Figure 1.
Polymer material classification with respect to their healing chemistry: autonomic self-healing systems and non-autonomic systems (adapted from [
7]).
Figure 1.
Polymer material classification with respect to their healing chemistry: autonomic self-healing systems and non-autonomic systems (adapted from [
7]).
Figure 2.
Synthetization process of PUF-DCPD microcapsules showing the (a) weighting of DCPD monomer at room temperature before phase transformation at 35 °C, (b) microcapsule formation at 50 °C, and (c) microcapsule suspension left to dry at room temperature for 24 h.
Figure 2.
Synthetization process of PUF-DCPD microcapsules showing the (a) weighting of DCPD monomer at room temperature before phase transformation at 35 °C, (b) microcapsule formation at 50 °C, and (c) microcapsule suspension left to dry at room temperature for 24 h.
Figure 3.
Process of PUF-DCPD microcapsule synthesis.
Figure 3.
Process of PUF-DCPD microcapsule synthesis.
Figure 4.
Sample taken during microcapsule synthesis process (optical image, 25× zoom).
Figure 4.
Sample taken during microcapsule synthesis process (optical image, 25× zoom).
Figure 5.
Optical images illustrating (a) microcapsules after 24 h drying at room temperature—25× zoom and (b) microcapsule dimensions—45× zoom (one division represents 100 µm).
Figure 5.
Optical images illustrating (a) microcapsules after 24 h drying at room temperature—25× zoom and (b) microcapsule dimensions—45× zoom (one division represents 100 µm).
Figure 6.
SEM images of (a) microcapsule agglomeration, (b) a 250 µm microcapsule, and (c) a broken microcapsule.
Figure 6.
SEM images of (a) microcapsule agglomeration, (b) a 250 µm microcapsule, and (c) a broken microcapsule.
Figure 7.
The reaction mixture employed for obtaining MUF microcapsules with embedded ENB healing agent.
Figure 7.
The reaction mixture employed for obtaining MUF microcapsules with embedded ENB healing agent.
Figure 8.
Microcapsules after a (a) 12 h and (b) 24 h drying period.
Figure 8.
Microcapsules after a (a) 12 h and (b) 24 h drying period.
Figure 9.
Schematic illustration of melamine-urea-formaldehyde microcapsule synthesis with embedded 5-ethylidene-2-norbornene as repair agent.
Figure 9.
Schematic illustration of melamine-urea-formaldehyde microcapsule synthesis with embedded 5-ethylidene-2-norbornene as repair agent.
Figure 10.
Optical image illustrating a (a) sample taken 30 min after the addition of ENB and a (b) sample after 12 h drying at room temperature (one division represents 100 µm).
Figure 10.
Optical image illustrating a (a) sample taken 30 min after the addition of ENB and a (b) sample after 12 h drying at room temperature (one division represents 100 µm).
Figure 11.
FT-IR spectra of the main (a) PUF-DCPD and (b) MUF-ENB microcapsule constituents.
Figure 11.
FT-IR spectra of the main (a) PUF-DCPD and (b) MUF-ENB microcapsule constituents.
Figure 12.
Thermogravimetric analysis (TGA)/differential thermal analysis (DTA) analysis of the PUF-DCPD system.
Figure 12.
Thermogravimetric analysis (TGA)/differential thermal analysis (DTA) analysis of the PUF-DCPD system.
Figure 13.
TGA/DTA analysis of the MUF-ENB system.
Figure 13.
TGA/DTA analysis of the MUF-ENB system.
Figure 14.
TEM images presenting the core-shell structure of synthetized (a) PUF-DCPD and (b) MUF-ENB microcapsules.
Figure 14.
TEM images presenting the core-shell structure of synthetized (a) PUF-DCPD and (b) MUF-ENB microcapsules.
Figure 15.
Illustration of the (a) cube-cell element and (b) finite element model with an embedded microcapsule.
Figure 15.
Illustration of the (a) cube-cell element and (b) finite element model with an embedded microcapsule.
Figure 16.
Von Mises illustration of the (a) cube-cell cross-section view, (b) cube-cell quarter view, (c) filled microcapsule, and (d) base cube-cell with an empty microcapsule.
Figure 16.
Von Mises illustration of the (a) cube-cell cross-section view, (b) cube-cell quarter view, (c) filled microcapsule, and (d) base cube-cell with an empty microcapsule.
Figure 17.
Von Mises distribution on the 20 µm microcapsule.
Figure 17.
Von Mises distribution on the 20 µm microcapsule.
Figure 18.
Von Mises stress distribution with respect to spherical inclusions (a) placed at 45° at 0.085 mm, (b) placed in the median plane at 0.05 mm, and (c) in an overlapping position in the perpendicular plane at 0.05 mm.
Figure 18.
Von Mises stress distribution with respect to spherical inclusions (a) placed at 45° at 0.085 mm, (b) placed in the median plane at 0.05 mm, and (c) in an overlapping position in the perpendicular plane at 0.05 mm.
Figure 19.
Specimens fabricated using (a) method 1, showing defects due to the high volumetric fraction of epoxy matrix and 250 µm PUF-DCPD microcapsules, and (b) method 2, presenting microcapsule agglomeration at the surface.
Figure 19.
Specimens fabricated using (a) method 1, showing defects due to the high volumetric fraction of epoxy matrix and 250 µm PUF-DCPD microcapsules, and (b) method 2, presenting microcapsule agglomeration at the surface.
Figure 20.
Specimens containing (a) MUF-DCPD and (b) PUF-ENB systems and Grubbs catalyst fabricated by method 6.
Figure 20.
Specimens containing (a) MUF-DCPD and (b) PUF-ENB systems and Grubbs catalyst fabricated by method 6.
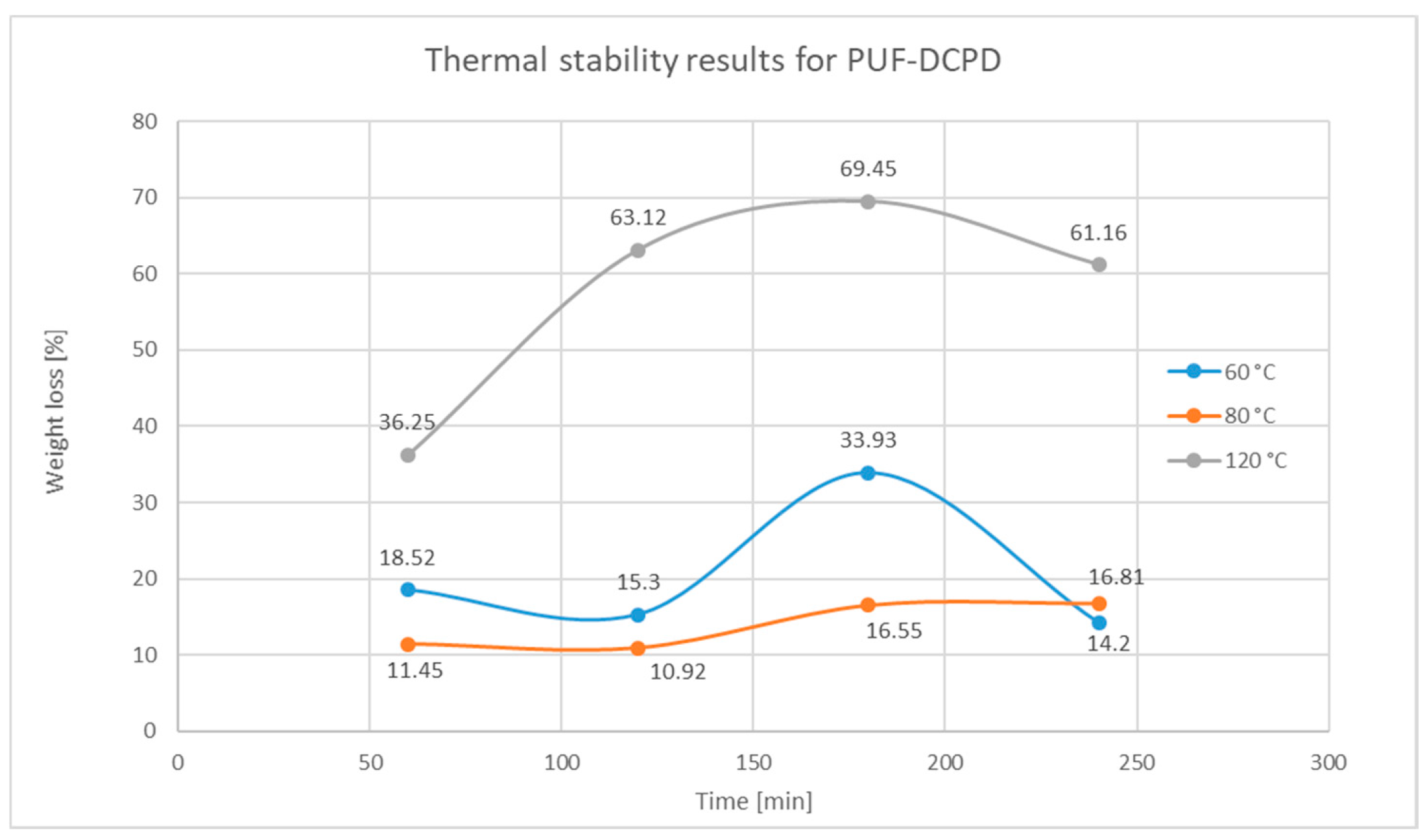
Figure 21.
Weight loss variation over time for PUF-DCPD after thermal exposure at 60, 80, and 120 °C.
Figure 21.
Weight loss variation over time for PUF-DCPD after thermal exposure at 60, 80, and 120 °C.
Figure 22.
Weight loss variation over time for MUF-ENB after thermal exposure at 60, 80, and 120 °C.
Figure 22.
Weight loss variation over time for MUF-ENB after thermal exposure at 60, 80, and 120 °C.
Figure 23.
Stress–strain curves for specimens cured at 60 °C for 16 h.
Figure 23.
Stress–strain curves for specimens cured at 60 °C for 16 h.
Figure 24.
Stress–strain curves for specimens cured at 80 °C for 2 h.
Figure 24.
Stress–strain curves for specimens cured at 80 °C for 2 h.
Figure 25.
Stress–strain curves for PUF-DCPD specimens fabricated with method 5.
Figure 25.
Stress–strain curves for PUF-DCPD specimens fabricated with method 5.
Figure 26.
Stress–strain curves for PUF-DCPD specimens fabricated with method 6.
Figure 26.
Stress–strain curves for PUF-DCPD specimens fabricated with method 6.
Figure 27.
Stress–strain curves for MUF-ENB specimens fabricated according to method 6.
Figure 27.
Stress–strain curves for MUF-ENB specimens fabricated according to method 6.
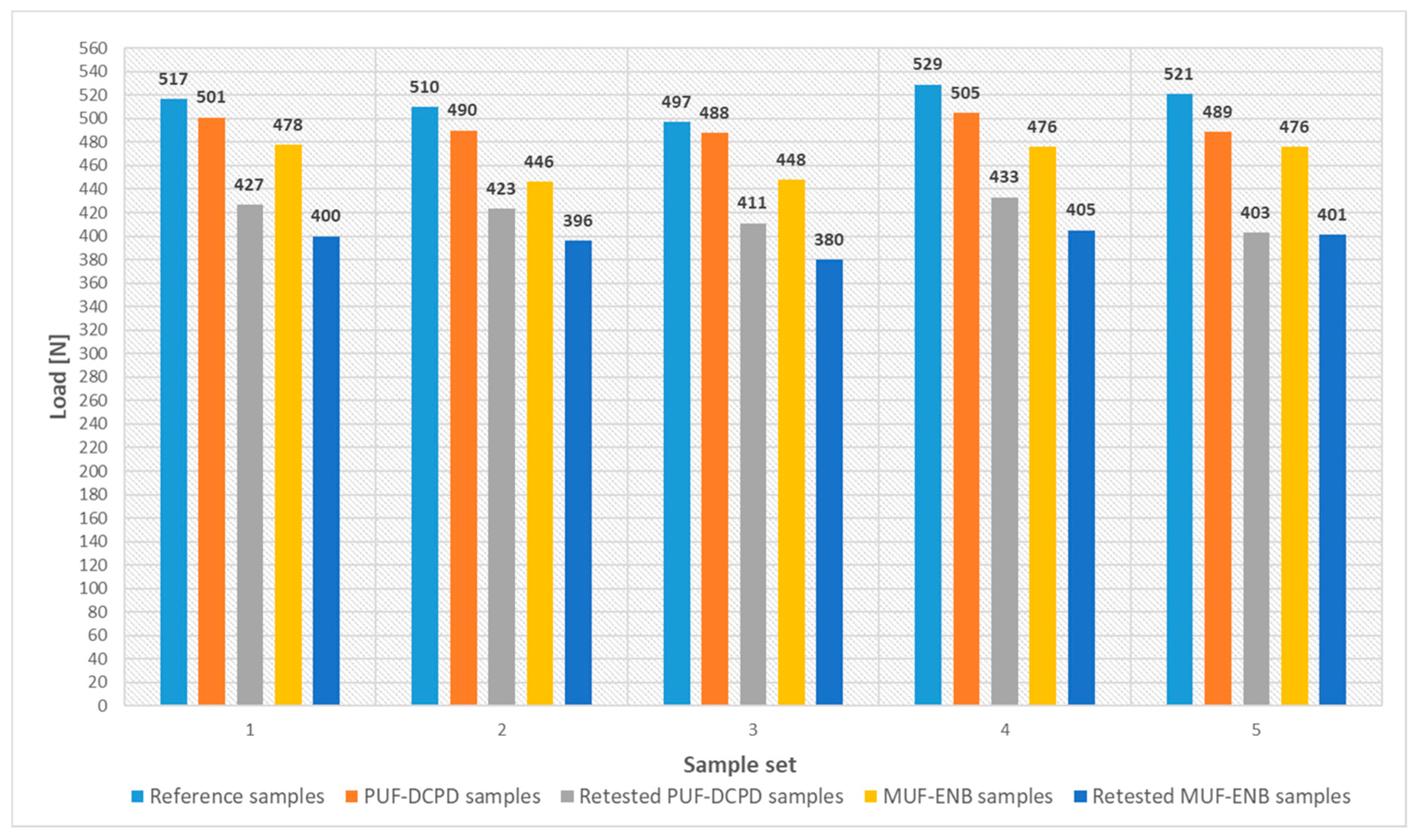
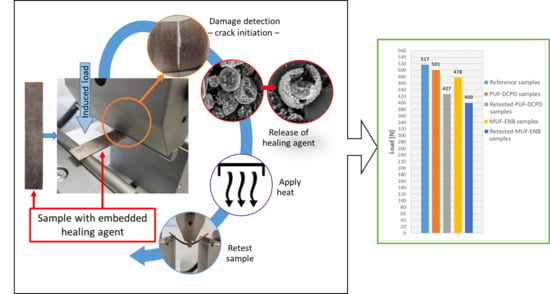
Figure 28.
Load values for the three-point bending tested specimens.
Figure 28.
Load values for the three-point bending tested specimens.
Figure 29.
Displacement values for the three-point bending tested specimens.
Figure 29.
Displacement values for the three-point bending tested specimens.
Table 1.
Overview of materials that can be used as healing agents and their respective delivery system.
Table 1.
Overview of materials that can be used as healing agents and their respective delivery system.
| Healing Agent | Delivery System | Catalyst Required | Used in FRP Composites |
|---|
| Microcapsule | HGF | Microvascular |
|---|
| DCPD/ENB | X (UF) | | X | X | X |
| Siloxanes | X (UF/PU) | | | X | |
| Epoxy | X (UF) | | | X | X |
| Amine-Epoxy | X (UF) | X | X | | X |
| Thiol-epoxy | X (MF) | | | X | X |
| Thiol-ene | X (UF) | | | X | |
| Thiol-isocyanate | X (MF/PU) | | | | |
| Azide-alkyne | X (UF) | | | X | |
| Methacrylates | | X | | X | |
| GMA | X (MF) | | | | |
| Melaimides | X (UF) | | | | X |
| Isocyanates | X (PU) | X | | | |
| Cyanoacrylates | X | X | | | X |
| Vinyl ester | X | | | X | X |
| Unsaturated polyester | X | | | X | X |
Table 2.
Chemical substances used in the production of poly-urea-formaldehyde (PUF) microcapsules with embedded dicyclopentadiene (DCPD).
Table 2.
Chemical substances used in the production of poly-urea-formaldehyde (PUF) microcapsules with embedded dicyclopentadiene (DCPD).
| Material | Molecular Formula | Physical Properties | Role |
|---|
| Urea | CH4N2O | Crystalline, white powder. Melting point at 133–135 °C | Formation of the capsule shell in the aqueous state |
| Resorcinol (1,3- benzenediol) | C6H4(OH)2 | White crystals. Melting point at 113 °C | Blending resin with formaldehyde |
| Formaldehyde | CH2O | Colorless aqueous solution | Formation of capsule shell |
| Dicyclopentadiene (DCPD) | C10H12 | Solid state (gel-like state at room temperature). Melting point at 32.5 °C | Monomer-capsule core |
| Maleic anhydride | C2H2(CO)2O | Solid state. White powder | Emulsifier |
| Ammonium chloride | NH4Cl | Solid state. White powder | Formaldehyde hardener |
| Sodium hydroxide | NaOH | Solid state. White powder | Rising solution pH |
| Hydrochloric acid | HCl | Aqueous solution with strong odor | Lowering solution pH |
Table 3.
Chemical substances used in the production of melamine-urea-formaldehyde (MUF) microcapsules with embedded 5-ethylidene-2-norbornene (ENB).
Table 3.
Chemical substances used in the production of melamine-urea-formaldehyde (MUF) microcapsules with embedded 5-ethylidene-2-norbornene (ENB).
| Material | Molecular Formula | Physical Properties | Role |
|---|
| Melamine | C3H6N6 | White powder. Melting point at 345 °C | Formation of capsule shell |
| Urea | CH4N2O | Crystalline white powder. Melting point at 133–135 °C | Formation of the capsule shell in the aqueous state |
| Formaldehyde | CH2O | Colorless aqueous solution | Formation of capsule shell |
| Sodium lauryl sulfate (SLS) | CH3(CH2)10CH2 (OCH2CH2)nOSO3Na | Powder soluble in water | Emulsifier—oil solidification |
| Polyvinyl alcohol (PVA) | (C2H4O)x | Melting point at 200 °C | Separation film |
Table 4.
Methods of microcapsule integration in the epoxy matrix.
Table 4.
Methods of microcapsule integration in the epoxy matrix.
| Method No. | Method for Optimization |
|---|
| 1 | Homogenization at 300 rpm |
| 2 | Heating at 40 °C and homogenization at 300 rpm |
| 3 | Heating at 40 °C and homogenization at 200 rpm |
| 4 | Heating at 40 °C and homogenization at 100 rpm |
| 5 | Preheating at 60 °C, homogenization at 100 rpm, and heating at 80 °C |
| 6 | 10 vol % acetone dilution, heating at 60 °C, and homogenization at 100 rpm, and then acetone evaporation at 80 °C |
Table 5.
Flexural tests for specimens cured at 60 °C for 16 h.
Table 5.
Flexural tests for specimens cured at 60 °C for 16 h.
| Specimen No. | Flexural Strength (MPa) | Load (N) | Elongation at Break (mm) |
|---|
| P1 | 65.21 | 32.03 | 17.60 |
| P2 | 61.79 | 32.32 | 16.44 |
| P3 | 60.72 | 30.66 | 15.99 |
| P4 | 67.19 | 33.10 | 17.52 |
| P5 | 60.16 | 31.50 | 16.58 |
| Measurement error (%) | ±3 | ±1 | ±0.5 |
Table 6.
Flexural tests for specimens cured at 80 °C for 2 h.
Table 6.
Flexural tests for specimens cured at 80 °C for 2 h.
| Specimen No. | Flexural Strength (MPa) | Load (N) | Elongation at Break (mm) |
|---|
| P1.1 | 59.62 | 30.53 | 15.97 |
| P2.1 | 62.55 | 30.73 | 15.40 |
| P3.1 | 59.02 | 27.16 | 15.67 |
| P4.1 | 61.48 | 29.18 | 16.63 |
| P5.1 | 62.21 | 29.40 | 15.90 |
| Measurement error (%) | ±3 | ±1 | ±0.5 |
Table 7.
Flexural tests for method 5 specimens.
Table 7.
Flexural tests for method 5 specimens.
| Specimen No. | Flexural Strength (MPa) | Load (N) | Elongation at Break (mm) |
|---|
| P1 | 2.33 | 0.13 | 0.01 |
| P2 | 22.99 | 15.71 | 14.14 |
| P3 | 2.26 | −0.05 | 0.04 |
| Measurement error (%) | ±3 | ±1 | ±0.5 |
Table 8.
Method 6 flexural test results for PUF-DCPD specimens.
Table 8.
Method 6 flexural test results for PUF-DCPD specimens.
| Specimen No. | Flexural Strength (MPa) | Load (N) | Elongation at Break (mm) |
|---|
| P1 | 48.07 | 24.89 | 15.41 |
| P2 | 48.31 | 25.12 | 15.38 |
| P3 | 50.24 | 24.16 | 16.05 |
| P4 | 52.40 | | |
| P5 | 43.31 | 22.60 | 16.33 |
| Measurement error (%) | ±3 | ±1 | ±0.5 |
Table 9.
Flexural test results for MUF-ENB specimens, cured at 80 °C for 2 h, according to method 5.
Table 9.
Flexural test results for MUF-ENB specimens, cured at 80 °C for 2 h, according to method 5.
| Specimen No. | Flexural Strength (MPa) | Load (N) | Elongation at Break (mm) |
|---|
| P1 | 16.32 | 9.40 | 4.91 |
| P2 | 17.87 | 10.81 | 5.34 |
| P3 | 19.59 | 10.30 | 4.66 |
| P4 | 22.13 | 10.48 | 5.08 |
| P5 | 24.45 | 16.71 | 5.00 |
| Measurement error (%) | ±3 | ±1 | ±0.5 |