1. Introduction
Tires have a complex structure that consists of more than 10 components, such as tread, belts, carcass, sidewall, and inner liner, etc., among which treads are used to directly affect tire performance. The rubber compound must satisfy fuel efficiency (rolling resistance), traction performance, and abrasion resistance, etc., simultaneously. Among these properties, traction performance can be used to determine the driving safety, and tire treads directly contacting with the road surfaces have a great influence [
1,
2,
3]. Therefore, several research studies have focused on applying styrene-butadiene rubber (SBR), which has excellent traction ability, to the tire tread rubber compound for passenger car radials (PCRs) [
4,
5,
6].
SBR is classified into emulsion styrene-butadiene rubber (ESBR) and solution styrene-butadiene rubber (SSBR), depending on the polymerization method. The polymerization of ESBR uses water as a solvent and SSBR uses an organic solvent, and they are polymerized by different mechanisms. The advantages of SSBR include microstructure control, chain-end functionalization, and narrow dispersity, but it has the disadvantages of having a high manufacturing cost and requiring the use of organic solvents. When compared to SSBR, ESBR is more environmentally friendly and it is easier to obtain a high molecular weight polymer, which has advantages in terms of mechanical properties [
7]. However, microstructure and/or chain-end functionalization is difficult to control for ESBR, and polymerization is carried out through free radical polymerization; therefore, the dispersity is broad [
8]. In general, tire properties are highly influenced by the macrostructure (molecular weight and dispersity, etc.) and microstructure (chain architecture, vinyl content, branch of the polymer chain, etc.) of the polymer that is used [
9]. In the case of ESBR, the dispersity is broad and chain branches are formed due to the radical polymerization characteristics. Consequently, the hysteresis loss increases, which causes unfavorable results compared to SSBR in terms of the dynamic viscoelastic properties [
10].
As the dynamic viscoelastic properties of tread compounds are directly related to the fuel efficiency of tires, the broad dispersity of ESBR needs to be improved. In general, a method of effectively narrowing the broad dispersity of polymers is to apply the reversible deactivation radical polymerization (RDRP) technique. There are three major types of living radical polymerizations, depending on the mechanism: atom transfer radical polymerization (ATRP) [
11], nitroxide-mediate radical polymerization (NMP) [
12], and reversible addition-fragmentation radical transfer (RAFT) polymerization [
13]. Among these polymerization techniques, RAFT polymerization can be applied to various reaction conditions and it can be performed in the conventional free radical polymerization set-up. In addition, the polymerization conditions (for example, solvents, temperature, etc.) are environmentally friendly when compared to other living radical polymerizations, as there are no metal ligands or toxic solvents [
14,
15,
16,
17,
18,
19].
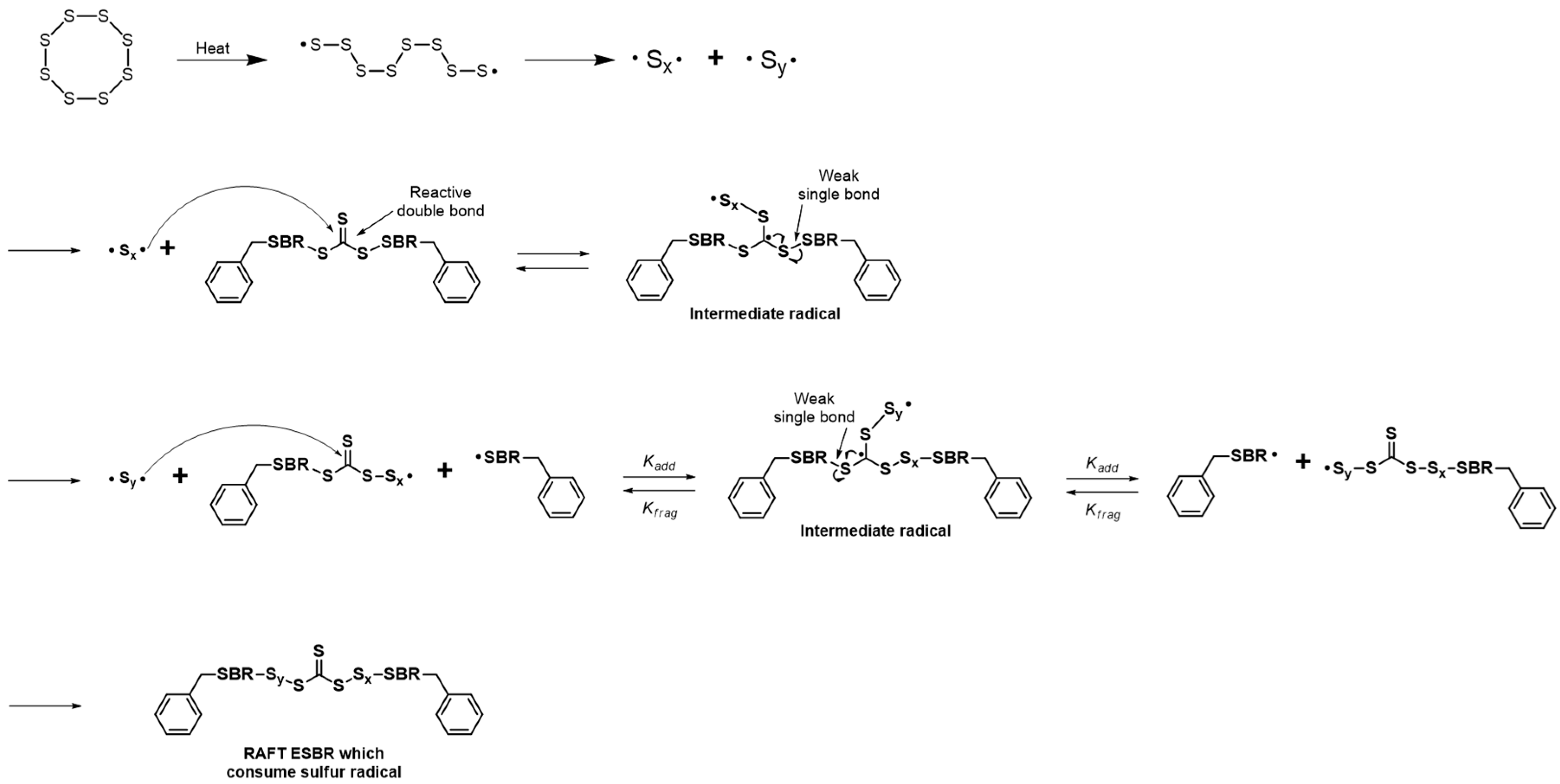
Usually, RAFT polymerization uses thiocarbonylthio compounds having a generic formula R–S–(C=S)–Z as a chain transfer agent (RAFT agent) [
20,
21,
22]. The RAFT agents are highly active chain transfer agents that allow for a dynamic equilibrium between propagating radicals and dormant species through fast degenerative chain transfer reactions [
23,
24]. This rapid dynamic equilibrium provides an equal probability of the polymer chains growing uniformly, and making the polymerized polymers have a narrow dispersity [
25] (
Figure 1). Furthermore, RAFT polymerization is most suitable for the polymers with large molecular weights and it can be applied to emulsion polymerization. Recently, research on RAFT emulsion polymerization while using the RAFT polymerization technique has been extensively studied [
26,
27,
28,
29,
30]
However, unlike solution or bulk polymerization, it has been reported that the application of the RAFT polymerization technique to emulsion polymerization causes some problems such as loss of molecular weight control, difficulties of coagulum formation, and phase separation [
31,
32,
33,
34,
35]. In the case of RAFT emulsion polymerization, polymerization occurs after the RAFT agent is diffused into the micelle particles due to the polymerization characteristics. Therefore, the above problems occur, depending on the structure and solubility of the RAFT agent. When the RAFT agent has a monomer-soluble structure, the RAFT agent has a good affinity with the monomer droplet. Thus, the diffusion rate of the RAFT agent into the micelle particles is low, such that the polymerization proceeds with the same mechanism as the conventional emulsion polymerization and it cannot mediate the polymerization reaction. In addition, when the RAFT agent has a water-soluble structure, a chain transfer reaction of the RAFT agent occurs in the water phase. Consequently, it takes a long time to form oligomeric radicals that can enter the micelle particles and act as a retardant of polymerization [
36].
Research on the polymerization of RAFT ESBR using this RAFT emulsion polymerization technique has been recently reported [
37]. In early RAFT ESRB polymerization, other RAFT agents, except
S,S-dibenzyl trithiocarbonate (DBTC), formed a gel and it could not effectively obtain a high molecular weight due to the problem of the slow diffusion rate of the RAFT agent into emulsion particles [
38]. Based on the reference 37 and 38, our researchers also carried out the polymerization of RAFT ESBR while using RAFT agents of various structures for the selection of appropriate RAFT agent. When the RAFT agent has hydrophilic functional groups (i.e.,
S-(thiobenzoyl) thioglycolic acid), the chain transfer radicals that formed by fragmentation were present in this water phase due to its water-soluble structure. Additionally, in the case of SBR polymerization, butadiene having a low propagation rate was used as a monomer. Therefore, when the RAFT agent with hydrophilic functional groups was used for ESBR polymerization, chain transfer radical acted as retardants, and polymerization conversion was low due to the water-soluble structure of RAFT agent and low propagation rate of butadiene monomer. In addition, when the RAFT agent has monomer-soluble structure (i.e., benzyl-benzodithioate or 2-phenyl-2-propyl-benzodithioate), gel was formed during the polymerization due to the slow diffusion rate of chain transfer radical into micelle particles. Accordingly, according to our research,
S,S-dibenzyl trithiocarbonate (DBTC) showed a proper capability of dispersity control for the polymerization of high molecular weight RAFT ESBR.
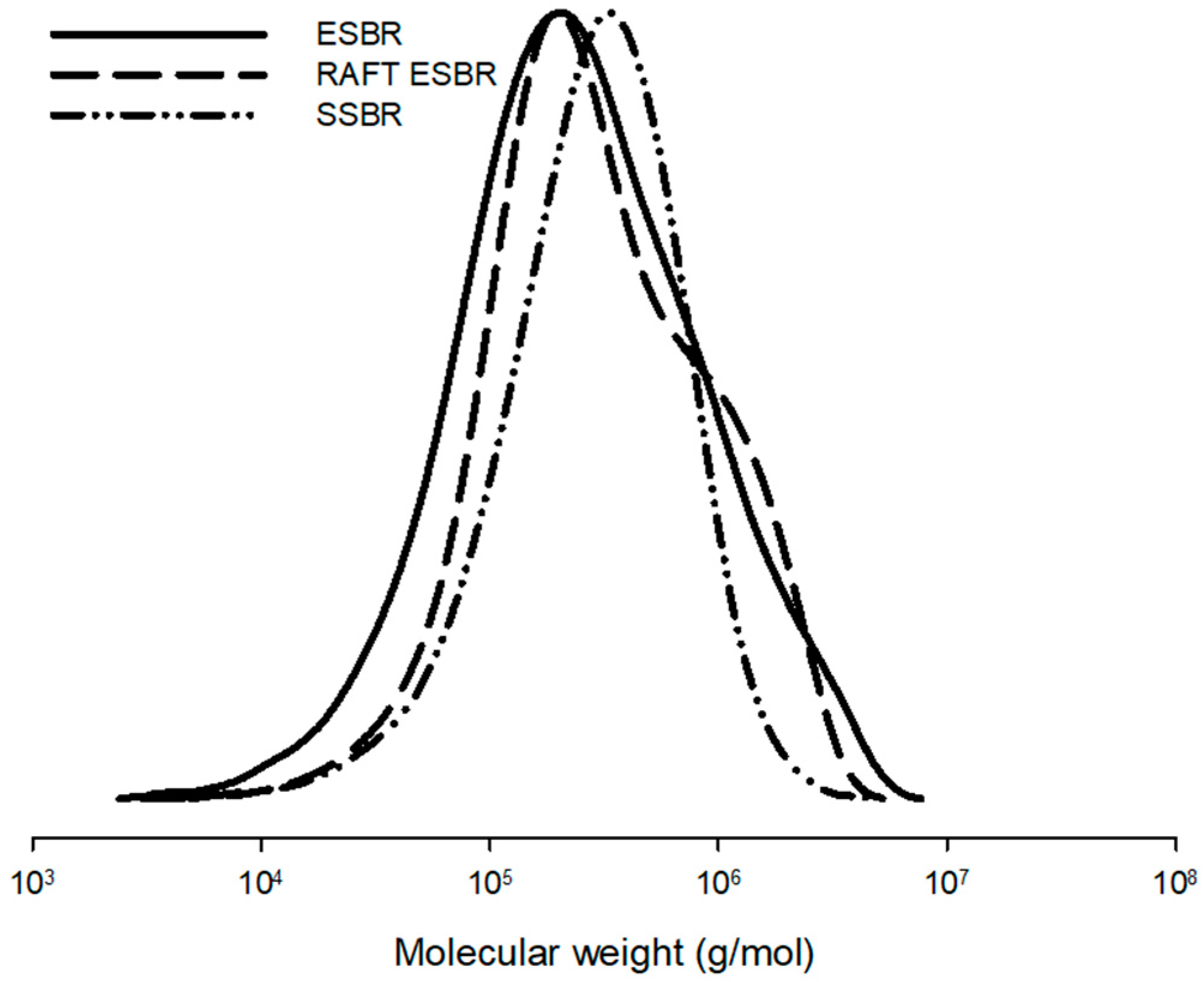
However, recently, RAFT ESBR polymerization studies reported solving these kinds of problem, and Yu et al. attempted to solve the problem of the slow diffusion rate of RAFT agents into micelle particles while using a mini emulsion polymerization technique [
39]. In addition, Mun et al. reported the results of polymerization of RAFT ESBR using DBTC as a RAFT agent, having a similar molecular weight as conventional ESBR, but with a narrower dispersity. The unfilled compounds were manufactured using the polymerized RAFT ESBR, and the properties were compared with conventional ESBR compounds. It was confirmed that RAFT ESBR has excellent abrasion resistance and fuel efficiency, despite the low crosslink density [
40]. According to Mun et al., the RAFT agent used as a chain transfer agent formed a polysulfide structure by reacting with sulfur, resulting in lower crosslink density of the compounds, or shortened SBR chain length, by reacting with a silane coupling agent. Consequently, unlike the unfilled compound, RAFT ESBR silica filled compounds showed unfavorable abrasion resistance and fuel efficiency as compared to the conventional ESBR. In the case of RAFT ESBR carbon black-filled compounds (which do not use the silane coupling agent), despite the low crosslink density due to the formation of polysulfide structure, RAFT ESBR showed similar abrasion resistance and excellent fuel efficiency due to having the same molecular weight and narrow dispersity [
41]. However, previous studies did not analyze the crosslink structure of carbon black-filled compounds or evaluate the properties in the compounds with the same crosslink density.
Therefore, in this study, the crosslink structure of RAFT ESBR carbon black-filled compounds was analyzed, and a carbon black-filled RAFT ESBR compound with the same crosslink density as the carbon black filled ESBR compound was manufactured in order to investigate the effect of the narrow dispersity of RAFT ESBR on the mechanical and dynamic viscoelastic properties. Accordingly, after the polymerization of RAFT ESBR having a high molecular weight and narrow dispersity, the RAFT ESBR carbon black-filled compound was manufactured and the polysulfide structure was broken by applying a mixture of propane-2-thiol and hexylamine. A swelling test was also carried out to quantitatively analyze the crosslink structure of the vulcanizates. Based on the crosslink density analysis results, the properties of the RAFT ESBR carbon black-filled compound, for which the crosslink density was controlled by cure system regulation, were compared with the conventional ESBR carbon black-filled compound. Finally, the effect of narrow dispersity of the RAFT ESBR on the mechanical and dynamic viscoelastic properties of carbon black-filled compounds was investigated.
2. Materials and Methods
2.1. Materials for ESBR Polymerization
For the polymerization of ESBR and RAFT ESBR, styrene (99.5%) was purchased from SAMCHUN Chemicals, Korea, and 1,3-butadiene, a surfactant (fatty soap, rosin soap), micelle stabilizer (KOH), and p-methane hydroperoxide (as an initiator) were supplied by Kumho Petrochemical Co. Ltd. (KKPC, Seoul, Korea) and then used without further purification. Tert-Dodecyl mercaptan (TDDM) as a chain transfer agent, S,S-dibenzyl trithiocarbonate (DBTC) (97%) as a RAFT agent, sodium hydrosulfite (SHS) as a reducing agent, sodium formaldehyde sulfoxylate (SFS, 86%) as a catalyst, ferrous sulfate (FES, 99%), ethylenediaminetetraacetic acid (EDTA, 99%), and diethylhydroxylamine (DEHA, 98%) as a shortstop agent, were purchased from Merck, Kenilworth, NJ, USA. As a coagulant, NaCl and H2SO4 (1 mol/L) were purchased from Daejung, Korea.
2.2. Materials for Manufacturing a Carbon Black-Filled Compound and Evaluating Crosslink Density
The ESBR and RAFT ESBR were used after coagulating latex with NaCl and H2SO4. Carbon black N330 (OCI, Korea) was used as a filler when manufacturing the carbon black-filled compound. Zinc oxide (ZnO) and stearic acid (CH3(CH2)16COOH) as additives, N-(1,3-dimethybutyl)-N’-phenyl-phenylenediamine (6PPD) as an antioxidant, sulfur, N-cyclohexyl benzothiazyl sulfenamide (CBS), and diphenyl guanidine (DPG) as vulcanization agents, were purchased from Merck, Kenilworth, NJ, USA.
Tetrahydrofuran (THF, Daejung, Korea) and n-hexane (Daejung, Busan, Korea) were used in order to remove organics from the vulcanizates prior to the swelling experiments. Toluene (Daejung, Korea) was used to confirm the crosslink density. Piperidine (Daejung, Korea), propane-2-thiol (Acros Organics, Waltham, MA, USA), and n-heptane (Samchun, Seoul, Korea) were used to destroy the vulcanizates structure.
2.3. Polymerization of ESBR
The ESBR and RAFT ESBR were polymerized by low-temperature emulsion polymerization through the following process. In a high-pressure stainless-steel stirrer reactor (2 L), styrene, water, rosin soap, fatty soap, electrolyte, sodium hydrosulfite, activate solution, and chain transfer agent were charged and purged with nitrogen gas. The TDDM was used for ESBR and DBTC was used for RAFT EBSR as chain transfer agents. TDDM, which is an irreversible chain transfer agent, can lose its activity to regulate the dispersity of a polymer after a transfer reaction, whereas DBTC, a reversible chain transfer agent, can reversibly transfer the active radical to a dormant species and make it reactive. Subsequently, the initiator was added and 1,3-butadiene (measured in a small chamber) was injected using a nitrogen pressure of 3-bar through a gas line connected to the reactor. Polymerization was carried out while maintaining the temperature of the reactor at 9 °C.
Table 1 lists a detailed polymerization recipe.
2.4. Conversion Measurement and Latex Coagulation
During the polymerization reaction, ESBR was sampled at 2 h intervals and RAFT ESBR, which had a relatively long polymerization time, was sampled at 4 h intervals. The conversion was calculated by measuring the total solids content of the latex that was sampled every interval while using a moisture dryer (MB45, OHAUS, Parsippany, NJ, USA). The reaction was terminated by injecting a shortstop agent when the conversion reached 65%.
NaCl was added up to 15% of the amount of rubber in the polymerized latex and the solution was slowly coagulated by dropping in an aqueous sulfuric acid solution. Coagulated ESBR and RAFT ESBR were washed three times with distilled water and then dried in a circulation hot air-drying oven at 55 °C for 24 h.
2.5. Characterization of ESBR and RAFT ESBR Raw Polymers
The contents of styrene, butadiene, and vinyl structure in the polymerized polymer were determined while using nuclear magnetic resonance spectrometer (1H-NMR; Varian, Unity Plus 300 spectrometer, Garden State Scientific, Morristown, NJ, USA).
The molecular weight and dispersity were determined by GPC (gel permeation chromatography, DGU 20A 3R, Shimazu, Kyoto, Japan) using a solvent delivery unit, refractive index detector, and styragel column (High Temperature [HT] 6E, 10 μm, Φ 7.8 mm × 6300 mm; High Molecular Weight [HMW] 7, 15–20 μm, Φ 7.8 mm × 300 mm; HMW 6E, 15–20 μm, Φ 7.8 mm × 300 mm). The GPC calibration curves were prepared using a polystyrene standard.
Mooney viscosity was measured using a Mooney viscometer (Vulchem IND Co., Korea), which is a type of rotatory viscometer. After preheating for 1 min. according to ASTM D 1646 conditions at 100 °C, the large disk (38.10 ± 0.05 mm, thickness 5.5 ± 0.05 mm) was measured by operating at 2 rpm for 4 min.
2.6. Manufacture of Compounds and Vulcanizates
The carbon black-filled compounds were manufactured by applying the formulation shown in
Table 2 to quantitatively analyze the crosslink structure of the manufactured vulcanizates and determine the effect of crosslink density of vulcanizates on mechanical and dynamic properties. The carbon black filler was variably added to clearly distinguish between total crosslink density and chemical crosslink density through the Kraus plot. The compound was manufactured by applying the formulation shown in
Table 3 to increase the crosslink density of carbon black-filled compound using RAFT ESBR.
The compounds were manufactured using an internal Kneader (300 cc, MIRAESI Company, Incheon, Korea), and the fill factor was set to 0.7 and proceeded in two steps. In the first stage, kneading commenced at 110 °C, and the carbon black masterbatch was kneaded for 12 min. at the dump temperature of 150–155 °C. After the first stage of kneading, sulfur and cure accelerator
N-
tert-
butyl-2-benzothiazyl sulfonamide (TBBS) were added and kneaded at 50 °C for 2 min. during the second stage.
Table 4 lists the detailed procedure.
2.7. Experimental Methods for Carbon Black-Filled Compounds
All of the experiments were repeated 3–4 times and the difference in each average value was insignificant. For this reason, error bars are not included with the graphs of the data.
2.7.1. Cure Characteristics
The cure characteristics of the manufactured carbon black-filled compounds were measured using a moving die rheometer (RLR-3; rotorless rheometer, Toyoseiki, Nagano, Japan) for 20 min. at 160 °C with an oscillation angle of ±1°. Through this experiment, it is possible to determine the minimum and maximum torque values and the optimum vulcanization time (t90). Subsequently, vulcanizates were manufactured while using a press with optimum vulcanization time at 160 °C.
2.7.2. Analysis of vulcanizate Structure (Swelling Test)
The crosslinked structure was evaluated by measuring the crosslink density. The vulcanizates were cut into 10 mm (length) × 10 mm (width) × 2 mm (thickness) sizes and then stored for 48 h in 30 mL of THF (tetrahydrofuran) and 30 mL of
n-hexane, respectively, to remove organic additives. After drying the samples for one day at room temperature to remove the organic additives, the samples were weighed, and were then swollen in toluene for 24 h and weighed again. Finally, the total crosslink density was calculated using the measured weight and the Flory–Rehner Equation (1), below.
Here, ν is the crosslink density (mol/g), MC is the average molecular weight between crosslink points (g/mol), V1 is the volume fraction of rubber in the swollen gel at equilibrium, V0 is the molar volume of solvent (cm3/mol), ρr is the density of the rubber sample (g/cm3), and χ is the polymer–solvent interaction parameter.
For further analysis of the crosslink structure, a mixture of n-heptane (50 mL), propane-2-thiol (0.4 M), and hexylamine (0.4 M) was prepared. The samples, after removing the organic additives, were stored at room temperature for 24 h in the mixture solution. Subsequently, the sample was removed and dried, and then swollen in toluene for 24 h. Di- and mono-crosslinks were measured using the difference in the sample weight before and after swelling [
42,
43].
The total crosslink density (chemical crosslink density + filler-rubber interaction) and chemical crosslink of the unfilled compound were obtained using the Flory–Rehner Equation (1) and Kraus Equation (2). The degree of filler-rubber interaction was determined by calculating the difference in the densities [
44,
45,
46,
47].
Here, φ is the volume fraction of filler, νr0 is the volume fraction of unfilled rubber in the swollen gel, and νr is the volume fraction of rubber in the swollen gel.
2.7.3. Mechanical Properties
Dumbbell-shaped specimens of 100 mm (length) × 25 mm (width) × 2 mm (thickness) specifications were prepared according to ASTM D412. The prepared specimens were measured for mechanical properties (tensile strength, elongation at break, and tensile modulus) of vulcanizates while using a universal testing machine (UTM, Model; KSU-05M-C, KSU Co., Korea).
2.7.4. Abrasion Loss
Cylindrical specimens of 16 mm diameter and 8 mm thickness were prepared according to DIN 53516 and the initial mass of the specimens were measured. Subsequently, the mass of the abraded specimen was measured to determine the abrasion loss after grinding the specimens for 40 m at 40 rpm using a Deutsche Industrie Normen (DIN) abrasion tester.
2.7.5. Dynamic Viscoelastic Properties
Specimens of 15.0 mm (length) × 5.0 mm (width) × 2.0 mm (thickness) were prepared for measuring the dynamic viscoelastic properties of vulcanizates. The glass transition temperature and dynamic viscoelastic properties of the vulcanizates were measured while using a dynamic mechanical thermal analyzer (DMTA, EPLEXOR 500N, GABO, München, Germany) in tension mode with an amplitude of 30 μm, frequency of 10 Hz, and temperature range of −80 °C to 80 °C.