Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers
Abstract
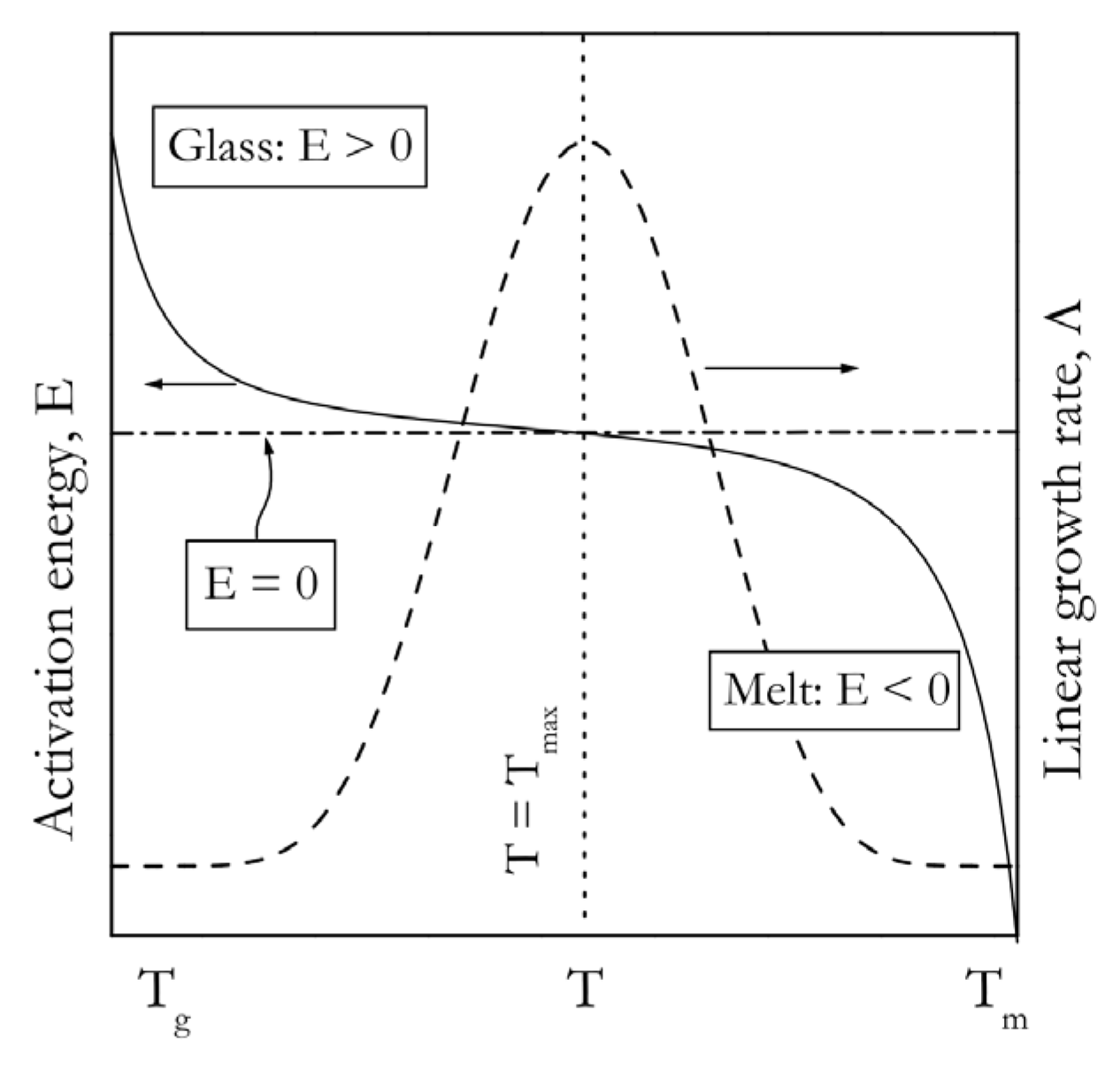
1. Introduction
2. Rate of Crystallization
2.1. Theoretical Considerations
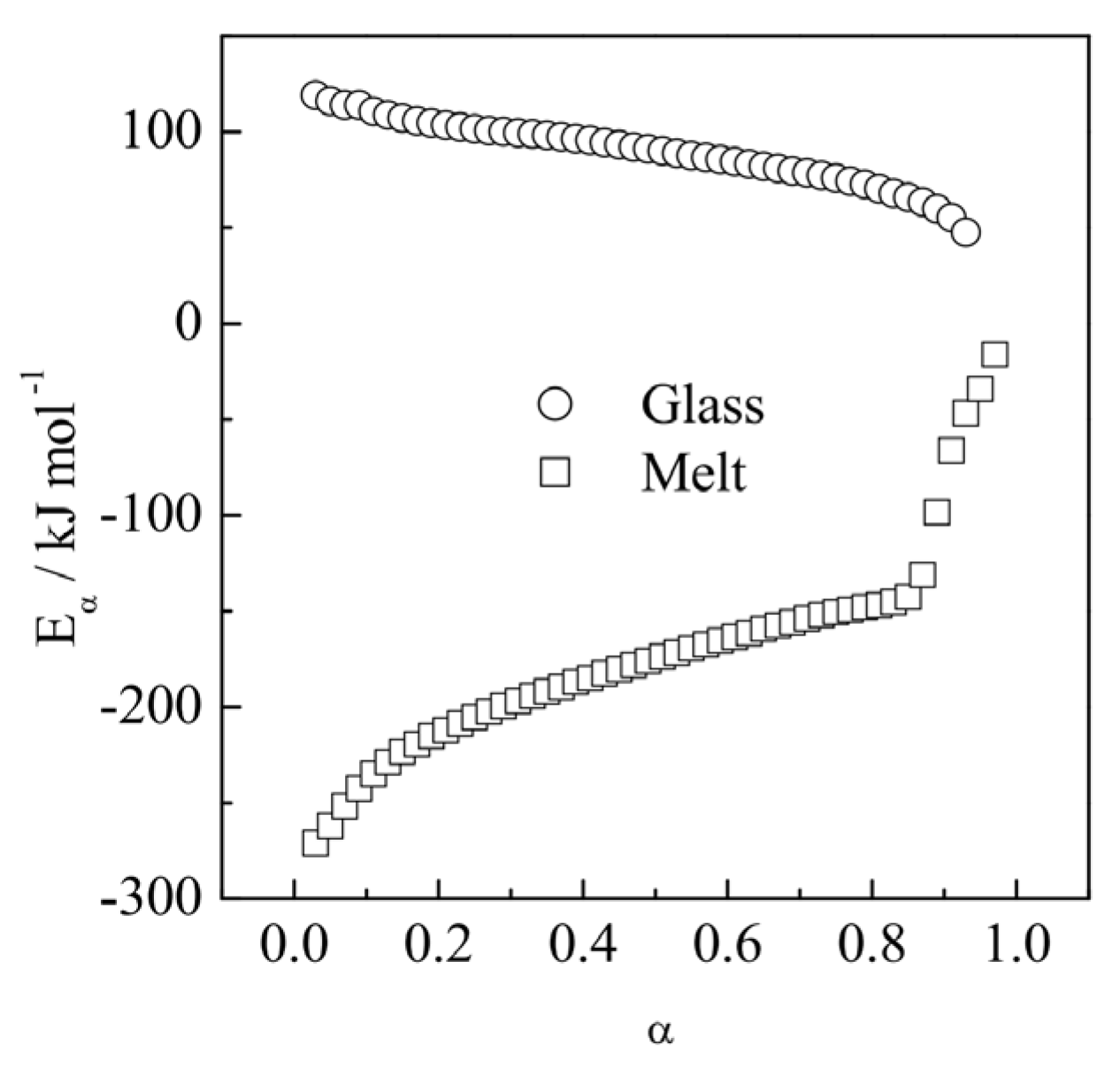
2.2. Practical Considerations
3. Rate of Melting
3.1. Theoretical Considerations
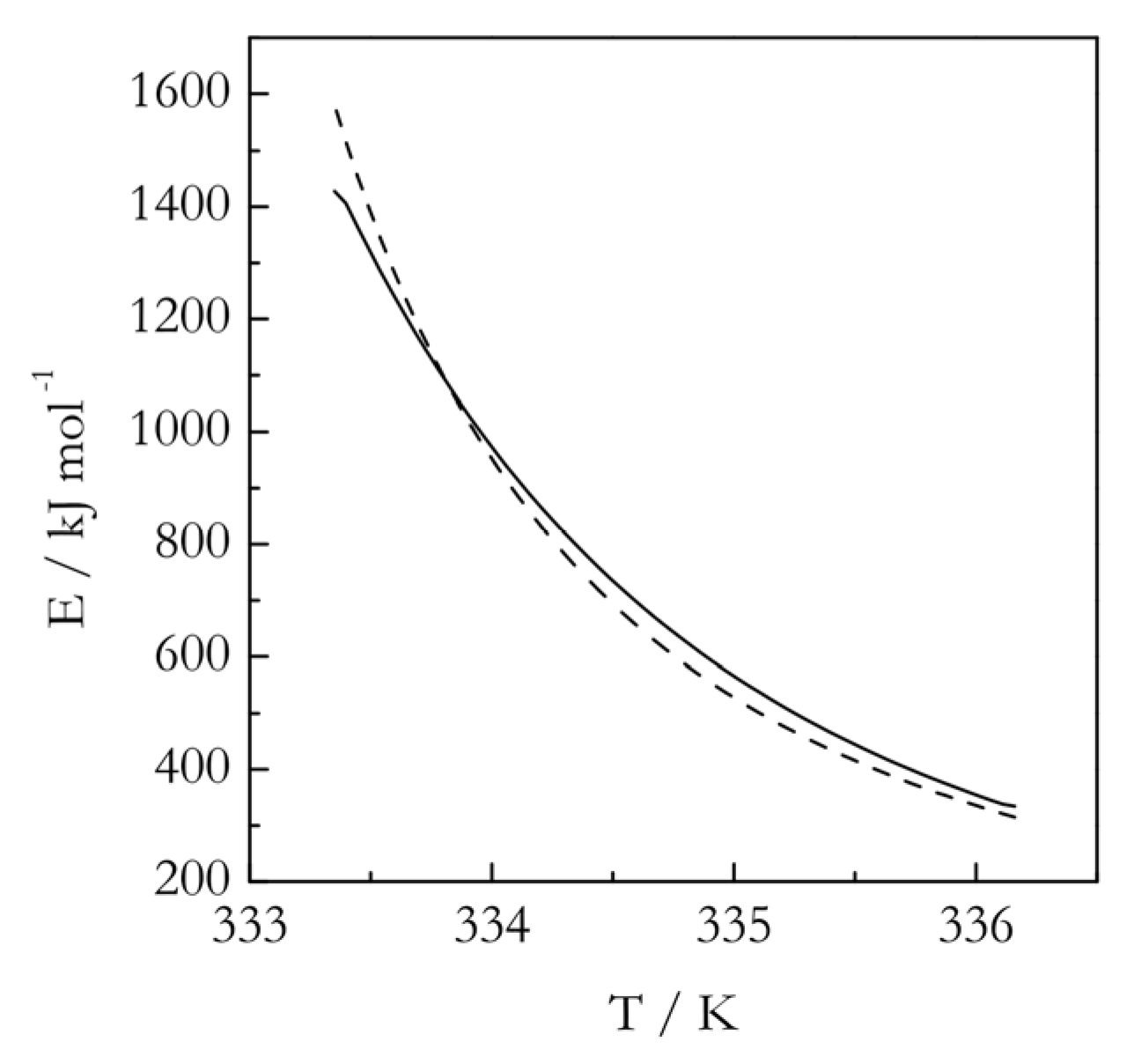
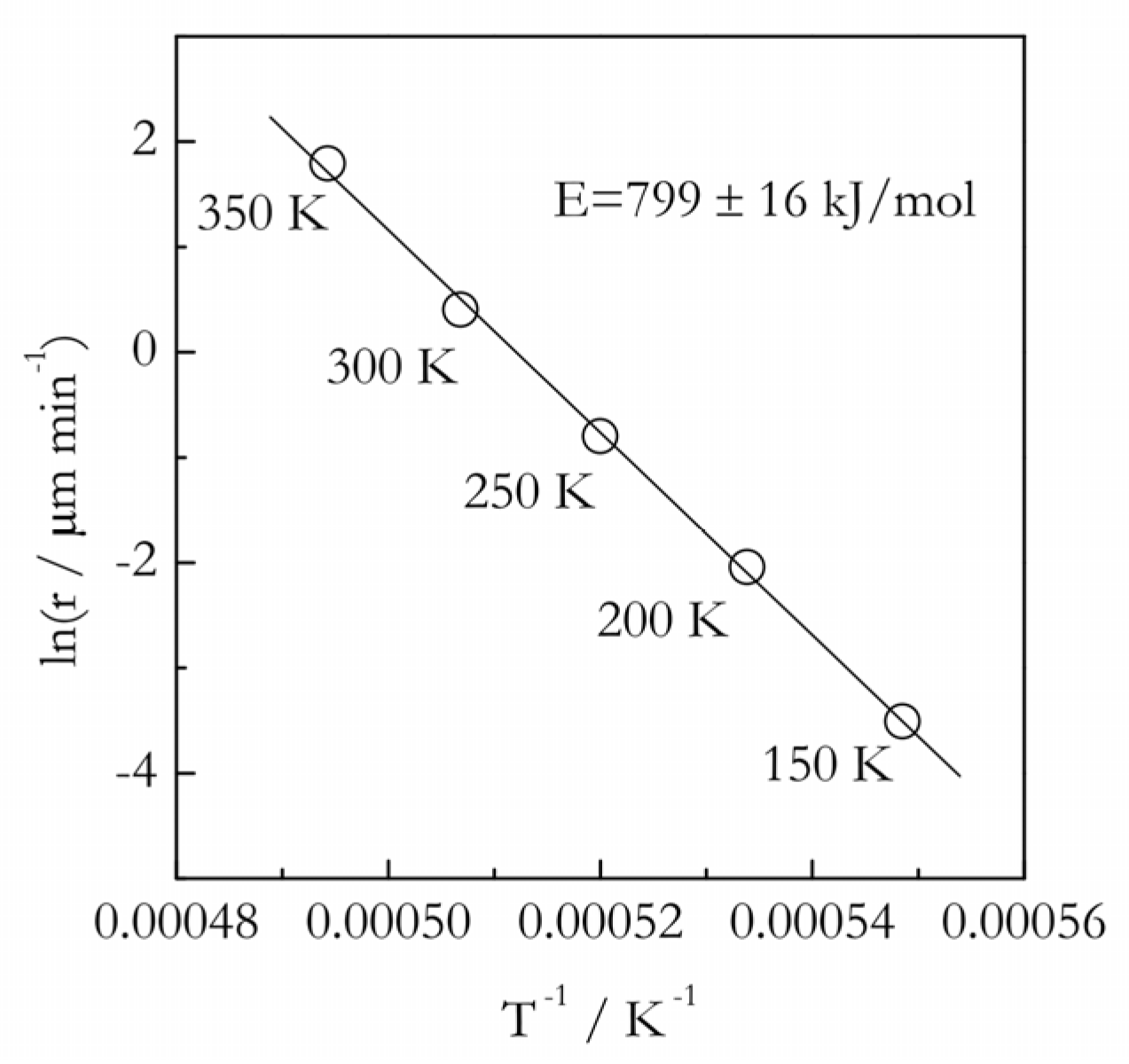
3.2. Practical Considerations
4. Conclusions
Funding
Conflicts of Interest
References
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Glasstone, S.; Laidler, K.J.; Eyring, H. The Theory of Rate Process; McGrow-Hill: New York, NY, USA, 1941. [Google Scholar]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Pergamon Press: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Jackson, K.A. Kinetic processes. In Crystal Growth, Diffusion, and Phase Transitions in Materials; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- van’t Hoff, J.H. Studies in Chemical Dynamics; F. Muller & Co: Amsterdam, The Netherlands, 1896. [Google Scholar]
- Atkins, P.; de Paula, J. Physical Chemistry, 10th ed.; W. H. Freeman: New York, NY, USA, 2014. [Google Scholar]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kinetic concepts of thermally stimulated reactions in solids: A view from a historical perspective. Int. Rev. Phys. Chem. 2000, 19, 45–60. [Google Scholar] [CrossRef]
- Vyazovkin, S. On the phenomenon of variable activation energy for condensed phase reactions. New J. Chem. 2000, 24, 913–917. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Borchardt, H.J.; Daniels, F. The application of differential thermal analysis to the study of reaction kinetics. J. Am. Chem. Soc. 1957, 79, 41–46. [Google Scholar] [CrossRef]
- Sestak, J. Ignoring heat inertia impairs accuracy of determination of activation energy in thermal analysis. Int. J. Chem. Kinet. 2019, 51, 74–80. [Google Scholar] [CrossRef]
- Vyazovkin, S. How much is the accuracy of activation energy affected by ignoring thermal inertia? Int. J. Chem. Kin. 2020, 52, 23–28. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Birx, L.; Vyazovkin, S. Thermal stability of malonic acid dissolved in polyvinylpyrrolidone and other polymeric matrices. Ind. Eng. Chem. Res. 2018, 57, 5228–5233. [Google Scholar] [CrossRef]
- Jelić, D.; Liavitskaya, T.; Paulechka, E.; Vyazovkin, S. Accelerating effect of poly (vinylpyrrolidone) matrix on thermal decomposition of malonic acid. Ind. Eng. Chem. Res. 2019, 58, 2891–2898. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Vyazovkin, S. Modern isoconversional kinetics: From Misconceptions to Advances. In The Handbook of Thermal Analysis & Calorimetry, Vol.6: Recent Advances, Techniques and Applications, 2nd ed.; Vyazovkin, S., Koga, N., Schick, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–172. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Nat. Bur. Stand. A 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional kinetics of polymers: The decade past. Macromol. Rapid Commun. 2017, 38, 1600615. [Google Scholar]
- Staudinger, H. Uber polymerization. Ber. Deutsch. Chem. Gesell. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Herzog, R.O.; Jancke, W. Roentgenspektrographische Beobachtungen an Zellulose. Z. Phys. 1920, 3, 196–198. [Google Scholar] [CrossRef]
- Staudinger, H.; Johner, H.; Signer, R.; Mie, G.; Hengstenberg, J. Der polymere Formaldehyd, ein Modell der Zellulose. Z. Phys. Chem. 1927, 126, 425–448. [Google Scholar] [CrossRef]
- Bekkedahl, N. Forms of rubber as indicated by temperature-volume relationship. J. Res. Nat. Bur. Stand. 1934, 13, 411–431. [Google Scholar] [CrossRef]
- Wood, L.A.; Bekkedahl, N. Crystallization of unvulcanized rubber at different temperatures. J. Appl. Phys. 1946, 17, 362–375. [Google Scholar] [CrossRef]
- Tammann, G. Ueber die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur. Z. Phys. Chem. 1898, 25, 441–479. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional kinetics. In The Handbook of Thermal Analysis & Calorimetry, Vol.5: Recent Advances, Techniques and Applications; Brown, M.E., Gallagher, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 503–538. [Google Scholar]
- Mandelkern, L. Crystalline polymer: Some reminiscences over the years. Thermochim. Acta 2006, 442, 31–34. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional analysis of nonisothermal crystallization of a polymer melt. Macromol. Rapid Commun. 2002, 23, 766–770. [Google Scholar] [CrossRef]
- Mandelkern, L.; Quinn, A.; Flory, P.J. Crystallization kinetics in high polymers. I. Bulk polymers. J. Appl. Phys. 1954, 25, 830–839. [Google Scholar] [CrossRef]
- Turnbull, D.; Fisher, J.C. Rate of nucleation in condensed systems. J. Chem. Phys. 1949, 17, 71–73. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A. Keimbildung in übersättigten Gebilden. Z. Phys. Chem. 1926, 119, 277–301. [Google Scholar] [CrossRef]
- Volmer, M. Kinetik der Phasenbildung; Verlag Theodor Steinkopff: Dresden, Germany, 1939. [Google Scholar]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth: Oxford, UK, 2004. [Google Scholar]
- Mandelkern, L. Crystallization of Polymers; Cambridge University Press: Cambridge, UK, 2004; Volume 2. [Google Scholar]
- Papon, P.; Leblond, J.; Meijer, P.H.E. The Physics of Phase Transitions; Springer: Berlin, Germany, 1999. [Google Scholar]
- Becker, R. Die Keimbildung bei der Ausscheidung in metallischen Mischkristallen. Ann. Phys. 1938, 424, 128–140. [Google Scholar] [CrossRef]
- Mullin, J.W.; Leci, C.L. Some nucleation characteristics of aqueous citric acid solutions. J. Cryst. Growth 1969, 5, 75–76. [Google Scholar] [CrossRef]
- Stanford, V.L.; McCulley, C.M.; Vyazovkin, S. Isoconversional kinetics of nonisothermal crystallization of salts from solutions. J. Phys. Chem. B 2016, 120, 5703–5709. [Google Scholar] [CrossRef] [PubMed]
- Stolte, I.; Androsch, R.; Di Lorenzo, M.L.; Schick, C. Effect of aging the glass of isotactic polybutene-1 on form II nucleation and cold crystallization. J. Phys. Chem. B 2013, 117, 15196–15203. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Schick, C.; Schmelzer, J.W.P. Sequence of enthalpy relaxation, homogeneous crystal nucleation and crystal growth in glassy polyamide 6. Eur. Polym. J. 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Hikima, T.; Hanaya, M.; Oguni, M. β-molecular rearrangement process, but not an α-process, as governing the homogeneous crystal-nucleation rate in a supercooled liquid. Bull. Chem. Soc. Jpn. 1996, 69, 1863–1868. [Google Scholar] [CrossRef]
- Hatase, M.; Hanaya, M.; Hikima, T.; Oguni, M. Discovery of homogeneous-nucleation-based crystallization in simple glass-forming liquid of toluene below its glass-transition temperature. J. Non-Cryst. Solids 2002, 307–310, 257–263. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Physical stability and relaxation of amorphous indomethacin. J. Phys. Chem. B 2005, 109, 18637–18644. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Effect of physical aging on nucleation of amorphous indomethacin. J. Phys. Chem. B 2007, 111, 7283–7287. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Davis, G.T.; Lauritzen, J.I., Jr. The Rate of Crystallization of Linear Polymers with Chain Folding. In Treatise on Solid State Chemistry; Hannay, N.B., Ed.; Plenum Press: New York, NY, USA, 1976; Volume 3, pp. 497–614. [Google Scholar]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional approach to evaluating the Hoffman-Lauritzen parameters (U* and Kg) from the overall rates of nonisothermal crystallization. Macromol. Rapid Commun. 2004, 25, 733–738. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I. Isoconversional analysis of combined melt and glass crystallization data. Macromol. Chem. Phys. 2006, 207, 20–25. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of nonisothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Vyazovkin, S. Nonisothermal crystallization of polymers: Getting more out of kinetic analysis of differential scanning calorimetry data. Polym. Cryst. 2018, 1, e10003. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Dong, Z. Comparison of the Ozawa and modified Avrami models of polymer crystallization under nonisothermal conditions using a computer simulation method. Thermochim. Acta 2007, 466, 22–28. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization pf poly (ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Bikiaris, D.N.; Chrissafis, K. A different approach for the study of the crystallization kinetics in polymers. Key study: Poly (ethylene terephthalate)/SiO2 nanocomposites. Polym. Int. 2010, 59, 1630–1638. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Vyazovkin, S. Is the Kissinger equation applicable to the processes that occur on cooling? Macromol. Rapid Commun. 2002, 23, 771–775. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Ortega, A. A simple and precise linear integral method for isoconversional data. Thermochim. Acta 2008, 474, 81–86. [Google Scholar] [CrossRef]
- Ainslie, N.G.; MacKenzie, D.; Turnbull, D. Melting kinetics of quartz and cristobalite. J. Phys. Chem. 1961, 65, 1718–1724. [Google Scholar] [CrossRef]
- Uhlmann, D.R. On the internal nucleation of melting. J. Non-Cryst. Solids 1980, 41, 347–357. [Google Scholar] [CrossRef]
- Vyazovkin, S. Power law and Arrhenius approaches to the melting kinetics of superheated crystals: Are they compatible? Cryst. Growth Des. 2018, 18, 6389–6392. [Google Scholar] [CrossRef]
- Tammann, G. Zur Uberhitzung von Kristallen. Z. Phys. Chem. 1910, 68, 257–269. [Google Scholar] [CrossRef]
- Prime, R.B.; Wunderlich, B.; Melillo, L. Extended-chain crystals. V. Thermal analysis and electron microscopy of the melting process in polyethylene. J. Pol. Sci Part A-2 1969, 7, 2091–2097. [Google Scholar] [CrossRef]
- Hellmuth, E.; Wunderlich, B. Superheating of linear high-polymer polyethylene crystals. J. Appl. Phys. 1965, 36, 3039–3044. [Google Scholar] [CrossRef]
- Matsuoka, S. Relaxation Phenomena in Polymers; Hanser Publishers: Munich, Germany, 1992. [Google Scholar]
- Kovacs, A.J.; Gonthier, A.; Straupe, C. Isothermal growth, thickening, and melting of poly (ethylene oxide) single crystals in the bulk. J. Polym. Sci. Polym. Symp. 1975, 50, 283–325. [Google Scholar] [CrossRef]
- Sanchez, I.C.; Colson, J.P.; Eby, R.K. Theory and observations of polymer crystal thickening. J. Appl. Phys. 1973, 44, 4332–4339. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Birx, L.; Vyazovkin, S. Melting kinetics of superheated crystals of glucose and fructose. Phys. Chem. Chem. Phys. 2017, 19, 26056–26064. [Google Scholar] [CrossRef]
- Czornyj, G.; Wunderlich, B. Preparation and study of separated single crystals. J. Polym. Sci. Polym. Phys. 1977, 15, 1905–1912. [Google Scholar] [CrossRef]
- Wunderlich, B.; Shu, H.-C. The crystallization and melting of selenium. J. Cryst. Growth 1980, 48, 227–239. [Google Scholar] [CrossRef]
- Maffezzoli, A.M.; Kenny, J.M.; Nicolais, L. Welding of PEEK/Carbon fiber composite laminates. SAMPE J. 1989, 25, 35–39. [Google Scholar]
- Lippits, D.R.; Rastogi, S.; Höhne, G.W.H. Melting kinetics in polymers. Phys. Rev. Lett. 2006, 96, 218303. [Google Scholar] [CrossRef]
- Cubeta, U.; Bhattacharya, D.; Sadtchenko, V. Melting of superheated molecular crystals. J. Chem. Phys. 2017, 147, 014505. [Google Scholar] [CrossRef]
- Toda, A.; Hikosaka, M.; Yamada, K. Superheating of the melting kinetics in polymer crystals: A possible nucleation mechanism. Polymer 2002, 43, 1667–1679. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Yancey, B.; Walker, K. Nucleation driven kinetics of poly (ethylene terephthalate) melting. Macromol. Chem. Phys. 2013, 214, 2562–2566. [Google Scholar] [CrossRef]
- Cormia, R.L.; Mackenzie, J.D.; Turnbull, D. Kinetics of melting and crystallization of phosphorus pentoxide. J. Appl. Phys. 1963, 34, 2239–2244. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Yancey, B.; Walker, K. Polymer melting kinetics appears to be driven by heterogeneous nucleation. Macromol. Chem. Phys. 2014, 215, 205–209. [Google Scholar] [CrossRef]
- Saini, D.R.; Shenoy, A.V. A new method for the determination of flow activation energy of polymer melt. J. Macromol. Sci. B 1983, 22, 437–449. [Google Scholar] [CrossRef]
- Ramkumar, D.H.S.; Bhattacharya, M. Steady shear and dynamic properties of biodegradabIe polyesters. Polym. Eng. Sci. 1998, 38, 1426–1435. [Google Scholar] [CrossRef]
- Bockris, J.O.; Mackenzie, J.D.; Kitchen, J.A. Viscous flow in silica and binary liquid silicates. Trans. Faraday Soc. 1955, 51, 1734–1748. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Thermodynamics and the velocity of irreversible processes. Part II. Trans. Faraday Soc. 1937, 33, 1198–1212. [Google Scholar] [CrossRef]
- Pace, R.J.; Datyner, A. Statistical mechanical model of diffusion of complex penetrants in polymers. II. Applications. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1693–1708. [Google Scholar] [CrossRef]
- Pace, R.J.; Datyner, A. Statistical mechanical model for diffusion of simple penetrants in polymers. III. Applications—Vinyl and related polymers. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 465–476. [Google Scholar] [CrossRef]
- Illers, K.-H. Die Ermittlung des Schmelzpunktes von Kristallen Polymeren Mittels Warmeflusskalorimetrie (DSC). Eur. Pol. J. 1974, 10, 911–916. [Google Scholar] [CrossRef]
- Drebushchak, V.A. Thermophysical theory of DSC melting peak. J. Therm. Anal. Calorim. 2012, 109, 545–553. [Google Scholar] [CrossRef]
- Toda, A. Heating rate dependence of melting peak temperature examined by DSC of heat flux type. J. Therm. Anal. Calorim. 2016, 123, 1795–1808. [Google Scholar] [CrossRef]
- Brown, M.E. Introduction to Thermal Analysis, 2nd ed.; Kluwer: Dodrecht, The Netherlands, 2001. [Google Scholar]
- Höhne, G.W.H.; Hemminger, W.F.; Flammersheim, H.J. Differential Scanning Calorimetry, 2nd ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Chen, K.; Baker, A.N.; Vyazovkin, S. Concentration effect on temperature dependence of gelation rate in aqueous solutions of methylcellulose. Macromol. Chem. Phys. 2009, 210, 211–216. [Google Scholar] [CrossRef]
- Farasat, R.; Vyazovkin, S. Coil-to-globule transition of poly (N-isopropylacrylamide) in aqueous solution: Kinetics in bulk and nanopores. Macromol. Chem. Phys. 2014, 215, 2112–2118. [Google Scholar] [CrossRef]
- Farasat, R.; Vyazovkin, S. Nanoconfined solid-solid transitions: Attempt to separate the size and surface effects. J. Phys. Chem. C 2015, 119, 9627–9636. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kinetic effects of pressure on decomposition of solids. Int. Rev. Phys. Chem. 2020, 39, 35–66. [Google Scholar] [CrossRef]





© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyazovkin, S. Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers. Polymers 2020, 12, 1070. https://doi.org/10.3390/polym12051070
Vyazovkin S. Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers. Polymers. 2020; 12(5):1070. https://doi.org/10.3390/polym12051070
Chicago/Turabian StyleVyazovkin, Sergey. 2020. "Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers" Polymers 12, no. 5: 1070. https://doi.org/10.3390/polym12051070
APA StyleVyazovkin, S. (2020). Activation Energies and Temperature Dependencies of the Rates of Crystallization and Melting of Polymers. Polymers, 12(5), 1070. https://doi.org/10.3390/polym12051070




