Preparation of Succinoglycan Hydrogel Coordinated With Fe3+ Ions for Controlled Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isolation and Purification of Succinoglycan
2.3. Rheological Measurements
2.4. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5. Circular Dichroism (CD) Spectropolarimetry
2.6. Preparation of Fe3+-SG Hydrogel Beads
2.7. Field Emission-Scanning Electron Microscopy (FE-SEM) Analysis
2.8. Spectrophotometric Detection of the Reduction of Fe3+ to Fe2+
2.9. Congo Red Loading and Release
2.10. Cytotoxicity Study
3. Results and Discussion
3.1. Rheological Measurements
3.2. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectra Analysis
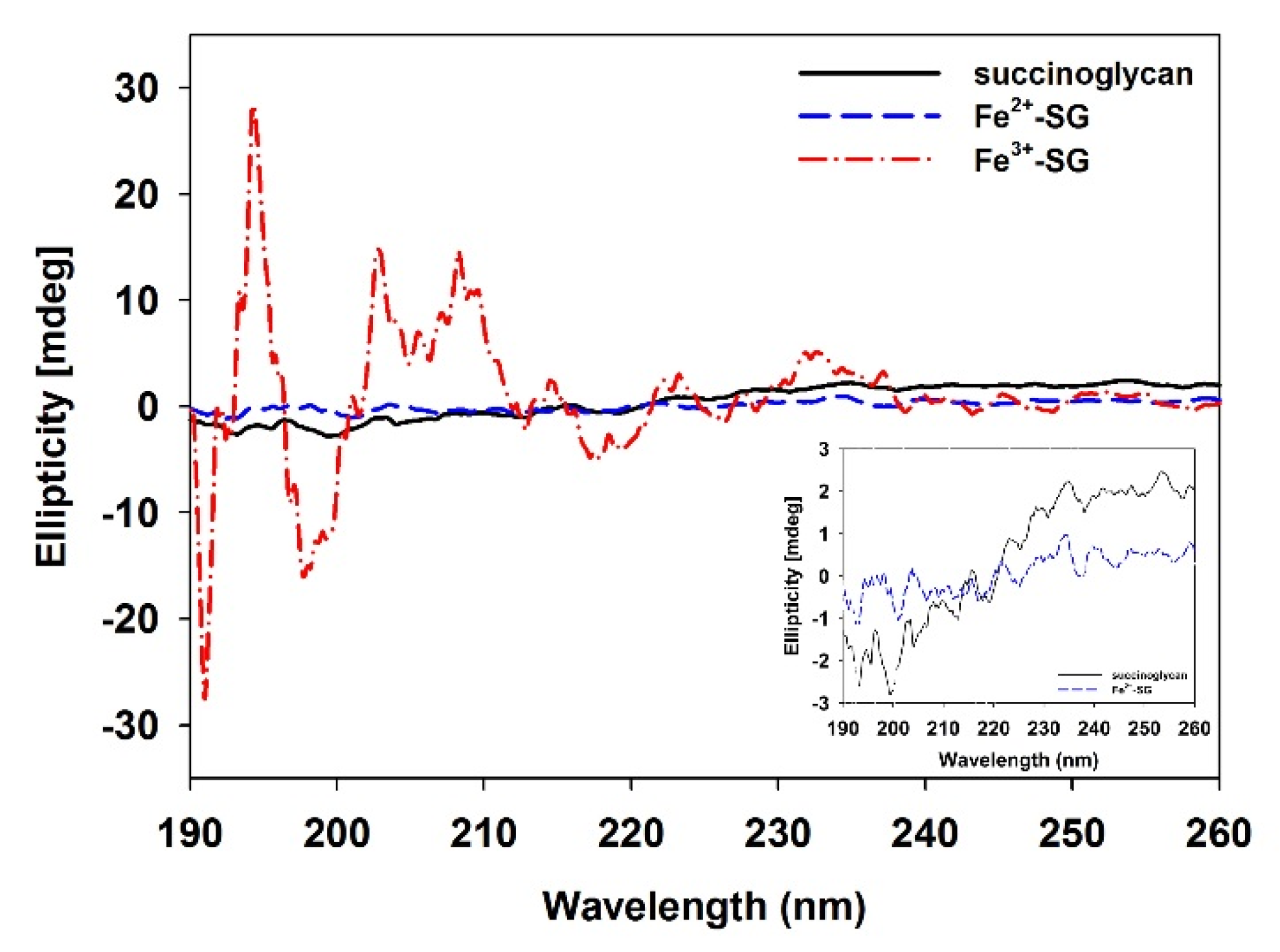
3.3. Circular Dichroism (CD) Analysis
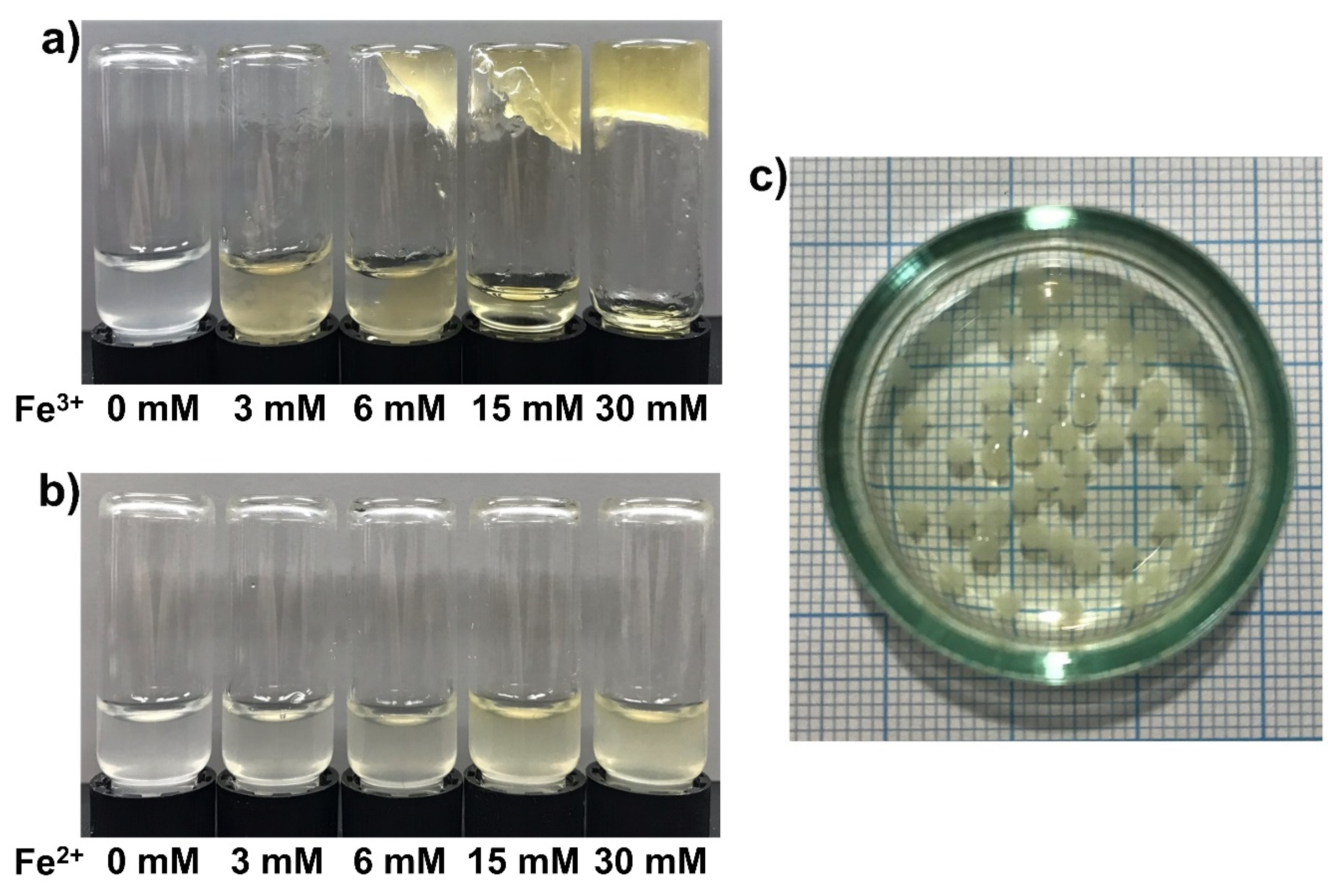
3.4. Fe3+-SG Hydrogel Bead Preparation
3.5. FE-SEM Micrograph Analysis
3.6. Redox-Responsive Fe3+-SG Hydrogel Beads
3.7. Congo Red Release
3.8. Cytotoxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, N.; Liu, C.; Chen, W. Facile Access to Guar Gum Based Supramolecular Hydrogels with Rapid Self-Healing Ability and Multistimuli Responsive Gel–Sol Transitions. J. Agric. Food Chem. 2018, 67, 746–752. [Google Scholar] [CrossRef]
- Guo, J.; Kim, Y.; Xie, V.; Smith, B.; Watson, E.; Lam, J.; Pearce, H.; Engel, P.; Mikos, A. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Luensmann, D.; van Doorn, K.; May, C.; Srinivasan, S.; Jones, L. The Impact of Cosmetics on the Physical Dimension and Optical Performance of Contemporary Silicone Hydrogel Contact Lenses. Eye Contact Lens 2019, 46, 166–173. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kawasaki, N.; Kubo, W.; Endo, K.; Attwood, D. Comparison of in situ gelling formulations for the oral delivery of cimetidine. Int. J. Pharm. 2001, 220, 161–168. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Wu, P. A multifunctional skin-like sensor based on a 3D printed thermo-responsive hydrogel. Mater. Horiz. 2017, 4, 694–700. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.M.; Moorjani, S.K.; Scranton, A.B. Methods for synthesis of hydrogel networks: A review. J. Macromol. Sci. Part C Polym. Rev. 1996, 36, 405–430. [Google Scholar] [CrossRef]
- Du, J.; Xu, S.; Feng, S.; Yu, L.; Wang, J.; Liu, Y. Tough dual nanocomposite hydrogels with inorganic hybrid crosslinking. Soft Matter 2016, 12, 1649–1654. [Google Scholar] [PubMed]
- Giammanco, G.E.; Sosnofsky, C.T.; Ostrowski, A.D. Light-responsive iron (III)–polysaccharide coordination hydrogels for controlled delivery. ACS Appl. Mater. Interfaces 2015, 7, 3068–3076. [Google Scholar] [CrossRef]
- Rendleman, J. Metal-polysaccharide complexes—Part I. Food Chem. 1978, 3, 47–79. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.; Oliveira, J.T.; Sousa, R.; Mano, J.; Reis, R. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef]
- Okajima, M.K.; Nguyen, Q.T.l.; Tateyama, S.; Masuyama, H.; Tanaka, T.; Mitsumata, T.; Kaneko, T. Photoshrinkage in polysaccharide gels with trivalent metal ions. Biomacromolecules 2012, 13, 4158–4163. [Google Scholar] [CrossRef]
- Rendleman, J. Metal-polysaccharide complexes—Part II. Food Chem. 1978, 3, 127–162. [Google Scholar] [CrossRef]
- Boutebba, A.; Milas, M.; Rinaudo, M. On the interchain associations in aqueous solutions of a succinoglycan polysaccharide. Int. J. Boil. Macromol. 1999, 24, 319–327. [Google Scholar] [CrossRef]
- Andhare, P.; Delattre, C.; Pierre, G.; Michaud, P.; Pathak, H. Characterization and rheological behaviour analysis of the succinoglycan produced by Rhizobium radiobacter strain CAS from curd sample. Food Hydrocoll. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Halder, U.; Banerjee, A.; Bandopadhyay, R. Structural and functional properties, biosynthesis, and patenting trends of Bacterial succinoglycan: A review. Indian J. Microbiol. 2017, 57, 278–284. [Google Scholar] [CrossRef]
- Chouly, C.; Colquhoun, I.J.; Jodelet, A.; York, G.; Walker, G.C. NMR studies of succinoglycan repeating-unit octasaccharides from Rhizobium meliloti and Agrobacterium radiobacter. Int. J. Boil. Macromol. 1995, 17, 357–363. [Google Scholar] [CrossRef]
- Cho, E.; Choi, J.M.; Kim, H.; Tahir, M.N.; Choi, Y.; Jung, S. Ferrous iron chelating property of low-molecular weight succinoglycans isolated from Sinorhizobium meliloti. Biometals 2013, 26, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Boil. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Che, P.; Ma, Y. More sensitive way to determine iron using an iron (II)− 1, 10-phenanthroline complex and capillary electrophoresis. J. Chromatogr. A 1996, 749, 287–294. [Google Scholar] [CrossRef]
- Lee, B.; Jeong, D.; Joo, S.W.; Choi, J.M.; Lee, J.Y.; Cho, E.; Park, S.; Jung, S. Preparation of Hydroxypropyl Cyclosophoraose/Dextran Microspheres for the Controlled Release of Ciprofloxacin. Bull. Korean Chem. Soc. 2016, 37, 1947–1954. [Google Scholar] [CrossRef]
- Bosio, V.E.; Basu, S.; Abdullha, F.; Villalba, M.E.C.; Güida, J.A.; Mukherjee, A.; Castro, G.R. Encapsulation of Congo Red in carboxymethyl guar gum–alginate gel microspheres. React. Funct. Polym. 2014, 82, 103–110. [Google Scholar] [CrossRef]
- Gan, M.; Zhang, W.; Wei, S.; Dang, H. The influence of mPEG-PCL and mPEG-PLGA on encapsulation efficiency and drug-loading of SN-38 NPs. Artif. Cells Nanomed. Biotechnol. 2017, 45, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cho, E.; Choi, J.M.; Kim, H.; Jang, A.; Choi, Y.; Yu, J.-H.; Jung, S. Intermolecular complexation of low-molecular-weight succinoglycans directs solubility enhancement of pindolol. Carbohydr. Polym. 2014, 106, 101–108. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, H.; Jeong, J.-P.; Dindulkar, S.D.; Cho, E.; Yang, Y.-H.; Jung, S. Cyclosophoraose/cellulose hydrogels as an efficient delivery system for galangin, a hydrophobic antibacterial drug. Cellulose 2016, 23, 2609–2625. [Google Scholar] [CrossRef]
- Matulová, M.; Toffanin, R.; Navarini, L.; Gilli, R.; Paoletti, S.; Cesàro, A. NMR analysis of succinoglycans from different microbial sources: Partial assignment of their 1H and 13C NMR spectra and location of the succinate and the acetate groups. Carbohydr. Res. 1994, 265, 167–179. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Läuger, J.; Stettin, H. Effects of instrument and fluid inertia in oscillatory shear in rotational rheometers. J. Rheol. 2016, 60, 393–406. [Google Scholar] [CrossRef]
- Chakraborty, I. Fe (III)-Coordinated Hydrogel and Photo-Induced Sol-Gel Process—A Case Study. Int. J. Eng. Sci. Math. 2018, 7, 1–11. [Google Scholar]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable iron (III) cross-linked alginate gels. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Zhao, D.; Shui, J.L.; Grabstanowicz, L.R.; Chen, C.; Commet, S.M.; Xu, T.; Lu, J.; Liu, D.J. Highly Efficient Non-Precious Metal Electrocatalysts Prepared from One-Pot Synthesized Zeolitic Imidazolate Frameworks. Adv. Mater. 2014, 26, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, V.S.; Wyandt, C.M. Rheology of microcrystalline cellulose and sodiumcarboxymethyl cellulose hydrogels using a controlled stress rheometer: Part II. Int. J. Pharm. 2005, 292, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Joo, S.-W.; Shinde, V.V.; Jung, S. Triple-crosslinkedβ-cyclodextrin oligomer self-healing hydrogel showing high mechanical strength, enhanced stability and pH responsiveness. Carbohydr. Polym. 2018, 198, 563–574. [Google Scholar] [CrossRef]
- Mahdi, M.; Diryak, R.; Kontogiorgos, V.; Morris, G.; Smith, A.M. In situ rheological measurements of the external gelation of alginate. Food Hydrocoll. 2016, 55, 77–80. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, W.; Cui, Z.; Zhu, H.; Wu, C. Journal of the Mechanical Behavior of Biomedical Materials. J. Mech. Behav. Biomed. Mater. 2018, 81, 106–119. [Google Scholar]
- Berthomieu, C.; Hienerwadel, R. Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Lee, S.-H.; Kyung, S.-G.; Jung, S.-H. Catalytic methanolysis induced by succinoglycan, a Rhizobial exopolysaccharide. Bull. Korean Chem. Soc. 2006, 27, 921–924. [Google Scholar]
- Moosavi-Nasab, M.; Taherian, A.R.; Bakhtiyari, M.; Farahnaky, A.; Askari, H. Structural and rheological properties of succinoglycan biogums made from low-quality date syrup or sucrose using agrobacterium radiobacter inoculation. Food Bioprocess Technol. 2012, 5, 638–647. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G. The influence of xanthan on the crystallization of calcium carbonate. J. Cryst. Growth 2011, 314, 231–238. [Google Scholar] [CrossRef]
- Su, T.; Qi, X.; Zuo, G.; Pan, X.; Zhang, J.; Han, Z.; Dong, W. Polysaccharide metallohydrogel obtained from Salecan and trivalent chromium: Synthesis and characterization. Carbohydr. Polym. 2018, 181, 285–291. [Google Scholar] [CrossRef]
- Wang, B.; Liao, L.; Huang, Q.; Cheng, Y. Adsorption behaviors of benzonic acid by carboxyl methyl konjac glucomannan gel micropheres cross-linked with Fe3+. J. Chem. Eng. Data 2012, 57, 72–77. [Google Scholar] [CrossRef]
- Swamy, B.Y.; Yun, Y.-S. In vitro release of metformin from iron (III) cross-linked alginate–carboxymethyl cellulose hydrogel beads. Int. J. Boil. Macromol. 2015, 77, 114–119. [Google Scholar] [CrossRef]
- Singh, T.; Trivedi, T.J.; Kumar, A. Dissolution, regeneration and ion-gel formation of agarose in room-temperature ionic liquids. Green Chem. 2010, 12, 1029–1035. [Google Scholar] [CrossRef]
- Dentini, M.; Crescenzi, V.; Fidanza, M.; Coviello, T. The aggregation and conformational states in aqueous solution of a succinoglycan polysaccharide. Macromolecules 1989, 22, 954–959. [Google Scholar] [CrossRef]
- Bejenariu, A.; Popa, M.; Picton, L.; Le Cerf, D. Effect of concentration, pH and temperature on xanthan conformation: A preliminary study before crosslinking. Rev. Roum. Chim. 2010, 55, 147–152. [Google Scholar]
- Sreeram, K.J.; Shrivastava, H.Y.; Nair, B.U. Studies on the nature of interaction of iron (III) with alginates. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004, 1670, 121–125. [Google Scholar] [CrossRef]
- Park, J.; Chakrabarti, B. Optical characteristics of carboxyl group in relation to the circular dichroic properties and dissociation constants of glycosaminoglycans. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1978, 544, 667–675. [Google Scholar] [CrossRef]
- Fidanza, M.; Dentini, M.; Crescenzi, V.; Del Vecchio, P. Influence of charged groups on the conformational stability of succinoglycan in dilute aqueous solution. Int. J. Boil. Macromol. 1989, 11, 372–376. [Google Scholar] [CrossRef]
- Bueno, V.B.; Petri, D.F.S. Xanthan hydrogel films: Molecular conformation, charge density and protein carriers. Carbohydr. Polym. 2014, 101, 897–904. [Google Scholar] [CrossRef]
- Betigeri, S.S.; Neau, S.H. Immobilization of lipase using hydrophilic polymers in the form of hydrogel beads. Biomaterials 2002, 23, 3627–3636. [Google Scholar] [CrossRef]
- Joung, Y.K.; You, S.S.; Park, K.M.; Go, D.H.; Park, K.D. In situ forming, metal-adhesive heparin hydrogel surfaces for blood-compatible coating. Colloids Surf. B Biointerfaces 2012, 99, 102–107. [Google Scholar] [CrossRef]
- Sui, X.; Feng, X.; Hempenius, M.A.; Vancso, G.J. Redox active gels: Synthesis, structures and applications. J. Mater. Chem. B 2013, 1, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.E.; Carrion, B.; Coleman, R.M.; Ostrowski, A.D. Photoresponsive polysaccharide-based hydrogels with tunable mechanical properties for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 14423–14429. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ohta, S.; Sugahara, A.; Okubo, M.; Yamada, A.; Ito, T. In vivo redox-responsive sol–gel/gel–sol transition of star block copolymer solution based on ionic cross-linking. Macromolecules 2017, 50, 5539–5548. [Google Scholar] [CrossRef]
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, N.; Kim, M.-G. Simultaneous determination of L-ascorbic acid and dehydroascorbic acid in human plasma. Anal. Methods 2015, 7, 9206–9210. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Corbella, L.; Senger, B.; Boulmedais, F.; Hernández, R. Photoresponsive Nanometer-Scale Iron Alginate Hydrogels: A Study of Gel–Sol Transition Using a Quartz Crystal Microbalance. Langmuir 2019, 35, 11397–11405. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Bosio, V.E.; López, A.G.; Mukherjee, A.; Mechetti, M.; Castro, G.R. Tailoring doxorubicin sustainable release from biopolymeric smart matrix using congo red as molecular helper. J. Mater. Chem. B 2014, 2, 5178–5186. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Jeong, D.; Kim, Y.; Kim, S.; Jung, S. Preparation of Succinoglycan Hydrogel Coordinated With Fe3+ Ions for Controlled Drug Delivery. Polymers 2020, 12, 977. https://doi.org/10.3390/polym12040977
Hu Y, Jeong D, Kim Y, Kim S, Jung S. Preparation of Succinoglycan Hydrogel Coordinated With Fe3+ Ions for Controlled Drug Delivery. Polymers. 2020; 12(4):977. https://doi.org/10.3390/polym12040977
Chicago/Turabian StyleHu, Yiluo, Daham Jeong, Yohan Kim, Seonmok Kim, and Seunho Jung. 2020. "Preparation of Succinoglycan Hydrogel Coordinated With Fe3+ Ions for Controlled Drug Delivery" Polymers 12, no. 4: 977. https://doi.org/10.3390/polym12040977
APA StyleHu, Y., Jeong, D., Kim, Y., Kim, S., & Jung, S. (2020). Preparation of Succinoglycan Hydrogel Coordinated With Fe3+ Ions for Controlled Drug Delivery. Polymers, 12(4), 977. https://doi.org/10.3390/polym12040977





