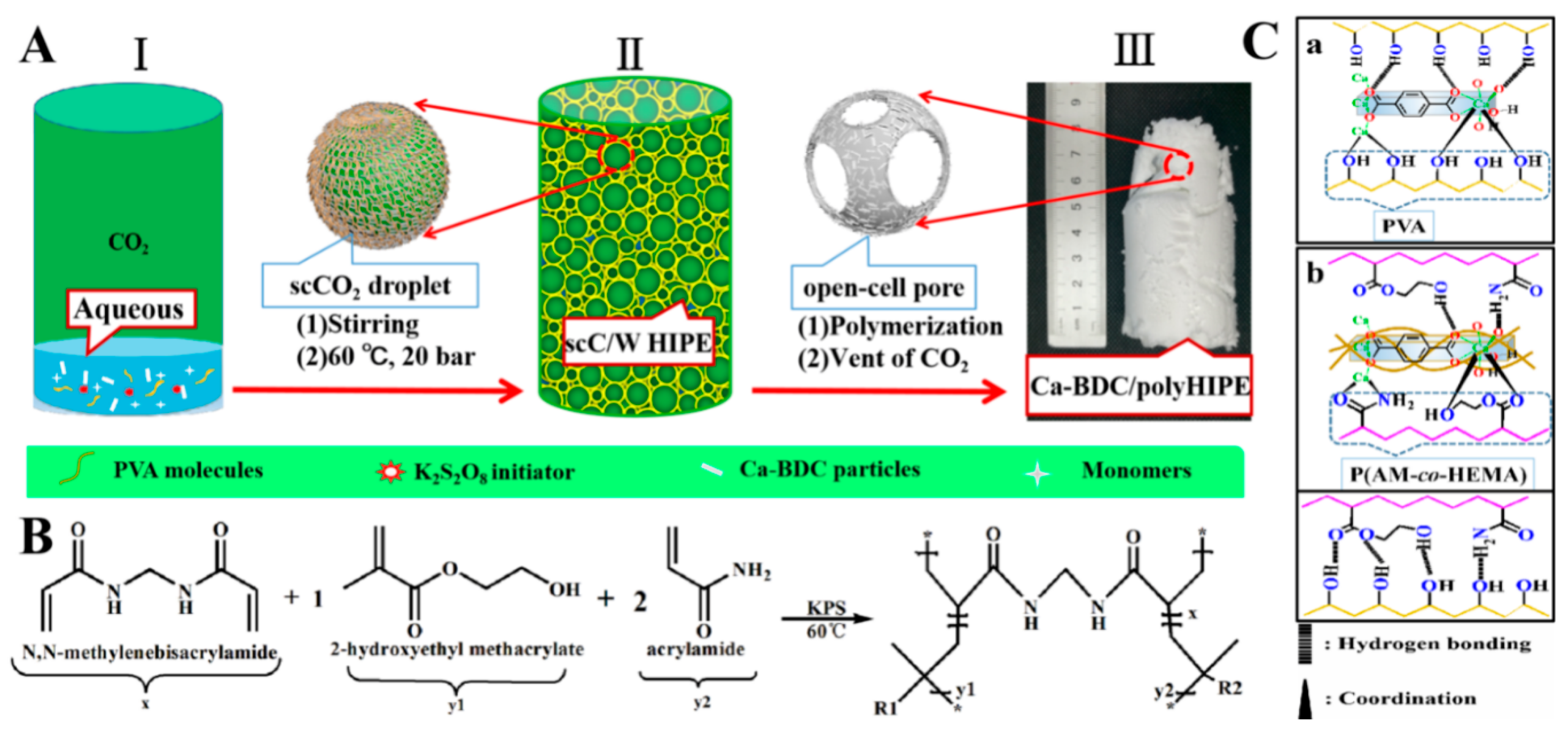
2.4. Preparations of Ca-BDC/PolyHIPEs Monolithic Composites
2.5. Characterization
2.6. Mechanical Properties Analysis
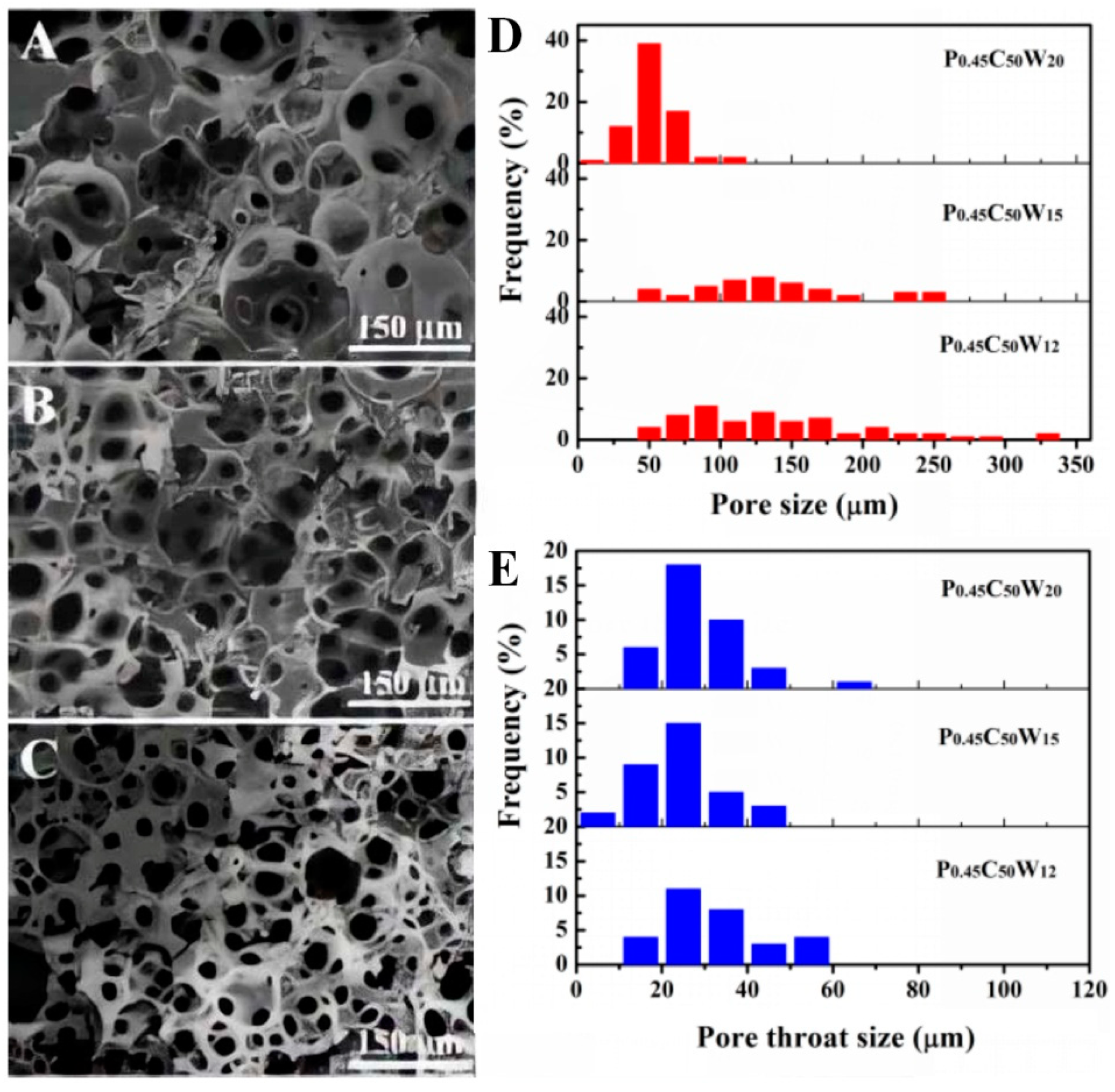
2.7. Determination of Pore and Pore Throats Size Distributions
2.8. Bio-Compatibility Test
2.9. Enzyme Immobilization
3. Results and Discussion
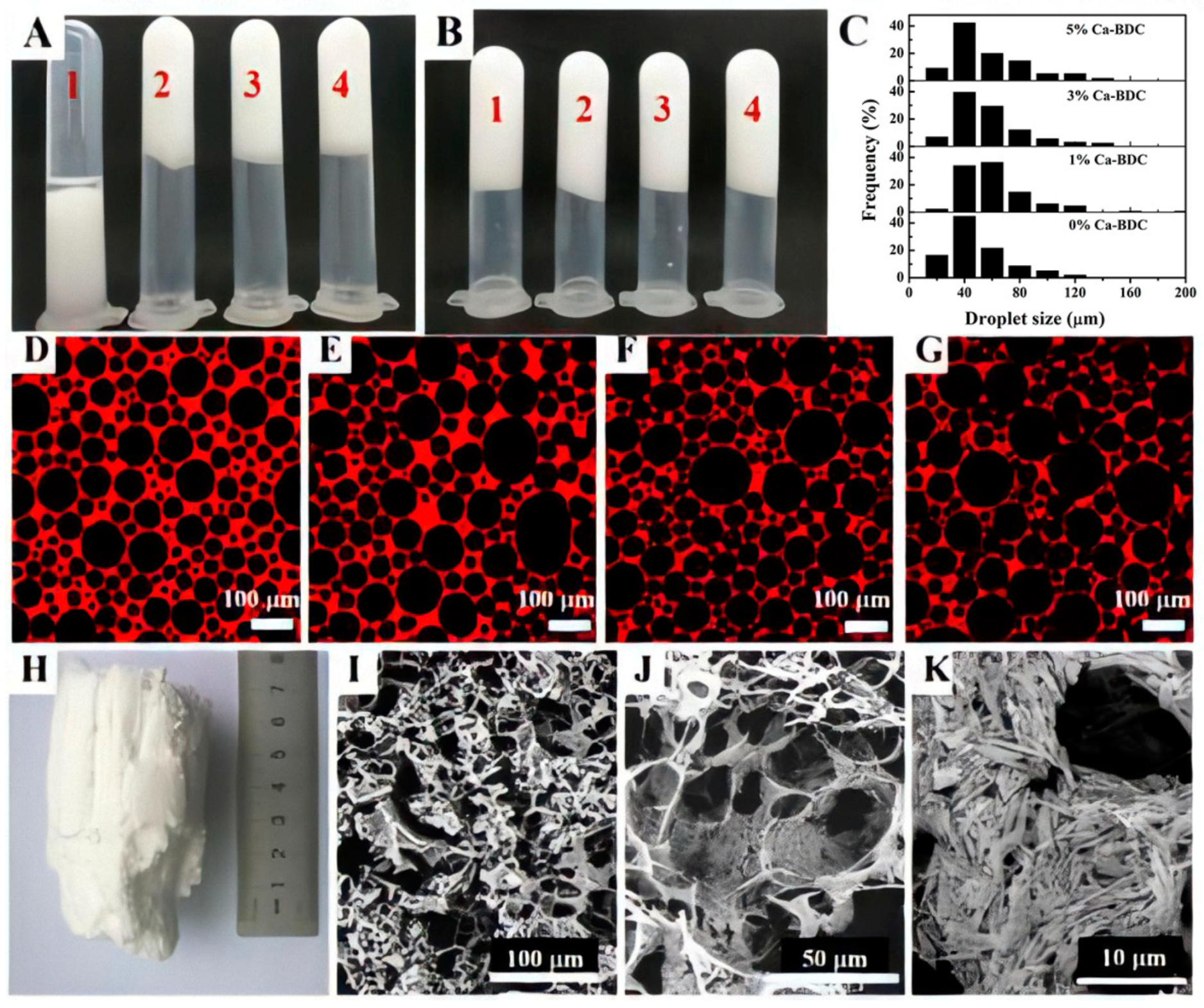
3.1. HIPEs Emulsified by Ca-BDC
3.2. Preparations of the Ca-BDC/PolyHIPEs Monolithic Composites
3.3. Mechanical Performances
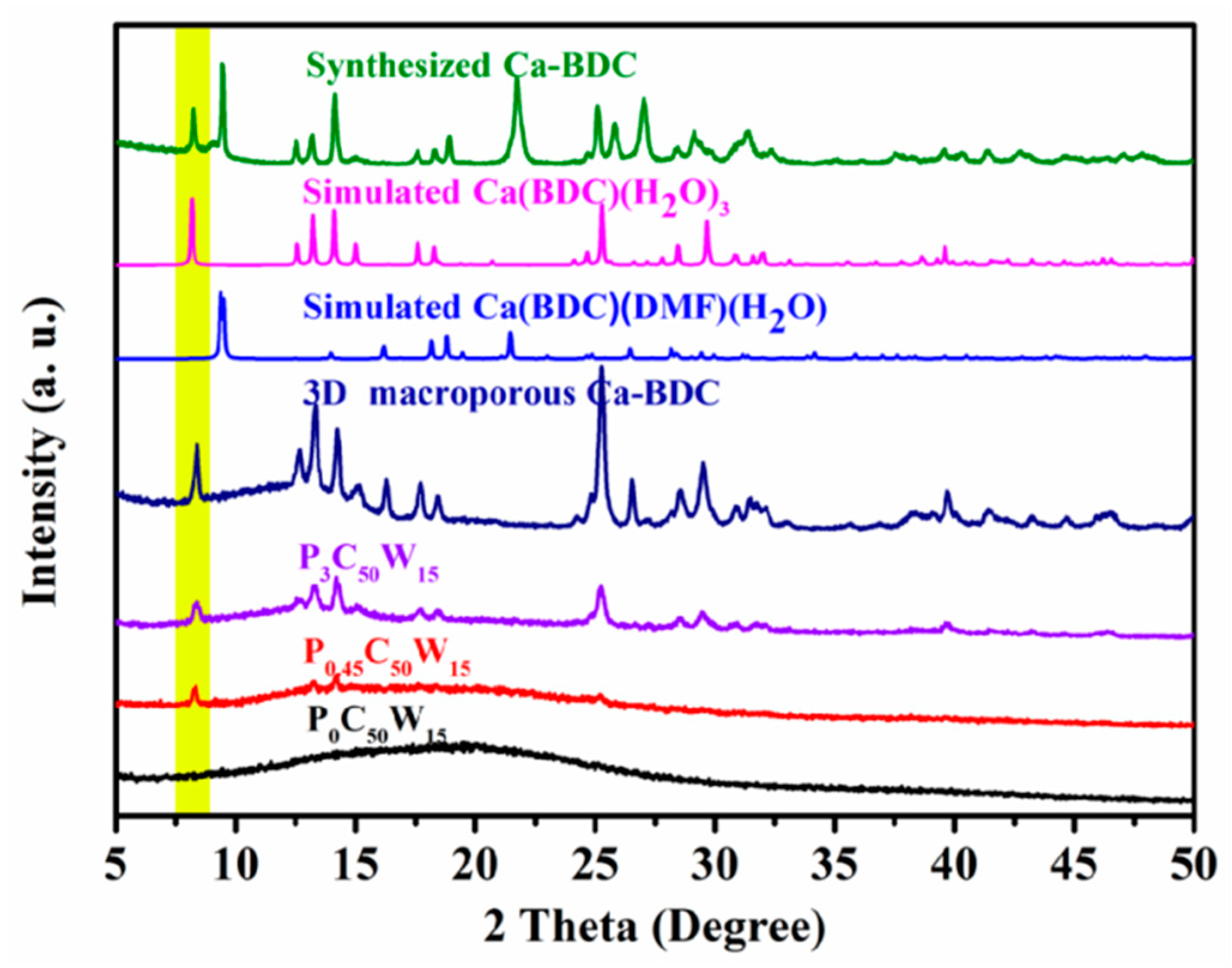
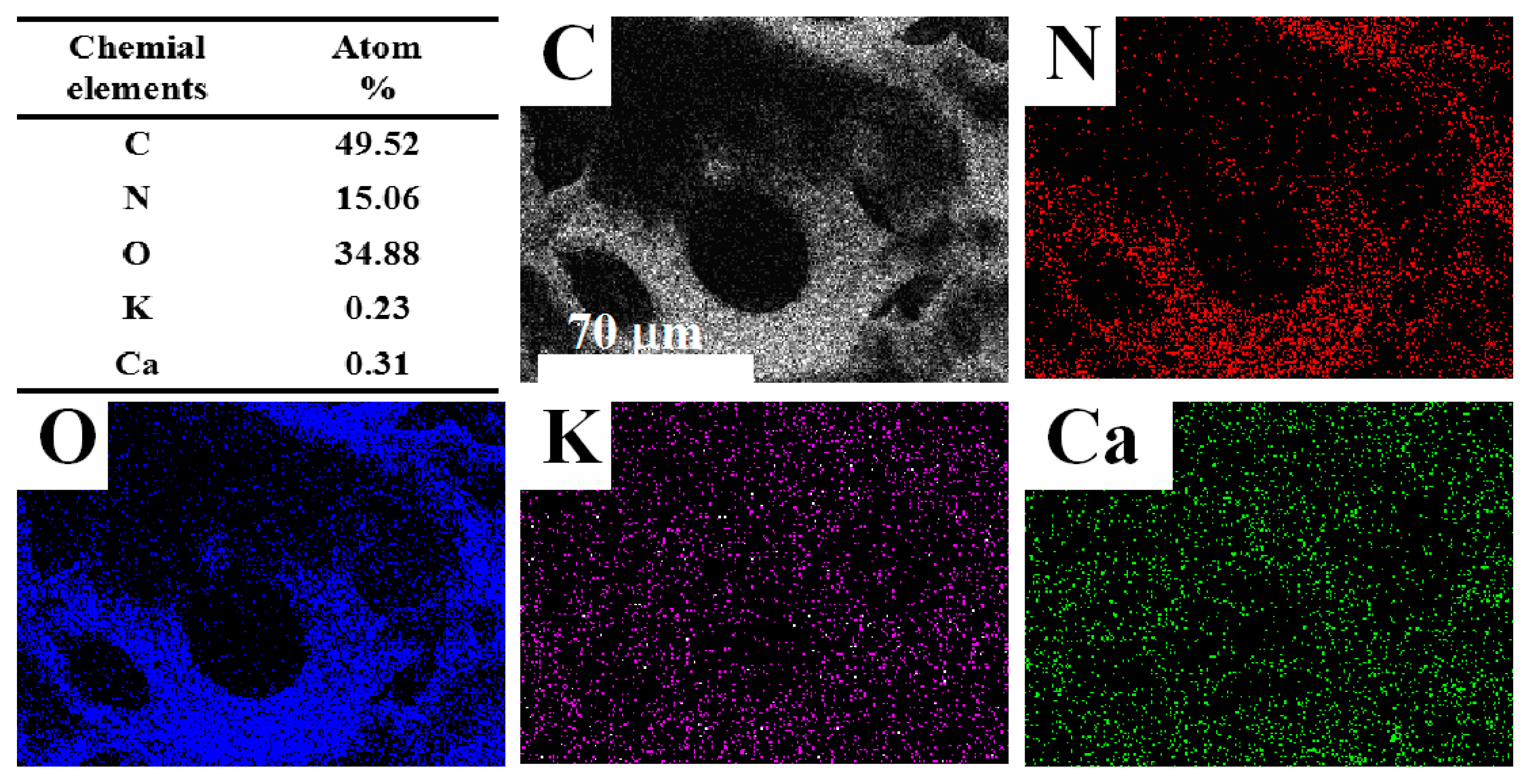
3.4. General Characterization
3.5. Bio-Compatibility Evaluations
3.6. Evaluation of β-amylases Immobilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayward, A.S.; Sano, N.; Przyborski, S.; Cameron, N.R. Acrylic-acid-functionalized PolyHIPE scaffolds for use in 3D cell culture. Macromol. Rapid Commun. 2013, 34, 1844–1849. [Google Scholar] [CrossRef]
- Barbetta, A.; Massimi, M.; Devirgiliis, L.C.; Dentini, M. Enzymatic cross-linking versus radical polymerization in the preparation of gelatin PolyHIPEs and their performance as scaffolds in the culture of hepatocytes. Biomacromolecules 2006, 7, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; John, D.; Grigoriev, D.; Puretskiy, N.; Böker, A. From 2D to 3D patches on multifunctional particles: How Microcontact Printing creates a new Dimension of Functionality. Soft Matter 2018, 14, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.; Bailey, T.S.; Rzayev, J. Large Pore Size Nanoporous Materials from the self-assembly of asymmetric bottlebrush block copolymers. Nano Lett. 2011, 11, 998–1001. [Google Scholar] [CrossRef]
- Murphy, R.; Walsh, D.P.; Hamilton, C.; Cryan, S.; Panhuis, M.I.H.; Heise, A. Degradable 3D-printed hydrogels based on star-shaped copolypeptides. Biomacromolecules 2018, 19, 2691–2699. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion templating: Porous polymers and beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Nischang, I.; Causon, T.J. Porous polymer monoliths: From their fundamental structure to analytical engineering applications. Trends Anal. Chem. 2016, 75, 108–117. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, Y.; Qi, Y.; Wang, W.; Wang, A. Magnetic chitosan–based adsorbent prepared via Pickering high internal phase emulsion for high-efficient removal of antibiotics. Int. J. Biol. Macromol. 2018, 106, 870–877. [Google Scholar] [CrossRef]
- Matos, M.; Gutierrez, G.; Martinezrey, L.; Iglesias, O.; Pazos, C. Encapsulation of resveratrol using food-grade concentrated double emulsions: Emulsion characterization and rheological behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Xu, Y.; Yin, S.; Liu, F.; Tang, C. Surface modification improves fabrication of pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Johnston, K.P.; Rocha, S.R.P.D. Colloids in supercritical fluids over the last 20 years and future directions. J. Supercrit. Fluids 2009, 47, 523–530. [Google Scholar] [CrossRef]
- Zhang, J.; Han, B. Supercritical or compressed CO2 as a stimulus for tuning surfactant aggregations. Acc. Chem. Res. 2013, 46, 425–433. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibanez, E.; Valle, J.M.D. Astaxanthin extraction from Haematococcuspluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Diazreinoso, B.; Moure, A.; Dominguez, H.; Parajo, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cooper, A.I. Synthesis and applications of emulsion-templated porous materials. Soft Matter 2005, 1, 107–113. [Google Scholar] [CrossRef]
- Butler, R.; Davies, C.M.; Cooper, A.I. Emulsion templating using High Internal Phase supercritical fluid emulsions. Adv. Mater. 2001, 13, 1459–1463. [Google Scholar] [CrossRef]
- Butler, R.; Hopkinson, I.; Cooper, A.I. Synthesis of porous emulsion-templated polymers using high internal phase CO2-in-water emulsions. J. Am. Chem. Soc. 2003, 125, 14473–14481. [Google Scholar] [CrossRef]
- Calvo, L.; Holmes, J.D.; Yates, M.Z.; Johnston, K.P. Steric stabilization of inorganic suspensions in carbon dioxide. J. Supercrit. Fluids 2000, 16, 247–260. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef]
- Menner, A.; Ikem, V.O.; Salgueiro, M.; Shaffer, M.S.P.; Bismarck, A. High internal phase emulsion templates solely stabilised by functionalisedtitania nanoparticles. Chem. Commun. 2007, 41, 4274–4276. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.L.; Binks, B.P.; Johnston, K.P. Stabilization of Carbon Dioxide-in-Water Emulsions with Silica Nanoparticles. Langmuir 2004, 20, 7976–7983. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Hartlieb, K.J.; Holcroft, J.M.; Moghadam, P.Z.; Vermeulen, N.A.; Algaradah, M.M.; Nassar, M.S.; Botros, Y.Y.; Snurr, R.Q.; Stoddart, J.F. CD-MOF: A versatile separation medium. J. Am. Chem. Soc. 2016, 138, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Ahmar, H.; Dehghani, A.; Bagheri, A.; Tadjarodi, A.; Fakhari, A.R. A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of L-cysteine. Biosens. Bioelectron. 2013, 42, 426–429. [Google Scholar] [CrossRef]
- Huxford, R.C.; Rocca, J.D.; Lin, W. Metal-Organic Frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Xamena, F.X.L.I. Metal Organic Framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Q.; Zhu, S. Assembly of Metal-Organic Framework into 3D hierarchical porous monoliths through Pickering High Internal Phase Emulsion template. Chem. Eur. J. 2016, 22, 8751–8755. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Luo, T.; Tan, X.; Liu, C.; Sang, X.; Ma, X.; Han, B.; Yang, G. High internal ionic liquid phase emulsion stabilized by metal–organic frameworks. Soft Matter 2016, 12, 8841–8846. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Zheng, L.; Zhang, J.; Sang, X.; Kang, X.; Zhang, B.; Luo, T.; Tan, X.; Han, B. Metal–Organic Framework for emulsifying carbon dioxide and water. Angew. Chem. 2016, 55, 11372–11376. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, L.; Li, J.; Dong, Y.; Wang, J. High-performance composite monolith synthesized via HKUST-1 stabilized HIPEs and its adsorptive properties. Macromol. Mater. Eng. 2018, 303, 1800426. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, L.; Li, J.; Yang, Y.; Wang, J. Facile preparation of UiO-66 /PAM monoliths via CO2-in-water HIPEs and their applications. RSC Adv. 2018, 8, 32358–32367. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, L.; Li, J.; Lin, J.; Wang, J. Facile synthesis of Cu-BDC/Poly(N-methylol acrylamide) HIPE monoliths via CO2-in-water emulsion stabilized by metal-organic framework. Polymer 2018, 153, 17–23. [Google Scholar] [CrossRef]
- Wickenheisser, M.; Janiak, C. Hierarchical embedding of micro-mesoporous MIL-101(Cr) in macroporous poly(2-hydroxyethyl methacrylate) high internal phase emulsions with monolithic shape for vapor adsorption applications. Microporous Mesoporous Mater. 2015, 204, 242–250. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, M.; Wang, J.; Ngai, T. Pure protein scaffolds from pickering high internal phase emulsion template. Macromol. Rapid Commun. 2013, 34, 169–174. [Google Scholar] [CrossRef]
- Kovacic, S.; Mazaj, M.; Jeselnik, M.; Pahovnik, D.; Žagar, E.; Slugovc, C.; Logar, N.Z. Synthesis and catalytic performance of hierarchically porous MIL-100(Fe)@polyHIPE hybrid membranes. Macromol. Rapid Commun. 2015, 36, 1605–1611. [Google Scholar] [CrossRef]
- Deshmukh, A.B.; Nalawade, A.C.; Karbhal, I.; Qureshi, M.S.; Shelke, M.V. Electrochemical capacitive energy storage in PolyHIPE derived nitrogen enriched hierarchical porous carbon nanosheets. Carbon 2018, 128, 287–295. [Google Scholar] [CrossRef]
- Szczurek, A.; Yuso, A.M.; Fierro, V.; Pizzi, A.; Celzard, A. Tannin-based monoliths from emulsion-templating. Mater. Des. 2015, 79, 115–126. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Liang, P.C.; Liu, H.K.; Yeh, C.T.; Lin, C.H.; Zima, V. Supramolecular assembly of Calcium Metal-Organic Frameworks with structural transformations. Cryst. Growth Des. 2011, 11, 699–708. [Google Scholar] [CrossRef]
- Zou, S.; Yu, Y.; Hao, L.; Wang, C. Synergistic stabilization and tunable structures of Pickering high internal phase emulsions by nanoparticles and surfactants. Colloids Surf. A. 2013, 436, 1–9. [Google Scholar] [CrossRef]
- Ikem, V.O.; Menner, A.; Horozov, T.S.; Bismarck, A. Highly permeable macroporous polymers synthesized from Pickering medium and High Internal Phase emulsion templates. Adv. Mater. 2010, 22, 3588–3592. [Google Scholar] [CrossRef] [PubMed]
- Ottenbrite, R.M.; Park, K.; Okano, T.; Peppas, N.A. Biomedical Applications of Hydrogels Handbook, 1st ed.; Springer: New York, NY, USA, 2010; pp. 1–50. [Google Scholar]
- Mazaj, M.; Logar, N.Z. Phase formation study of Ca-terephthalate MOF-type materials. Cryst. Growth Des. 2015, 15, 617–624. [Google Scholar] [CrossRef]
- Mazaj, M.; Mali, G.; Rangus, M.; Žunkovic, E.; Kaucic, V.; Logar, N.Z. Spectroscopic studies of structural dynamics induced by heating and hydration: A case of Calcium-Terephthalate Metal–Organic Framework. Phys. Chem. C 2013, 117, 7552–7564. [Google Scholar] [CrossRef]
- Atia, K.S.; Ismail, S.A.; Dessouki, A.M. Immobilization of β-amylase using polyacrylamide polymer derivatives. J. Chem. Technol. Biotechnol. 2010, 78, 891–898. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandezlorente, G.; Guisan, J.M.; Fernandezlafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Warren, J.C.; Cheatum, S.G. Effect of neutral salts on Enzyme activity and structure. Biochemistry 1966, 5, 1702–1707. [Google Scholar] [CrossRef]









| Samples | PxC50W15 a | P0.45C50Wy b | P0.45CzW15 c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | 0.15 | 0.45 | 0.75 | 12 | 15 | 20 | 40 | 55 | 70 |
| d/D (%) | 47 | 36 | 47 | 33 | 29 | 42 | 23 | 22 | 44 |
| d (μm) | 18 | 26 | 28 | 38 | 36 | 22 | 30 | 17 | 12 |
| D (μm) | 38 | 72 | 60 | 115 | 125 | 52 | 128 | 75 | 27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Hao, Y.; Cao, L. Bio-Compatible Ca-BDC/Polymer Monolithic Composites Templated from Bio-Active Ca-BDC Co-Stabilized CO2-in-Water High Internal Phase Emulsions. Polymers 2020, 12, 931. https://doi.org/10.3390/polym12040931
Yang X, Hao Y, Cao L. Bio-Compatible Ca-BDC/Polymer Monolithic Composites Templated from Bio-Active Ca-BDC Co-Stabilized CO2-in-Water High Internal Phase Emulsions. Polymers. 2020; 12(4):931. https://doi.org/10.3390/polym12040931
Chicago/Turabian StyleYang, Xule, Youwei Hao, and Liqin Cao. 2020. "Bio-Compatible Ca-BDC/Polymer Monolithic Composites Templated from Bio-Active Ca-BDC Co-Stabilized CO2-in-Water High Internal Phase Emulsions" Polymers 12, no. 4: 931. https://doi.org/10.3390/polym12040931
APA StyleYang, X., Hao, Y., & Cao, L. (2020). Bio-Compatible Ca-BDC/Polymer Monolithic Composites Templated from Bio-Active Ca-BDC Co-Stabilized CO2-in-Water High Internal Phase Emulsions. Polymers, 12(4), 931. https://doi.org/10.3390/polym12040931





