Marine-Derived Polymeric Materials and Biomimetics: An Overview
Abstract
:1. Introduction
2. The Ocean as a Source of Biotechnological Tools
2.1. Marine Biomacromolecules
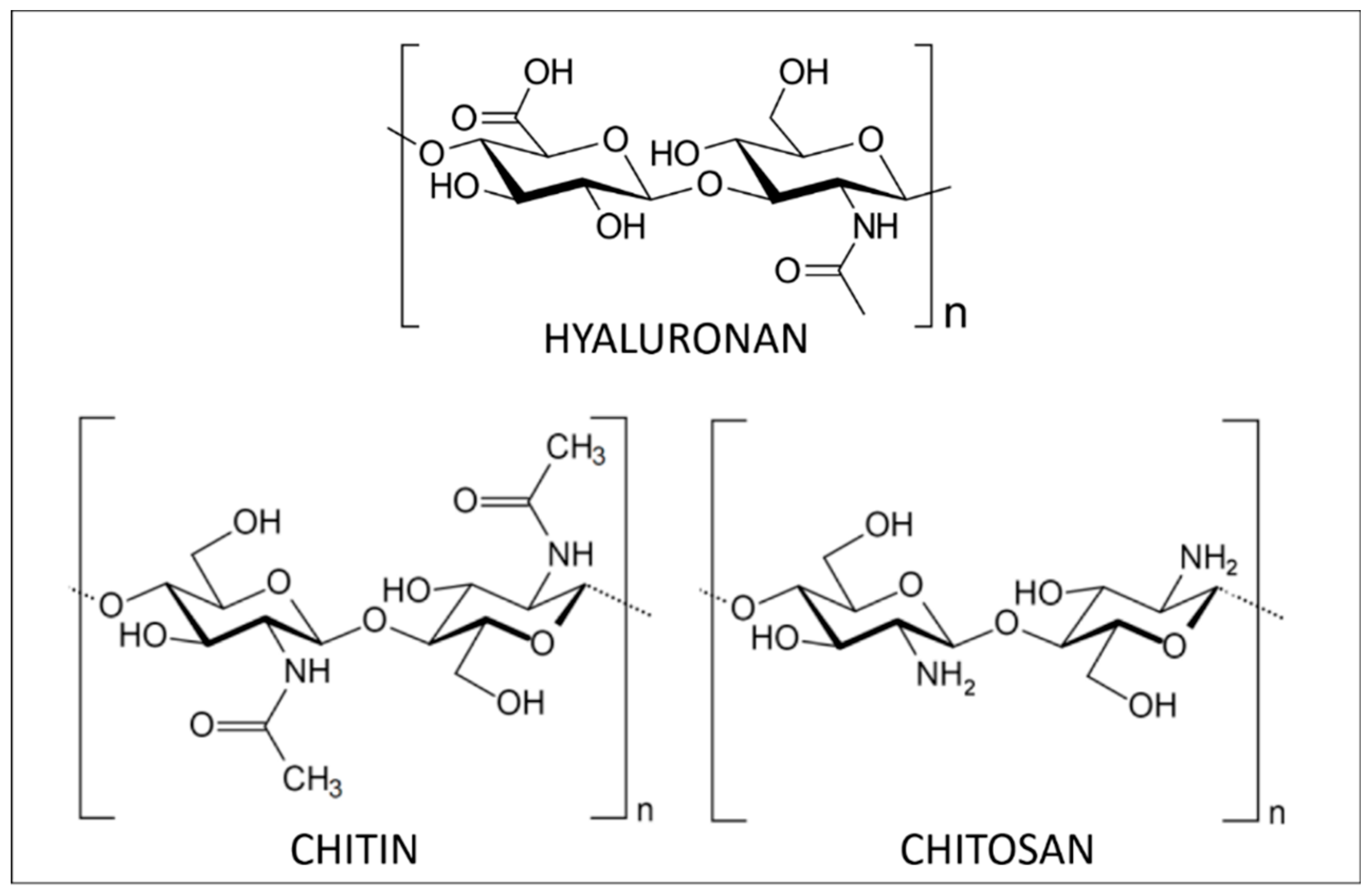
2.1.1. Polysaccharides from Marine Animals
2.1.2. Marine Proteins
2.1.3. Biopolymers from Macroalgae
2.2. Marine Secondary Metabolites
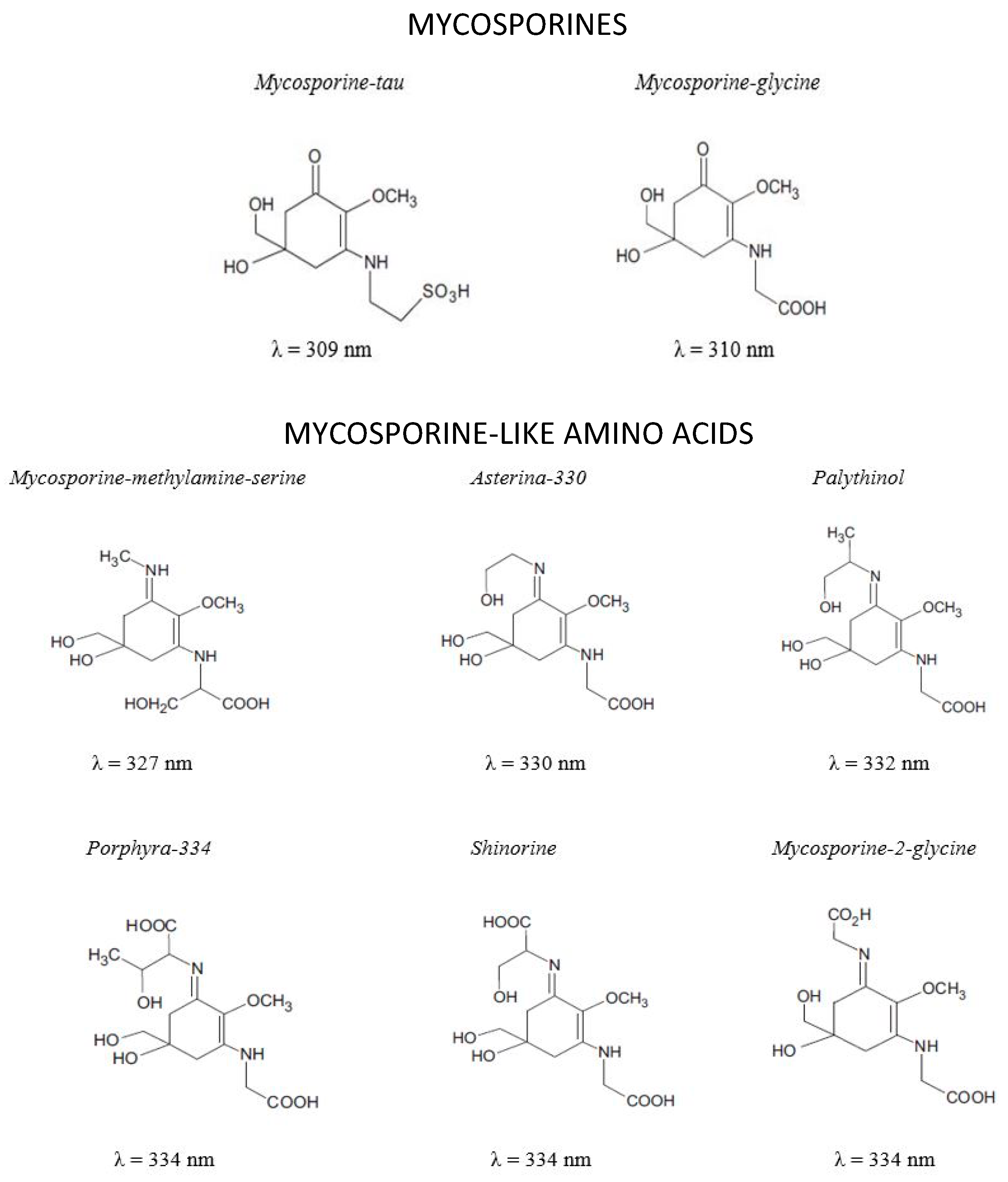
Mycosporines and Mycosporine-Like Amino Acids
2.3. Marine Molecules as Tools for Biocomposite Materials and Their Applications
2.3.1. Marine-Based Biocomposites for Food Packaging
2.3.2. Marine Nanofillers for Structural Applications
2.3.3. Marine-Based Biocomposites for Water Treatment
2.3.4. Marine Biomaterials in Biomedical Applications
3. The Ocean as a Source of Bio-Inspired Materials
3.1. Mimicking the Functionalization of Reef Fish Mucus to Fight UVR-Induced Damages-Protection
3.2. Adhesive Surfaces and Materials Inspired from Marine Organisms
3.2.1. Marine Mussel Adhesion
3.2.2. Sandcastle Worms
3.3. Structural Colouration in the Marine Environment
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Johnson, K.; Dalton, G.; Masters, I. Building Industries at Sea: “Blue Growth” and the New Maritime Economy; River Publishers: Gistrup, Denmark, 2018; pp. 39–70. [Google Scholar]
- Trincone, A. Enzymatic Processes in Marine Biotechnology. Mar. Drugs 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, J.M.; Arnaud-Haond, S.; Duarte, C.M. What lies underneath: Conserving the oceans’ genetic resources. Proc. Natl. Acad. Sci. USA 2010, 107, 18318–18324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [Green Version]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine organisms as a source of natural matrix for bone tissue engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Kim, S.-K. (Ed.) Marine Proteins and Peptides: Biological Activities and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 978-1-118-37508-2. [Google Scholar]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Nakano, K.; Sim, J.S. A Simple Rapid Method to Estimate Hyaluronic Acid Concentrations in Rooster Comb and Wattle Using Cellulose Acetate Electrophoresis. J. Agric. Food Chem. 1994, 42, 2766–2768. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Schiller, J.; Mendichi, R. Hyaluronic Acid: Its Function and Degradation in in vivo Systems. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 34, pp. 789–882. [Google Scholar]
- Cardoso, M.; Costa, R.; Mano, J. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giji, S.; Arumugam, M. Isolation and Characterization of Hyaluronic Acid from Marine Organisms. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 61–77. ISBN 978-0-12-800269-8. [Google Scholar]
- Sadhasivam, G.; Muthuvel, A.; Pachaiyappan, A.; Thangavel, B. Isolation and characterization of hyaluronic acid from the liver of marine stingray Aetobatus narinari. Int. J. Biol. Macromol. 2013, 54, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; Rodríguez-Amado, I.; Montemayor, M.; Fraguas, J.; González, M.; Murado, M. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passi, A.; Vigetti, D. Hyaluronan as tunable drug delivery system. Adv. Drug Deliv. Rev. 2019, 146, 83–96. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Huh, H.W.; Zhao, L.; Kim, S.Y. Biomineralized biomimetic organic/inorganic hybrid hydrogels based on hyaluronic acid and poloxamer. Carbohydr. Polym. 2015, 126, 130–140. [Google Scholar] [CrossRef]
- Chantre, C.O.; Gonzalez, G.M.; Ahn, S.; Cera, L.; Campbell, P.H.; Hoerstrup, S.P.; Parker, K.K. Porous Biomimetic Hyaluronic Acid and Extracellular Matrix Protein Nanofiber Scaffolds for Accelerated Cutaneous Tissue Repair. ACS Appl. Mater. Interfaces 2019, 11, 45498–45510. [Google Scholar] [CrossRef]
- Nakod, P.S.; Kim, Y.; Rao, S.S. Three-dimensional biomimetic hyaluronic acid hydrogels to investigate glioblastoma stem cell behaviors. Biotechnol. Bioeng. 2019. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.; Jang, M.; Lim, A.; Hardy, J.G.; Park, H.S.; Lee, J.Y. Versatile biomimetic conductive polypyrrole films doped with hyaluronic acid of different molecular weights. Acta Biomater. 2018, 80, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Lee, E.A.; Yoon, J.J.; Park, T.G. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 2005, 26, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F. Chitin Chemistry; Macmillan International Higher Education: Montgomery, AL, USA, 1992; ISBN 978-1-349-11545-7. [Google Scholar]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.G.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. What Is the Real Value of Chitosan’s Surface Energy? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Oliveira, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Desbriéres, J. Novel transparent nanocomposite films based on chitosan and bacterial cellulose. Green Chem. 2009, 11, 2023. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Desbriéres, J.; Blanc, S.; Ferreira, R.A.S.; Carlos, L.D. A study of the distribution of chitosan onto and within a paper sheet using a fluorescent chitosan derivative. Carbohydr. Polym. 2009, 78, 760–766. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Desbrières, J.; Gandini, A.; Neto, C.P. Production of Coated Papers with Improved Properties by Using a Water-Soluble Chitosan Derivative. Ind. Eng. Chem. Res. 2010, 49, 6432–6438. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Microwave assisted synthesis of poly (N-vinylimidazole) grafted chitosan as an effective adsorbent for mercury (II) removal from aqueous solution: Equilibrium, kinetic, thermodynamics and regeneration studies. J. Dispers. Sci. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Pascoal Neto, C.; Gandini, A. Novel materials based on chitosan and cellulose. Polym. Int. 2011, 60, 875–882. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog. Org. Coat. 2012, 75, 8–13. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Fernandes, S.C.M.; Freire, C.S.R.; Sadocco, P.; Causio, J.; Neto, C.P.; Trindade, T. Antibacterial activity of optically transparent nanocomposite films based on chitosan or its derivatives and silver nanoparticles. Carbohydr. Res. 2012, 348, 77–83. [Google Scholar] [CrossRef]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Properties and energetics for design and characterization of chitosan nanoparticles used for drug encapsulation. RSC Adv. 2014, 4, 12653. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review: Chitosan adsorbents for dye removal. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Kyzas, G.; Bikiaris, D. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D. Formulation and In-Vitro Characterization of Chitosan-Nanoparticles Loaded with the Iron Chelator Deferoxamine Mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michailidou, G.; Christodoulou, E.; Nanaki, S.; Barmpalexis, P.; Karavas, E.; Vergkizi-Nikolakaki, S.; Bikiaris, D.N. Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol. Carbohydr. Polym. 2019, 208, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, M.C.; Koliakou, I.G.; Kaloyianni, M.G.; Terzopoulou, Z.N.; Siska, E.K.; Karakassides, M.A.; Boccaccini, A.R.; Bikiaris, D.N. New N-(2-carboxybenzyl) chitosan composite scaffolds containing nanoTiO 2 or bioactive glass with enhanced cell proliferation for bone-tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 65, 140–148. [Google Scholar] [CrossRef]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.; Kostoglou, M.; Bikiaris, D.; Lambropoulou, D. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef] [Green Version]
- Labidi, A.; Salaberria, A.; Fernandes, S.; Labidi, J.; Abderrabba, M. Functional Chitosan Derivative and Chitin as Decolorization Materials for Methylene Blue and Methyl Orange from Aqueous Solution. Materials 2019, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Choi, G.; Kim, J.; Jang, J.; Choi, B.; Kim, J.; Jeong, S.; Leem, S.; Kwon, H.; Hwang, H.; et al. Biomimetic Chitin–Silk Hybrids: An Optically Transparent Structural Platform for Wearable Devices and Advanced Electronics. Adv. Funct. Mater. 2018, 28, 1705480. [Google Scholar] [CrossRef]
- Ehrlich, H. Extreme Biomimetics; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-45340-8. [Google Scholar]
- Wysokowski, M.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Szalaty, T.J.; Bazhenov, V.V.; Stelling, A.L.; Beyer, J.; Heitmann, J.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing: Collagen-Based Biomaterials. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Development of Biomimetic Tilapia Collagen Nanofibers for Skin Regeneration through Inducing Keratinocytes Differentiation and Collagen Synthesis of Dermal Fibroblasts. ACS Appl. Mater. Interfaces 2015, 7, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Venkatesan, J.; Bhatnagar, I.; Shim, Y.-B.; Kim, S.-K. Application of Marine Collagen–Based Scaffolds in Bone Tissue Engineering. In Marine Biomaterials; CRC Press: Boca Raton, FL, USA, 2013; pp. 519–528. ISBN 978-1-4665-0564-3. [Google Scholar]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Kim, S.-K.; Toldrá, F. (Eds.) Marine Enzymes Biotechnology: Production and Industrial Applications, Part I–Production of Enzymes, 1st ed.; Marine Enzymes Biotechnology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-803847-5. [Google Scholar]
- Debashish, G.; Malay, S.; Barindra, S.; Joydeep, M. Marine Enzymes. In Marine Biotechnology I; Ulber, R., Le Gal, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 96, pp. 189–218. ISBN 978-3-540-25659-5. [Google Scholar]
- Rao, T.E.; Imchen, M.; Kumavath, R. Marine Enzymes. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 149–163. ISBN 978-0-12-809587-4. [Google Scholar]
- Vingiani, G.M.; De Luca, P.; Ianora, A.; Dobson, A.D.W.; Lauritano, C. Microalgal Enzymes with Biotechnological Applications. Mar. Drugs 2019, 17, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doron, L.; Segal, N.; Shapira, M. Transgene Expression in Microalgae—From Tools to Applications. Front. Plant Sci. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Brasil, B.D.S.A.F.; de Siqueira, F.G.; Salum, T.F.C.; Zanette, C.M.; Spier, M.R. Microalgae and cyanobacteria as enzyme biofactories. Algal Res. 2017, 25, 76–89. [Google Scholar] [CrossRef]
- Blamey, J.M.; Fischer, F.; Meyer, H.-P.; Sarmiento, F.; Zinn, M. Enzymatic Biocatalysis in Chemical Transformations. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 347–403. ISBN 978-0-12-803725-6. [Google Scholar]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic Recovery of Metabolites from Seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 279–320. ISBN 978-0-12-408062-1. [Google Scholar]
- Ihua, M.; Guihéneuf, F.; Mohammed, H.; Margassery, L.; Jackson, S.; Stengel, D.; Clarke, D.; Dobson, A. Microbial Population Changes in Decaying Ascophyllum nodosum Result in Macroalgal-Polysaccharide-Degrading Bacteria with Potential Applicability in Enzyme-Assisted Extraction Technologies. Mar. Drugs 2019, 17, 200. [Google Scholar] [CrossRef] [Green Version]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei): Enzyme assisted extraction of chitin from shrimp shells (L. vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Shimanaka, K.; Ikai, K.; Kato, I.; Sakai, T.; Ishizuka, K. Structures of Oligosaccharides Derived from Cladosiphon okamuranus Fucoidan by Digestion with Marine Bacterial Enzymes. Mar. Biotechnol. 2003, 5, 536–544. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kusaykin, M.I.; Zakharenko, A.M.; Menshova, R.V.; Khanh, H.H.N.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B Enzym. 2014, 102, 154–160. [Google Scholar] [CrossRef]
- Cao, H.; Mikkelsen, M.; Lezyk, M.; Bui, L.; Tran, V.; Silchenko, A.; Kusaykin, M.; Pham, T.; Truong, B.; Holck, J.; et al. Novel Enzyme Actions for Sulphated Galactofucan Depolymerisation and a New Engineering Strategy for Molecular Stabilisation of Fucoidan Degrading Enzymes. Mar. Drugs 2018, 16, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Ning, L.; Jiang, Y.; Ge, L. Biochemical Characterization and Degradation Pattern of a Novel Endo-Type Bifunctional Alginate Lyase AlyA from Marine Bacterium Isoptericola halotolerans. Mar. Drugs 2018, 16, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, X.; Bi, X.; Ren, Y.; Han, Q.; Zhou, Y.; Han, Y.; Yao, R.; Li, S. Characterization of an Alkaline Alginate Lyase with pH-Stable and Thermo-Tolerance Property. Mar. Drugs 2019, 17, 308. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, X.; Liu, D.; Wu, R.; Wu, C.; Zhang, J.; Huang, J.; Liao, B.; He, H. Optimization of Collagenase Production by Pseudoalteromonas sp. SJN2 and Application of Collagenases in the Preparation of Antioxidative Hydrolysates. Mar. Drugs 2017, 15, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from Seaweeds; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hong, I.K.; Jeon, H.; Lee, S.B. Comparison of red, brown and green seaweeds on enzymatic saccharification process. J. Ind. Eng. Chem. 2014, 20, 2687–2691. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K. Introduction to Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–9. [Google Scholar]
- Popper, Z.A.; Tuohy, M.G. Beyond the Green: Understanding the Evolutionary Puzzle of Plant and Algal Cell Walls. Plant Physiol. 2010, 153, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.-C.; Hofbauer, L.C. Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on Biological Activities and Molecular Characteristics of Sulfated Polysaccharides from Marine Green Algae in Recent Years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between Sulfate Groups and Biological Activities of Fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2015, 4, 6162. [Google Scholar] [CrossRef]
- Lin, C.-C. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. RSC Adv. 2015, 5, 39844–39853. [Google Scholar] [CrossRef] [Green Version]
- Denny, M.W.; King, F.A. The extraordinary joint material of an articulated coralline alga. I. Mechanical characterization of a key adaptation. J. Exp. Biol. 2016, 219, 1833–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denny, M.W.; King, F.A. The extraordinary joint material of an articulated coralline alga. II. Modeling the structural basis of its mechanical properties. J. Exp. Biol. 2016, 219, 1843–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci. Rep. 2019, 9, 11869. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Nurul Fazita, M.R.; Hafiidz, J.M.; Banerjee, A.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, K.; Sharma, A.; Garg, A.; Kumar, S.; Purohit, A. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Phcog Rev 2011, 5, 138. [Google Scholar] [CrossRef] [Green Version]
- Bandaranayake, W. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Nishijima, M.; Adachi, K.; Sano, H. Isolation and Structure of a UV Absorbing Substance from the Marine Bacterium Micrococcus sp. AK-334; Marine Biotechnology Institute: Tokyo, Japan, 1992; pp. 88–94. [Google Scholar]
- Arpin, N.; Bouillant, M. Light and Mycosporines; Academic Press: London, UK, 1981. [Google Scholar]
- Trione, E.J.; Leach, C.M.; Mutch, J.T. Sporogenic Substances Isolated from Fungi. Nature 1966, 212, 163–164. [Google Scholar] [CrossRef]
- Leach, C.M. Ultraviolet-absorbing substances associated with light-induced sporulation in fungi. Can. J. Bot. 1965, 43, 185–200. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV-Absorbing, Mycosporine-Like Compounds among Cyanobacterial Isolates and an Estimate of Their Screening Capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, U.; Garcia-Pichel, F. Carotenoids and Mycosporine-like Amino Acid Compounds in Members of the Genus Microcoleus (Cyanobacteria): A Chemosystematic Study. Syst. Appl. Microbiol. 1996, 19, 285–294. [Google Scholar] [CrossRef]
- Vernet, M.; Whitehead, K. Release of ultraviolet-absorbing compounds by the red-tide dinoflagellateLingulodinium polyedra. Mar. Biol. 1996, 127, 35–44. [Google Scholar] [CrossRef]
- Shashar, N.; Banaszak, A.; Lesser, M.; Amrami, D. Coral Endolithic Algae: Life in a Protected Environment. Pac. Sci. 1997, 51, 167–173. [Google Scholar]
- Carreto, J.I.; Carignan, M.O.; Daleo, G.; Marco, S.G.D. Occurrence of mycosporine-like amino acids in the red-tide dinoflagellate Alexandrium excavatum: UV-photoprotective compounds? J. Plankton Res. 1990, 12, 909–921. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Shick, J.M. Review-ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biochemical and environmental perspective. J. Phycol. 1998, 34, 418–430. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. UV Photoprotectants From Algae—Synthesis and Bio-Functionalities. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. ISBN 978-0-444-63784-0. [Google Scholar]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Wu Won, J.J.; Chalker, B.E.; Rideout, J.A. Two New UV-Absorbing Compounds from Stylophora pistillata: Sulfate Esters of Mycosporine-like Amino Acids. Tetrahedron Lett. 1997, 38, 2525–2526. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, Y.; Qian, Y.; Wang, J.; Zhu, S. Long-Acting and Safe Sunscreens with Ultrahigh Sun Protection Factor via Natural Lignin Encapsulation and Synergy. ACS Appl. Bio Mater. 2018, 1, 1276–1285. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen Products as Emerging Pollutants to Coastal Waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Derm. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Pandika, M. Looking to Nature for New Sunscreens. ACS Cent. Sci. 2018, 4, 788–790. [Google Scholar] [CrossRef] [Green Version]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. ELife 2015, 4, e05919. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, K.; Long, P.F.; Young, A.R. Compositions Et Procédés Utilisant De La Palythine. WO2017013441A1, 26 January 2017. [Google Scholar]
- Richa, R.R.; Kumari, S.; Singh, K.L.; Kannaujiya, V.K.; Singh, G.; Kesheri, M.; Sinha, R.P. Biotechnological Potential of Mycosporine-like Amino Acids and Phycobiliproteins of Cyanobacterial Origin. Biotechnol. Bioinf. Bioeng. 2011, 1, 159–171. [Google Scholar]
- Kim, S.-K. Marine Microbiology: Bioactive Compounds and Biotechnological Applications; Wiley-VCH, Verlag GmbH & Co., KGaA: Weinheim, Germany, 2013; ISBN 978-3-527-33327-1. [Google Scholar]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-K.; Venkatesan, J. Introduction to Marine Biomaterials. In Marine Biomaterials; CRC Press: Boca Raton, FL, USA, 2013; pp. 3–16. ISBN 978-1-4665-0564-3. [Google Scholar]
- Dongre, R.S. Marine Polysaccharides in Medicine. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-2859-5. [Google Scholar]
- Kim, S.-K.; Venkatesan, J. Introduction to Marine Biotechnology. In Hb25_Springer Handbook of Marine Biotechnology., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–10. ISBN 978-3-642-53970-1. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Sirviö, J.A.; Kolehmainen, A.; Liimatainen, H.; Niinimäki, J.; Hormi, O.E.O. Biocomposite cellulose-alginate films: Promising packaging materials. Food Chem. 2014, 151, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Coimbra, M.A.; Ferreira, P. Tailoring Functional Chitosan-Based Composites for Food Applications. Chem. Rec. 2018, 18, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Silva, M.A.; Rodrigues, A.; Amado, O.; Coimbra, J.; Silva, D.; et al. Chitosan-genipin film, a sustainable methodology for wine preservation. Green Chem. 2016, 18, 5331–5341. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sain, M.M. Carbon storage potential in natural fiber composites. Resour. Conserv. Recycl. 2003, 39, 325–340. [Google Scholar] [CrossRef]
- Davies, P.; Morvan, C.; Sire, O.; Baley, C. Structure and properties of fibres from sea-grass (Zostera marina). J. Mater. Sci. 2007, 42, 4850–4857. [Google Scholar] [CrossRef] [Green Version]
- Vellanoweth, R.L.; Lambright, M.R.; Erlandson, J.M.; Rick, T.C. Early New World maritime technologies: Sea grass cordage, shell beads, and a bone tool from Cave of the Chimneys, San Miguel Island, California, USA. J. Archaeol. Sci. 2003, 30, 1161–1173. [Google Scholar] [CrossRef]
- Lacoste, C.; El Hage, R.; Bergeret, A.; Corn, S.; Lacroix, P. Sodium alginate adhesives as binders in wood fibers/textile waste fibers biocomposites for building insulation. Carbohydr. Polym. 2018, 184, 1–8. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing valuable renewable nanocomposite films based exclusively on oceanic biomass—Chitin nanofillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Fernandes, S.C.M.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Paillet, M.; Dufresne, A. Chitin Whisker Reinforced Thermoplastic Nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Salaberria, A.M.; H Diaz, R.; Andrés, M.; Fernandes, S.; Labidi, J. The Antifungal Activity of Functionalized Chitin Nanocrystals in Poly (Lactid Acid) Films. Materials 2017, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Salaberría, A.M.; Teruel-Juanes, R.; Badia, J.D.; Fernandes, S.C.M.; Sáenz de Juano-Arbona, V.; Labidi, J.; Ribes-Greus, A. Influence of chitin nanocrystals on the dielectric behaviour and conductivity of chitosan-based bionanocomposites. Compos. Sci. Technol. 2018, 167, 323–330. [Google Scholar] [CrossRef]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooresmaeil, M.; Namazi, H. Application of polysaccharide-based hydrogels for water treatments. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 411–455. ISBN 978-0-12-816421-1. [Google Scholar]
- Shao, Z.; Huang, X.; Yang, F.; Zhao, W.; Zhou, X.; Zhao, C. Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydr. Polym. 2018, 187, 85–93. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu( ii ), Zn( ii ), and Pb( ii ) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef] [Green Version]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Periolatto, M.; Ferrero, F. Cotton Filter Fabrics Functionalization by Chitosan UV grafting for Removal of Dyes; The Italian Association of Chemical Engineering, Ed.; Associazione Italiana di Ingegneria Chimica: Florence, Italy, 2013; ISBN 978-88-95608-23-5. [Google Scholar]
- Pisitsak, P.; Phamonpon, W.; Soontornchatchavet, P.; Sittinun, A.; Ummartyotin, S.; Buajarern, S.; Inprasit, T. The use of water hyacinth fibers to develop chitosan-based biocomposites with improved Cu2+ removal efficiency. Compos. Commun. 2019, 16, 1–4. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.M.; Cooper, P.R.; Lawson, M.; Triffitt, J.T.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, D.P.; de Torre-Minguela, C.; Gomez, A.I.; Águas, A.P.; Barbosa, M.A.; Pelegrín, P.; Barbosa, J.N. 3D chitosan scaffolds impair NLRP3 inflammasome response in macrophages. Acta Biomater. 2019, 91, 123–134. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Crosslinking of hybrid scaffolds produced from collagen and chitosan. Int. J. Biol. Macromol. 2019, 139, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73–85. [Google Scholar] [CrossRef]
- Doench, I.; Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N.; et al. Cellulose Nanofiber-Reinforced Chitosan Hydrogel Composites for Intervertebral Disc Tissue Repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.J.; Choi, S.M.; Kang, H.Y.; Min, H.-J.; Lee, R.; Ikram, M.; Subhan, F.; Jin, S.W.; Jeong, Y.H.; Kwak, J.-Y.; et al. Bioactive fish collagen/polycaprolactone composite nanofibrous scaffolds fabricated by electrospinning for 3D cell culture. J. Biotechnol. 2015, 205, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C 2016, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.L.B.M.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: Wound model studies. J. Biomed. Mater. Res. 2003, 66A, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sikareepaisan, P.; Ruktanonchai, U.; Supaphol, P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr. Polym. 2011, 83, 1457–1469. [Google Scholar] [CrossRef]
- Ogawa, Y.; Azuma, K.; Izawa, H.; Morimoto, M.; Ochi, K.; Osaki, T.; Ito, N.; Okamoto, Y.; Saimoto, H.; Ifuku, S. Preparation and biocompatibility of a chitin nanofiber/gelatin composite film. Int. J. Biol. Macromol. 2017, 104, 1882–1889. [Google Scholar] [CrossRef]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- D’Ayala, G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [Green Version]
- Jameela, S.R.; Jayakrishnan, A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery vehicle: Studies on the in tit..o release of mitoxantrone and in viva degradation of microspheres in rat muscle. Biomaterials 1995, 16, 769–775. [Google Scholar] [CrossRef]
- Chandy, T.; Mooradian, D.L.; Rao, G.H.R. Chitosan/polyethylene glycol-alginate microcapsules for oral delivery of hirudin. J. Appl. Polym. Sci. 1998, 70, 2143–2153. [Google Scholar] [CrossRef]
- Malesu, V.K.; Sahoo, D.; Nayak, P.L. Chitosan–Sodium Alginate Nanocomposites Blended with Cloisite 30B As a Novel Drug Delivery System for Anticancer Drug Curcumin. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 402–411. [Google Scholar]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.G.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef] [Green Version]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection: Emergence of non-conventional absorbers. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In Vivo Skin Penetration of Quantum Dot Nanoparticles in the Murine Model: The Effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef] [Green Version]
- Nohynek, G.J.; Dufour, E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health? Arch. Toxicol. 2012, 86, 1063–1075. [Google Scholar] [CrossRef]
- Klisch, M.; Richter, P.; Puchta, R.; Häder, D.-P.; Bauer, W. The Stereostructure of Porphyra-334: An Experimental and Calculational NMR Investigation. Evidence for an Efficient ‘Proton Sponge’. Helv. Chim. Acta 2007, 90, 488–511. [Google Scholar] [CrossRef]
- Klisch, M.; Häder, D.-P. Mycosporine-Like Amino Acids and Marine Toxins—The Common and the Different. Mar. Drugs 2008, 6, 147–163. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Williams, D.M.; Chalker, B.E.; Banaszak, A.T. Biochemical photoadaptation in vision: UV-absorbing pigments in fish eye tissues. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1989, 93, 601–607. [Google Scholar] [CrossRef]
- Zamzow, J.P.; Losey, G.S. Ultraviolet Radiation Absorbance by Coral Reef Fish Mucus: Photo-protection and Visual Communication. Environ. Biol. Fishes 2002, 63, 41–47. [Google Scholar] [CrossRef]
- Böhm, G.A.; Pfleiderer, W.; Böger, P.; Scherer, S. Structure of a Novel Oligosaccharide-Mycosporine-Amino Acid Ultraviolet A/B Sunscreen Pigment from the Terrestrial Cyanobacterium Nostoc commune. J. Biol. Chem. 1995, 270, 8536–8539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, N.; Zeng, H.; Wu, J. Marine mussel adhesion: Biochemistry, mechanisms, and biomimetics. J. Adhes. Sci. Technol. 2013, 27, 2139–2162. [Google Scholar] [CrossRef]
- Flammang, P.; Santos, R. Biological adhesives: From biology to biomimetics. Interface Focus 2015, 5, 20140086. [Google Scholar] [CrossRef] [Green Version]
- Autumn, K. Mechanisms of Adhesion in Geckos. Integr. Comp. Biol. 2002, 42, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Gorb, S.N. Biological attachment devices: Exploring nature’s diversity for biomimetics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 1557–1574. [Google Scholar] [CrossRef]
- Persson, B.N.J. Biological adhesion for locomotion: Basic principles. J. Adhes. Sci. Technol. 2007, 21, 1145–1173. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Seong, K.-Y.; Yang, S.Y.; Seo, S. Advances in medical adhesives inspired by aquatic organisms’ adhesion. Biomater. Res. 2017, 21, 16. [Google Scholar] [CrossRef] [Green Version]
- Balkenende, D.W.R.; Winkler, S.M.; Messersmith, P.B. Marine-inspired polymers in medical adhesion. Eur. Polym. J. 2019, 116, 134–143. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl. Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Li, L.; Zeng, H. Marine mussel adhesion and bio-inspired wet adhesives. Biotribology 2016, 5, 44–51. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, C.V.; Waite, J.H. Location and analysis of byssal structural proteins of Mytilus edulis. J. Morphol. 1986, 189, 171–181. [Google Scholar] [CrossRef]
- Quan, W.Y.; Hu, Z.; Liu, H.Z.; Ouyang, Q.Q.; Zhang, D.Y.; Li, S.D.; Li, P.W.; Yang, Z.M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Hwang, J.; Deming, T.J. Role of L -3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, H.J.; Jeong, Y.; Joo, K.I.; Cha, H.J. Biomimetic Surface Engineering of Biomaterials by Using Recombinant Mussel Adhesive Proteins. Adv. Mater. Interfaces 2018, 5, 1800068. [Google Scholar] [CrossRef]
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- d’Ischia, M.; Ruiz-Molina, D. Bioinspired Catechol-Based Systems: Chemistry and Applications. Biomimetics 2017, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez Rodriguez, N.R.; Das, S.; Kaufman, Y.; Israelachvili, J.N.; Waite, J.H. Interfacial pH during mussel adhesive plaque formation. Biofouling 2015, 31, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef] [Green Version]
- Chan Choi, Y.; Choi, J.S.; Jung, Y.J.; Cho, Y.W. Human gelatin tissue-adhesive hydrogels prepared by enzyme-mediated biosynthesis of DOPA and Fe 3+ ion crosslinking. J. Mater. Chem. B 2014, 2, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Pan, J.; Claesson, P.M. Electrochemical and AFM studies of mussel adhesive protein (Mefp-1) as corrosion inhibitor for carbon steel. Electrochim. Acta 2011, 56, 1636–1645. [Google Scholar] [CrossRef]
- Cui, M.; Wang, B.; Wang, Z. Nature-Inspired Strategy for Anticorrosion. Adv. Eng. Mater. 2019, 21, 1801379. [Google Scholar] [CrossRef] [Green Version]
- Wilke, P.; Helfricht, N.; Mark, A.; Papastavrou, G.; Faivre, D.; Börner, H.G. A Direct Biocombinatorial Strategy toward Next Generation, Mussel-Glue Inspired Saltwater Adhesives. J. Am. Chem. Soc. 2014, 136, 12667–12674. [Google Scholar] [CrossRef]
- Wang, C.S.; Stewart, R.J. Localization of the bioadhesive precursors of the sandcastle worm, Phragmatopoma californica (Fewkes). J. Exp. Biol. 2012, 215, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Sun, C.; Stewart, R.J.; Waite, J.H. Cement Proteins of the Tube-building Polychaete Phragmatopoma californica. J. Biol. Chem. 2005, 280, 42938–42944. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.J. The tube cement of Phragmatopoma californica: A solid foam. J. Exp. Biol. 2004, 207, 4727–4734. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A Water-Borne Adhesive Modeled after the Sandcastle Glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winslow, B.D.; Shao, H.; Stewart, R.J.; Tresco, P.A. Biocompatibility of adhesive complex coacervates modeled after the sandcastle glue of Phragmatopoma californica for craniofacial reconstruction. Biomaterials 2010, 31, 9373–9381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Weerasekare, G.M.; Stewart, R.J. Multiphase Adhesive Coacervates Inspired by the Sandcastle Worm. ACS Appl. Mater. Interfaces 2011, 3, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.; Pereira, M.J.; Lee, Y.; Friehs, I.; Vasilyev, N.V.; Feins, E.N.; Ablasser, K.; O’Cearbhaill, E.D.; Xu, C.; Fabozzo, A.; et al. A Blood-Resistant Surgical Glue for Minimally Invasive Repair of Vessels and Heart Defects. Sci. Transl. Med. 2014, 6, 218ra6. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Xu, C.; Sebastin, M.; Lee, A.; Holwell, N.; Xu, C.; Miranda Nieves, D.; Mu, L.; Langer, R.S.; Lin, C.; et al. Bioinspired Nanoparticulate Medical Glues for Minimally Invasive Tissue Repair. Adv. Healthc. Mater. 2015, 4, 2587–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellenc, Q.; Touma, J.; Coscas, R.; Edorh, G.; Pereira, M.; Karp, J.; Castier, Y.; Desgranges, P.; Alsac, J.M. Preclinical and clinical evaluation of a novel synthetic bioresorbable, on demand light activated sealant in vascular reconstruction. J. Cardiovasc. Surg. 2019, 60, 599. [Google Scholar] [CrossRef]
- Pereira, M.J.N.; Ouyang, B.; Sundback, C.A.; Lang, N.; Friehs, I.; Mureli, S.; Pomerantseva, I.; McFadden, J.; Mochel, M.C.; Mwizerwa, O.; et al. A Highly Tunable Biocompatible and Multifunctional Biodegradable Elastomer. Adv. Mater. 2013, 25, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Jia, Y.; Sun, S.; Xu, Y.; Minsky, B.B.; Stuart, M.A.C.; Cölfen, H.; von Klitzing, R.; Guo, X. Mineral-Enhanced Polyacrylic Acid Hydrogel as an Oyster-Inspired Organic–Inorganic Hybrid Adhesive. ACS Appl. Mater. Interfaces 2018, 10, 10471–10479. [Google Scholar] [CrossRef]
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. Caddisfly Inspired Phosphorylated Poly(ester urea)-Based Degradable Bone Adhesives. Biomacromolecules 2016, 17, 3016–3024. [Google Scholar] [CrossRef]
- Nakano, M.; Shen, J.-R.; Kamino, K. Self-Assembling Peptide Inspired by a Barnacle Underwater Adhesive Protein. Biomacromolecules 2007, 8, 1830–1835. [Google Scholar] [CrossRef]
- Favi, P.M.; Yi, S.; Lenaghan, S.C.; Xia, L.; Zhang, M. Inspiration from the natural world: From bio-adhesives to bio-inspired adhesives. J. Adhes. Sci. Technol. 2014, 28, 290–319. [Google Scholar] [CrossRef]
- Cholewinski, A.; Yang, F.; Zhao, B. Algae–mussel-inspired hydrogel composite glue for underwater bonding. Mater. Horiz. 2019, 6, 285–293. [Google Scholar] [CrossRef]
- Han, K.; Park, T.Y.; Yong, K.; Cha, H.J. Combinational Biomimicking of Lotus Leaf, Mussel, and Sandcastle Worm for Robust Superhydrophobic Surfaces with Biomedical Multifunctionality: Antithrombotic, Antibiofouling, and Tissue Closure Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 9777–9785. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Messersmith, P.B. The Present and Future of Biologically Inspired Adhesive Interfaces and Materials. Langmuir 2012, 28, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, P.; Stambles, J.R. Photonic Structures in Biology. Nature 2003, 24, 497–517. [Google Scholar] [CrossRef]
- Parker, A.R. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea). Proc. R. Soc. B Biol. Sci. 1995, 262, 349–355. [Google Scholar]
- Chae, J.; Kita-Tsukamoto, K.; Nishida, S.; Ohwada, K. Chemical Composition of the Integumental Reflecting Platelets in the Iridescent Copepods of the Family Sapphirinidae (Poecilostomatoida). J. Crustacean Biol. 1996, 16, 20. [Google Scholar] [CrossRef]
- Bok, M.J.; Porter, M.L.; Place, A.R.; Cronin, T.W. Biological Sunscreens Tune Polychromatic Ultraviolet Vision in Mantis Shrimp. Curr. Biol. 2014, 24, 1636–1642. [Google Scholar] [CrossRef] [Green Version]
- Denton, E.J.; Nicol, J.A.C. A survey of reflectivity in silvery teleosts. J. Mar. Biol. Assoc. UK 1966, 46, 685–722. [Google Scholar] [CrossRef]
- Shand, J. Corneal iridescence in fishes: Light-induced colour changes in relation to structure. J. Fish Biol. 1988, 32, 625–632. [Google Scholar] [CrossRef]
- Lythgoe, J.N.; Shand, J. The Structural Basis for Iridescent Colour Changes in Dermal and Corneal Irddophores in Fish. J. Exp. Biol. 1989, 141, 313–325. [Google Scholar]
- McKenzie, D.R.; Yin, Y.; McFall, W.D. Silvery Fish Skin as an Example of a Chaotic Reflector. Proc. R. Soc. A Math. Phys. Eng. Sci. 1995, 451, 579–584. [Google Scholar]
- Holt, A.L.; Vahidinia, S.; Gagnon, Y.L.; Morse, D.E.; Sweeney, A.M. Photosymbiotic giant clams are transformers of solar flux. J. R. Soc. Interface 2014, 11, 20140678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoshal, A.; Eck, E.; Gordon, M.; Morse, D.E. Wavelength-specific forward scattering of light by Bragg-reflective iridocytes in giant clams. J. R. Soc. Interface 2016, 13, 20160285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäthger, L.M.; Denton, E.J.; Marshall, N.J.; Hanlon, R.T. Mechanisms and behavioural functions of structural coloration in cephalopods. J. R. Soc. Interface 2009, 6, S149–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwick, W.H.; Lang, N.J. Structural, Chemical and Ecological Studies on Iridescence in Iridaea (Rhodophyta). J. Phycol. 1977, 13, 121–127. [Google Scholar] [CrossRef]
- Chandler, C.J.; Wilts, B.D.; Vignolini, S.; Brodie, J.; Steiner, U.; Rudall, P.J.; Glover, B.J.; Gregory, T.; Walker, R.H. Structural colour in Chondrus crispus. Sci. Rep. 2015, 5, 11645. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, M.; Masters, N.; O’Brien, H.E.; Lennon, J.; Atkinson, G.; Cryan, M.J.; Oulton, R.; Whitney, H.M. Light-induced dynamic structural color by intracellular 3D photonic crystals in brown algae. Sci. Adv. 2018, 4, eaan8917. [Google Scholar] [CrossRef] [Green Version]
- Chandler, C.J.; Wilts, B.D.; Brodie, J.; Vignolini, S. Structural Color in Marine Algae. Adv. Opt. Mater. 2017, 5, 1600646. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.R.; Townley, H.E. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2007, 2, 347–353. [Google Scholar] [CrossRef]
- Kientz, B.; Vukusic, P.; Luke, S.; Rosenfeld, E. Iridescence of a marine bacterium and classification of prokaryotic structural colors. Appl. Environ. Microbiol. 2012, 78, 2092–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Bhushan, B.; Tong, J. Structural coloration in nature. RSC Adv. 2013, 3, 14862. [Google Scholar] [CrossRef]
- Cuthill, I.C.; Allen, W.L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A.; et al. The biology of color. Science 2017, 357, eaan0221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.; Zhang, W.; Xu, K.; Zhao, Y. Bio-inspired Intelligent Structural Color Materials. Mater. Horiz. 2019, 6, 945–958. [Google Scholar] [CrossRef]
- Li, L.; Kolle, S.; Weaver, J.C.; Ortiz, C.; Aizenberg, J.; Kolle, M. A highly conspicuous mineralized composite photonic architecture in the translucent shell of the blue-rayed limpet. Nat. Commun. 2015, 6, 6322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xu, J.; Liu, X.; Zhang, H.; Wang, D.; Chen, Z.; Zhang, D.; Fan, T. Bio-Inspired Photonic Materials: Prototypes and Structural Effect Designs for Applications in Solar Energy Manipulation. Adv. Funct. Mater. 2018, 28, 1705309. [Google Scholar] [CrossRef]
- Baba, K.; Iwasaka, M. Intense light scattering by cooperative relaxation of magnetically-aligned organic crystal particles. Aip Adv. 2019, 9, 035127. [Google Scholar] [CrossRef] [Green Version]
- Iwasaka, M.; Mizukawa, Y.; Roberts, N.W. Magnetic Control of the Light Reflection Anisotropy in a Biogenic Guanine Microcrystal Platelet. Langmuir 2016, 32, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.A.; Hirsch, A.; Brumfeld, V.; Aflalo, E.D.; Pinkas, I.; Sagi, A.; Rosenne, S.; Oron, D.; Leiserowitz, L.; Kronik, L.; et al. Optically functional isoxanthopterin crystals in the mirrored eyes of decapod crustaceans. Proc. Natl. Acad. Sci. USA 2018, 115, 2299–2304. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Su, H.; Chen, J.; Zhang, D.; Moon, W.J. Bioinspired ultraviolet reflective photonic structures derived from butterfly wings (Euploea). Appl. Phys. Lett. 2011, 99, 2011–2014. [Google Scholar] [CrossRef]
- Kim, H.-N.; Vahidinia, S.; Holt, A.L.; Sweeney, A.M.; Yang, S. Geometric Design of Scalable Forward Scatterers for Optimally Efficient Solar Transformers. Adv. Mater. 2017, 29, 1702922. [Google Scholar] [CrossRef] [PubMed]
- Harris, O.K.; Kingston, A.C.N.; Wolfe, C.S.; Ghoshroy, S.; Johnsen, S.; Speiser, D.I. Core–shell nanospheres behind the blue eyes of the bay scallop Argopecten irradians. J. R. Soc. Interface 2019, 16, 20190383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, L.E.; Ouyang, L.; Elfwing, A.; Hedblom, M.; Wulff, A.; Inganäs, O. Diatom frustules protect DNA from UV light. Sci. Rep. 2017, 8, 5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Lenau, T.A.; Gundersen, E.; Kirkensgaard, J.J.K.; Maibohm, C.; Pinti, J.; Ellegaard, M. The UV filtering potential of drop-casted layers of frustules of three diatom species. Sci. Rep. 2018, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, E.; Congestri, R.; Dardano, P.; De Luca, A.C.; Managò, S.; Rea, I.; De Stefano, M. UV-shielding and wavelength conversion by centric diatom nanopatterned frustules. Sci. Rep. 2018, 8, 16285. [Google Scholar] [CrossRef] [PubMed]
- Romann, J.; Valmalette, J.-C.; Chauton, M.S.; Tranell, G.; Einarsrud, M.-A.; Vadstein, O. Wavelength and orientation dependent capture of light by diatom frustule nanostructures. Sci. Rep. 2015, 5, 17403. [Google Scholar] [CrossRef]
- Ingalls, A.E.; Whitehead, K.; Bridoux, M.C. Tinted windows: The presence of the UV absorbing compounds called mycosporine-like amino acids embedded in the frustules of marine diatoms. Geochim. Cosmochim. Acta 2010, 74, 104–115. [Google Scholar] [CrossRef]
| Taxa | Crystalline | Hemicelluloses | Matrix Carboxylic | Matrix-Sulfated | Storage |
|---|---|---|---|---|---|
| Chlorophyceae | Cellulose | Xyloglucan Mannans Glucuronan (1-3) β-glucan | Ulvans | Ulvans | Inlulin (fructan) Laminaran Strach |
| Rhodophytae | Cellulose (1-4)-β-d-mannans (1-4)-β-d-xylans (1-3)-β-d-xylan Chitin | Glucomannan Sulfated mixed-linkage glucan (1-3)(1-4)-β-d-xylan | - | Agars Carrageenans Porphyran Mannans | Floridean glycogen |
| Phaeophyceae | Cellulose | Sulfated xylofucoglucan Sulfated xylofuco-glucouronan (1-3)-β-glucan | Alginates | Homofucans | Laminaran |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. https://doi.org/10.3390/polym12051002
Claverie M, McReynolds C, Petitpas A, Thomas M, Fernandes SCM. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers. 2020; 12(5):1002. https://doi.org/10.3390/polym12051002
Chicago/Turabian StyleClaverie, Marion, Colin McReynolds, Arnaud Petitpas, Martin Thomas, and Susana C. M. Fernandes. 2020. "Marine-Derived Polymeric Materials and Biomimetics: An Overview" Polymers 12, no. 5: 1002. https://doi.org/10.3390/polym12051002
APA StyleClaverie, M., McReynolds, C., Petitpas, A., Thomas, M., & Fernandes, S. C. M. (2020). Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers, 12(5), 1002. https://doi.org/10.3390/polym12051002







