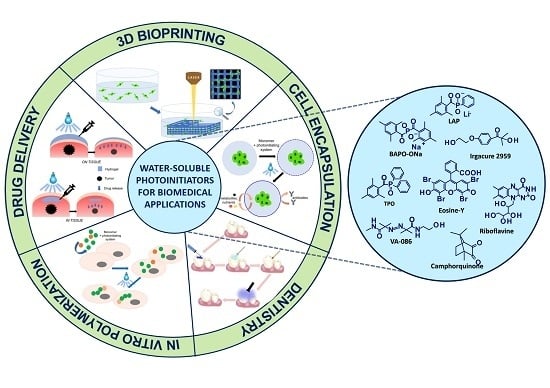
Water-Soluble Photoinitiators in Biomedical Applications
Abstract
:1. Introduction
2. The Dynamics of the Development of Water-Soluble Photoinitiators
3. Types of Photoinitiators for Photopolymerization Processes
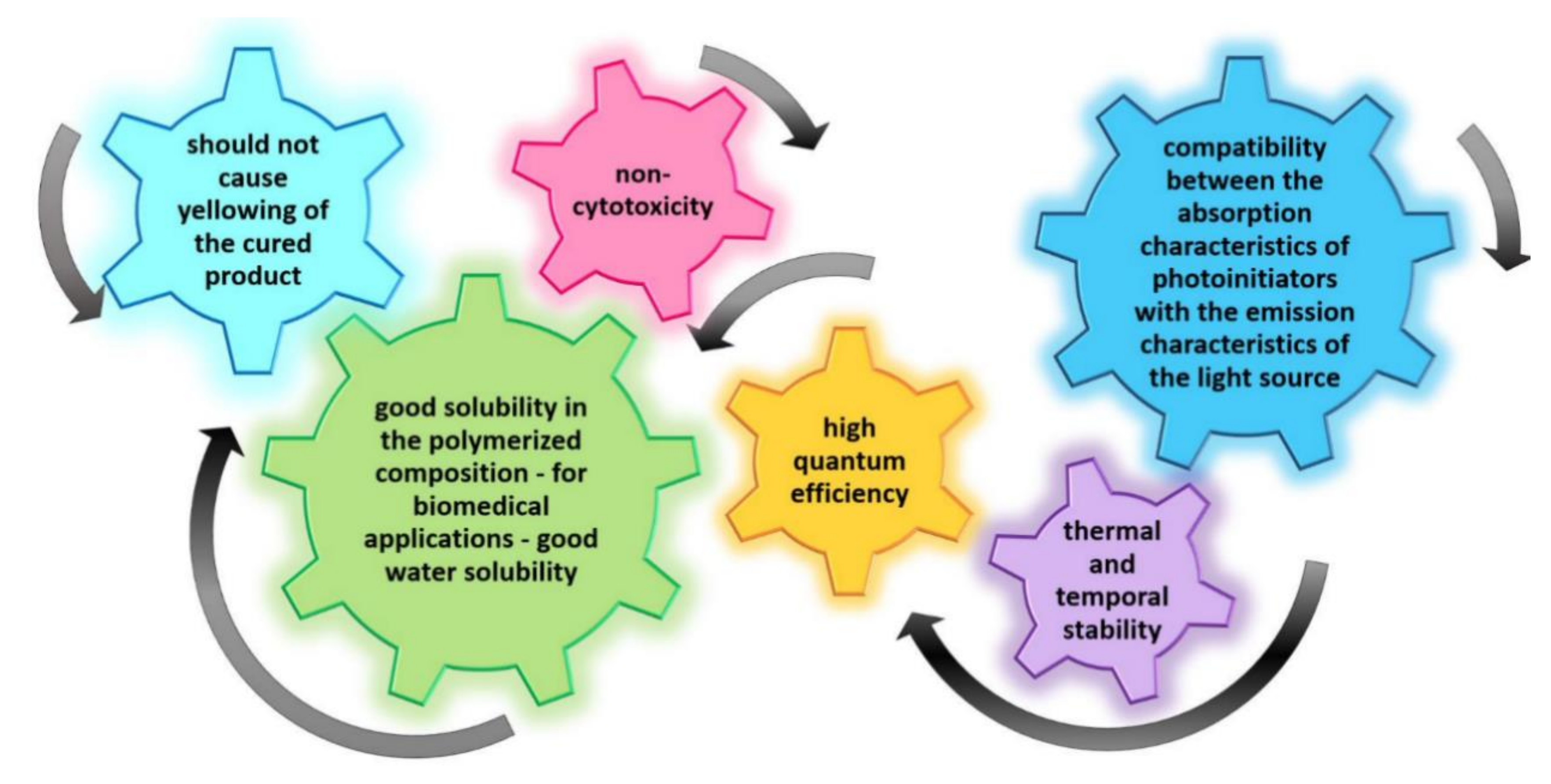
- compatibility between the absorption characteristics of photoinitiators and the emission characteristics of the light source,
- high quantum efficiency,
- good solubility in the polymerized composition – for biomedical applications – and good water solubility,
- non-cytotoxicity,
- should not cause yellowing of the cured product, and
- thermal and temporal stability.
- where the photosensitiser acts as an electron donor, the transfer of the electron to the co-initiator creates a cationic radical of the sensitizer particle and an anionic radical of the co-initiator;
- where the photosensitiser is an electron acceptor, it undergoes photoreduction, and the electron transfer products are the anionic radical formed on the sensitizer molecule and the cationic radical formed on the co-initiator.
4. Type I Initiating System for Free-Radical Photopolymerization
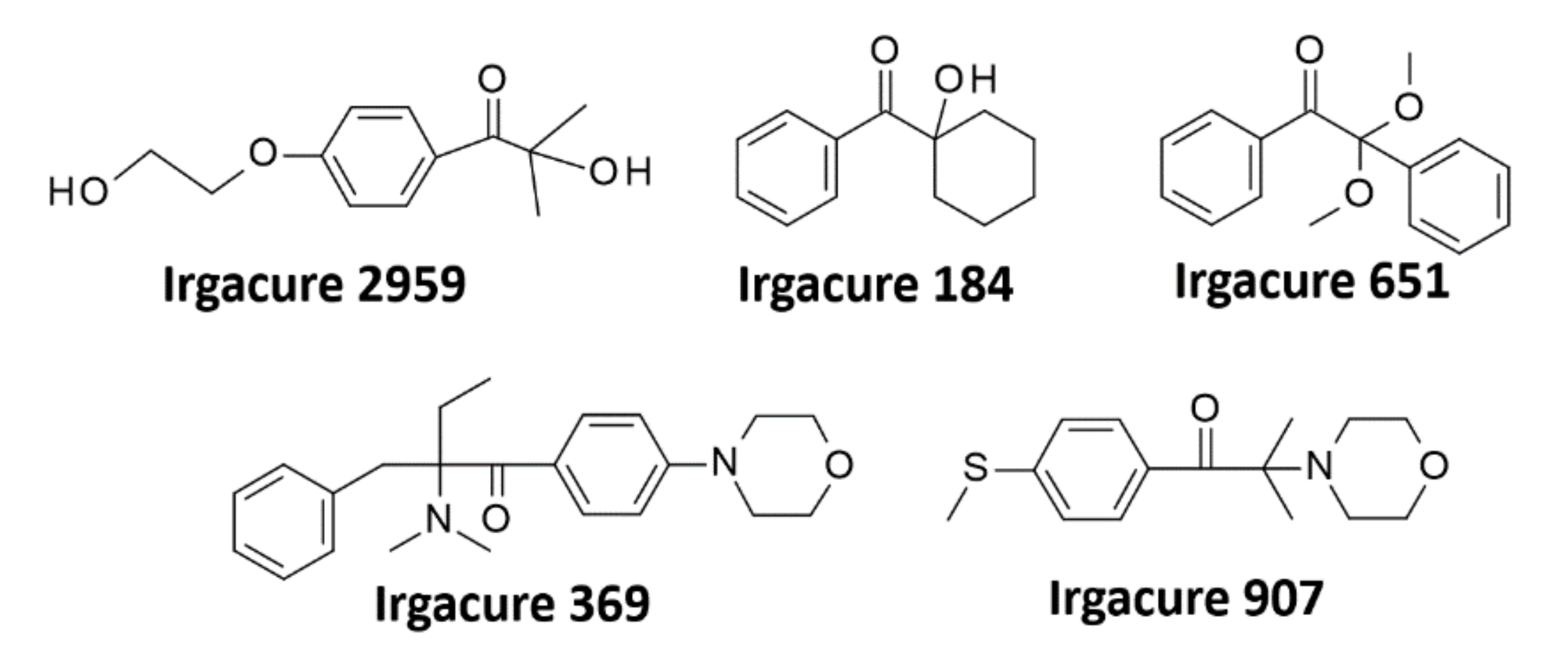
4.1. α-hydroxyketones and Their Derivatives
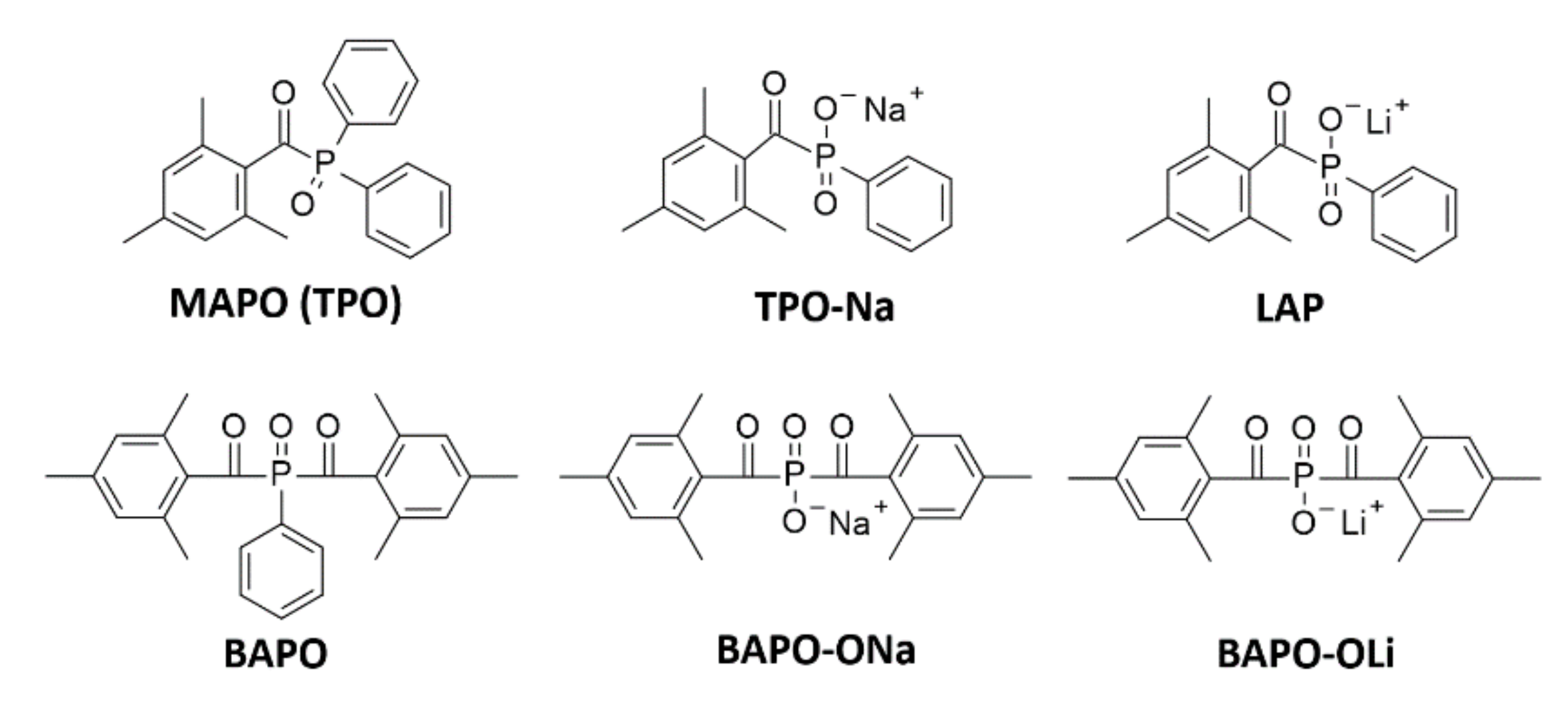
4.2. Phosphine Derivatives
4.3. Azo-Initiators
5. Type II Initiating System for Free-Radical Photopolymerization
5.1. Eosin-Y
5.2. Riboflavin (B2)
5.3. Camphorquinone and Its Modifications
6. Two-Photon Photoinitiators (2PP) for Free-Radical Photopolymerizations in Biomedical Applications
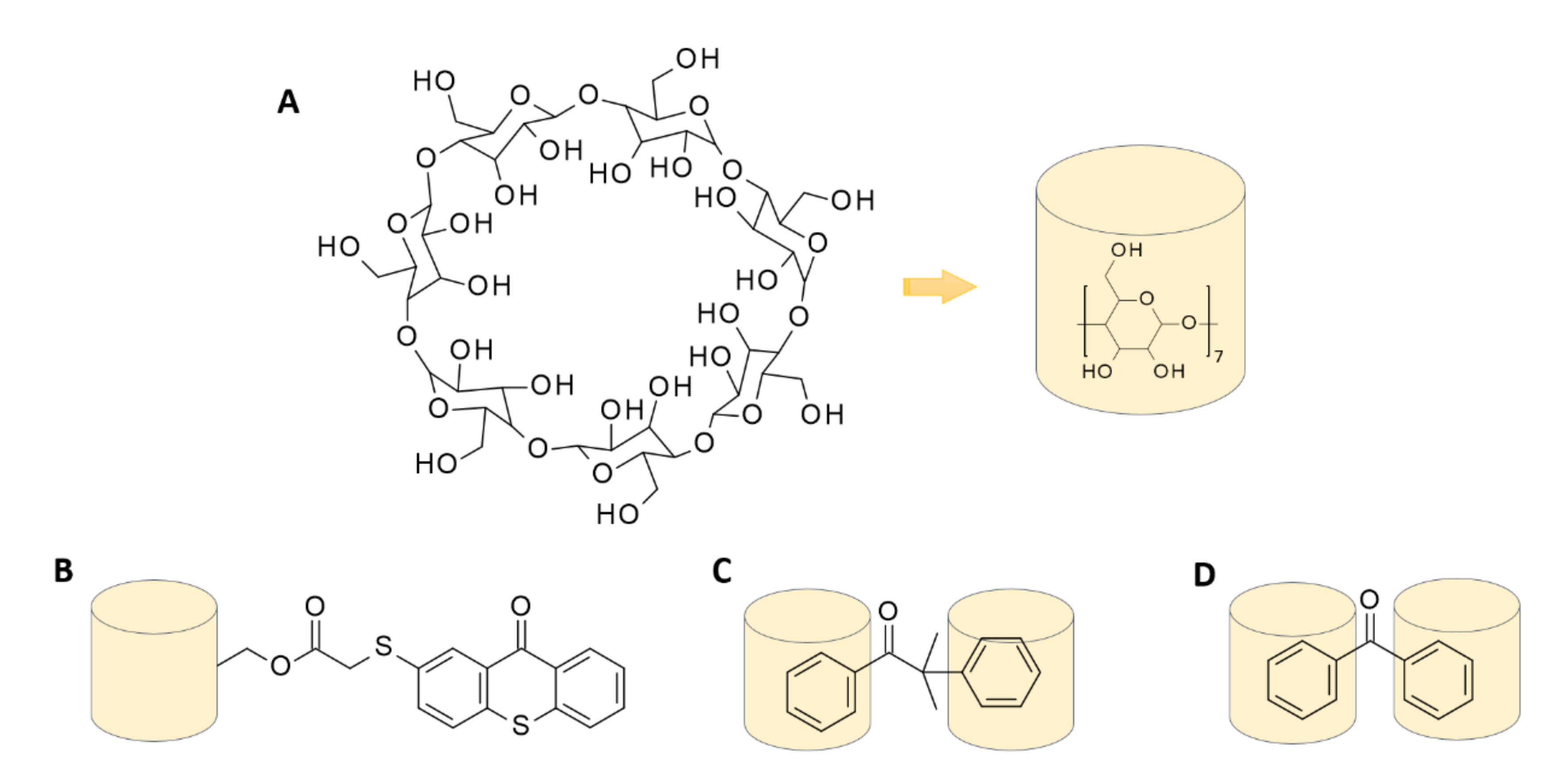
7. Inclusion Complexes of the Host-Guest Type: Photoinitiator—Cyclodextrin
8. Multi-Component Water-Soluble Photo Initiating Systems
9. Fields of Application for Water-Soluble Photoinitiators
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The power of light in polymer science: Photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Kostrzewska, K.; Ortyl, J.; Dobosz, R.; Kabatc, J. Squarylium dye and onium salts as highly sensitive photoradical generators for blue light. Polym. Chem. 2017, 8, 3464–3474. [Google Scholar] [CrossRef]
- Glöckner, P.; Struck, S.; Jung, T.; Studer, K. Radiation Curing: Coatings and Printing Inks; Technical Basics, Applications and Trouble Shooting; Vincentz Network: Hannover, Germany, 2008; ISBN 9783866309074. [Google Scholar]
- Green, W.A. Industrial Photoinitiators—A technical Guide; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439827451. [Google Scholar]
- Czech, Z.; Kowalczyk, A.; Ortyl, J.; Swiderska, J. Acrylic pressure-sensitive adhesives containing SiO2 nanoparticles. Polish J. Chem. Technol. 2013, 15, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.T.; Zhou, C.; Zhao, C.; Lee, R. Photopolymer-based waveguide holograms for optoelectronic interconnect applications. In Proceedings of the Polymers in Optics: Physics, Chemistry, and Applications: A Critical Review; SPIE: Bellingham, WA, USA, 1996; Volume 10285, p. 1028504. [Google Scholar]
- Liska, R.; Schuster, M.; Inführ, R.; Turecek, C.; Fritscher, C.; Seidl, B.; Schmidt, V.; Kuna, L.; Haase, A.; Varga, F.; et al. Photopolymers for rapid prototyping. J. Coatings Technol. Res. 2007, 4, 505–510. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Tomal; Pilch; Chachaj-Brekiesz; Ortyl Development of New High-Performance Biphenyl and Terphenyl Derivatives as Versatile Photoredox Photoinitiating Systems and Their Applications in 3D Printing Photopolymerization Processes. Catalysts 2019, 9, 827. [CrossRef] [Green Version]
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol-ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC Adv. 2020, 10, 7509–7522. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts 2020, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, e1706344. [Google Scholar] [CrossRef]
- Baroli, B. Photopolymerization of biomaterials: Issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. J. Chem. Technol. Biotechnol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Fisher, J.P.; Dean, D.; Engel, P.S.; Mikos, A.G. Photoinitiated Polymerization of Biomaterials. Annu. Rev. Mater. Res. 2001, 31, 171–181. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Restor. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Atai, M.; Nourbakhsh, M.S. Photopolymerization of dental resin as restorative material using an argon laser. Lasers Med. Sci. 2008, 23, 399–406. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Pietra, A.D.; Rengo, S.; Nicolais, L.; Valletta, G. Photopolymerization of dental composite matrices. Biomaterials 1994, 15, 1221–1228. [Google Scholar] [CrossRef]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization kinetics of methacrylate dental resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef]
- Censi, R.; Schuurman, W.; Malda, J.; di Dato, G.; Burgisser, P.E.; Dhert, W.J.A.; van Nostrum, C.F.; di Martino, P.; Vermonden, T.; Hennink, W.E. A Printable Photopolymerizable Thermosensitive p(HPMAm-lactate)-PEG Hydrogel for Tissue Engineering. Adv. Funct. Mater. 2011, 21, 1833–1842. [Google Scholar] [CrossRef]
- Gonen-Wadmany, M.; Oss-Ronen, L.; Seliktar, D. Protein-polymer conjugates for forming photopolymerizable biomimetic hydrogels for tissue engineering. Biomaterials 2007, 28, 3876–3886. [Google Scholar] [CrossRef]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Quick, D.J.; Anseth, K.S. Gene Delivery in Tissue Engineering: A Photopolymer Platform to Coencapsulate Cells and Plasmid DNA. Pharm. Res. 2003, 20, 1730–1737. [Google Scholar] [CrossRef]
- Herr, A.E.; Singh, A.K. Photopolymerized cross-linked polycrylamide gels for on-chip protein sizing. Anal. Chem. 2004, 76, 4727–4733. [Google Scholar] [CrossRef]
- Cao, Q.; Heil, T.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Visible-light induced emulsion photopolymerization with carbon nitride as a stabilizer and photoinitiator. Polym. Chem. 2019, 10, 5315–5323. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.F.; Bártolo, P.J. Photopolymerizable hydrogels in regenerative medicine and drug delivery. In Hot Topics in Biomaterials; Future Medicine Ltd.: London, UK, 2014; pp. 6–28. ISBN 9781909453708. [Google Scholar]
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236. [Google Scholar] [CrossRef]
- Elisseeff, J.; Anseth, K.; Sims, D.; Mcintosh, W.; Randolph, M.; Langer, R. Transdermal photopolymerization for minimally invasive implantation. Proc. Natl. Acad. Sci. USA 1999, 96, 3104–3107. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Anseth, K.S. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J. Control. Release 1999, 57, 291–300. [Google Scholar] [CrossRef]
- Ye, Q.; Park, J.; Topp, E.; Spencer, P. Effect of Photo-Initiators on the In Vitro Performance of a Dentin Adhesive Exposed to Simulated Oral Environment. Dent. Mater. 2009, 25, 452. [Google Scholar] [CrossRef] [Green Version]
- Kunio, I.; Takeshi, E. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent. Mater. J. 2010, 29, 481–501. [Google Scholar]
- Salgado, V.E.; Cavassoni, D.; Gonçalves, A.P.R.; Pfeifer, C.; Moraes, R.R.; Schneider, L.F. Photoinitiator system and water effects on C=C conversion and solubility of experimental etch-and-rinse dental adhesives. Int. J. Adhes. Adhes. 2017, 72, 6–9. [Google Scholar] [CrossRef]
- Albuquerque, P.P.A.C.; Moreira, A.D.L.; Moraes, R.R.; Cavalcante, L.M.; Schneider, L.F.J. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J. Dent. 2013, 41, e67–e72. [Google Scholar] [CrossRef]
- Clapper, J.D.; Skeie, J.M.; Mullins, R.F.; Guymon, C.A. Development and characterization of photopolymerizable biodegradable materials from PEG-PLA-PEG block macromonomers. Polymer 2007, 48, 6554–6564. [Google Scholar] [CrossRef]
- Wang, K.; Lu, J.; Yin, R.; Chen, L.; Du, S.; Jiang, Y.; Yu, Q. Preparation and properties of cyclic acetal based biodegradable gel by thiol-ene photopolymerization. Mater. Sci. Eng. C 2013, 33, 1261–1266. [Google Scholar] [CrossRef]
- Kim, B.S.; Hrkach, J.S.; Langer, R. Biodegradable photo-crosslinked poly(ether-ester) networks for lubricious coatings. Biomaterials 2000, 21, 259–265. [Google Scholar] [CrossRef]
- Claeyssens, F.; Hasan, E.A.; Gaidukeviciute, A.; Achilleos, D.S.; Ranella, A.; Reinhardt, C.; Ovsianikov, A.; Shizhou, X.; Fotakis, C.; Vamvakaki, M.; et al. Three-dimensional biodegradable structures fabricated by two-photon polymerization. Langmuir 2009, 25, 3219–3223. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhou, W.; Zhong, Z.; Wouters, M.; Feijen, J. Rapidly in situ forming biodegradable robust hydrogels by combining stereocomplexation and photopolymerization. J. Am. Chem. Soc. 2007, 129, 9918–9926. [Google Scholar] [CrossRef]
- Elisseeff, J.; McIntosh, W.; Fu, K.; Blunk, T.; Langer, R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J. Orthop. Res. 2001, 19, 1098–1104. [Google Scholar] [CrossRef]
- Mann, B.K.; Gobin, A.S.; Tsai, A.T.; Schmedlen, R.H.; West, J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials 2001, 22, 3045–3051. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nicodemus, G.D.; Villanueva, I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharm. Res. 2008, 25, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Schweitzer, K.; Habib, M.; Gepstein, L.; Seliktar, D. A photopolymerizable hydrogel for 3-D culture of human embryonic stem cell-derived cardiomyocytes and rat neonatal cardiac cells. J. Mol. Cell. Cardiol. 2009, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Y.; An, B.; Zhang, W.; Sun, M.; Fang, Z.; Yuan, W.; Khan, M. Fabrication of PU/PEGMA crosslinked hybrid scaffolds by in situ UV photopolymerization favoring human endothelial cells growth for vascular tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Tytgat, L.; Baudis, S.; Ottevaere, H.; Liska, R.; Thienpont, H.; Dubruel, P.; Van Vlierberghe, S. Photopolymerizable Materials for Cell Encapsulation. In 3D Printing and Biofabrication; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–43. [Google Scholar]
- Younes, H.M. Photopolymerization of Polymeric Composites in Drug Delivery, Tissue Engineering, and Other Biomedical Applications. In Lecture Notes in Bioengineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 271–297. [Google Scholar]
- Zhong, C.; Wu, J.; Reinhart-King, C.A.; Chu, C.C. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan-polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater. 2010, 6, 3908–3918. [Google Scholar] [CrossRef]
- Zhou, D.; Ito, Y. Visible light-curable polymers for biomedical applications. Sci. China Chem. 2014, 57, 510–521. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Comparison of covalently and physically cross-linked polyethylene glycol-based hydrogels for the prevention of postoperative adhesions in a rat model. Biomaterials 1995, 16, 1153–1156. [Google Scholar] [CrossRef]
- De Paepe, I.; Declercq, H.; Cornelissen, M.; Schacht, E. Novel hydrogels based on methacrylate-modified agarose. Polym. Int. 2002, 51, 867–870. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Van Vlierberghe, S.; Salamon, A.; Peters, K.; Thienpont, H.; Dubruel, P. Photo-crosslinkable biopolymers targeting stem cell adhesion and proliferation: the case study of gelatin and starch-based IPNs. J. Mater. Sci. Mater. Med. 2015, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization; Elsevier: Oxford, UK, 2005; ISBN 9780080442884. [Google Scholar]
- Hyun, J.; Chilkoti, A. Surface-initiated free radical polymerization of polystyrene micropatterns on a self-assembled monolayer on gold. Macromolecules 2001, 34, 5644–5652. [Google Scholar] [CrossRef]
- Heller, C.; Schwentenwein, M.; Russmüller, G.; Koch, T.; Moser, D.; Schopper, C.; Varga, F.; Stampfl, J.; Liska, R. Vinylcarbonates and vinylcarbamates: Biocompatible monomers for radical photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 650–661. [Google Scholar] [CrossRef]
- Uygun, M.; Kahveci, M.U.; Odaci, D.; Timur, S.; Yagci, Y. Antibacterial Acrylamide Hydrogels Containing Silver Nanoparticles by Simultaneous Photoinduced Free Radical Polymerization and Electron Transfer Processes. Macromol. Chem. Phys. 2009, 210, 1867–1875. [Google Scholar] [CrossRef]
- Ward, J.H.; Peppas, N.A. Preparation of controlled release systems by free-radical UV polymerizations in the presence of a drug. J. Control. Release 2001, 71, 183–192. [Google Scholar] [CrossRef]
- Eguiburu, J.L.; Fernandez-Berridi, M.J.; San Román, J. Graft copolymers for biomedical applications prepared by free radical polymerization of poly(L-lactide) macromonomers with vinyl and acrylic monomers. Polymer 1996, 37, 3615–3622. [Google Scholar] [CrossRef]
- Ortyl, J. Chapter 3: Cationic Photoinitiators. In RSC Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Ficek, B.A. The potential of cationic photopolymerization’s long lived active centers. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2008. [Google Scholar]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Huang, R.; Ficek, B.A.; Glover, S.O.; Scranton, A.B. Effect of Water in Cationic Photopolymerizations: Reversible Inhibition. Tech. Pap. 2007, 30–35. [Google Scholar]
- Ranaweera, R.A.A.U.; Schuman, T.P.; Wang, R.; Miller, B.D.; Kilway, K.V. Effect of moisture on cationic polymerization of silicone epoxy monomers. J. Appl. Polym. Sci. 2015, 132, 41831. [Google Scholar] [CrossRef]
- Barker, P.; Guthrie, J.T.; Davis, M.J.; Godfrey, A.A.; Green, P.N. Sensitized photoinitiated grafting of N-vinyl-2-prrolidone (NVP) to woolen substrates. J. Appl. Polym. Sci. 1981, 26, 521–527. [Google Scholar] [CrossRef]
- Ferreira, P.; Coelho, J.F.J.; Almeida, J.F.; Gill, M.H. Photocrosslinkable Polymers for Biomedical Applications. In Biomedical Engineering—Frontiers and Challenges; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Dumur, F. Recent advances on carbazole-based photoinitiators of polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Lougnot, D.J.; Turck, C.; Fouassier, J.P. Water-Soluble Polymerization Initiators Based on the Thioxanthone Structure: A Spectroscopic and Laser Photolysis Study. Macromolecules 1989, 22, 108–116. [Google Scholar] [CrossRef]
- Allen, N.S.; Howells, E.M.; Lam, E.; Catalina, F.; Green, P.N.; Green, W.A.; Chen, W. Photopolymerisation and flash photolysis of a water soluble benzophenone photoinitiator: Influence of tertiary amine. Eur. Polym. J. 1988, 24, 591–593. [Google Scholar] [CrossRef]
- Eibel, A.; Fast, D.E.; Gescheidt, G. Choosing the ideal photoinitiator for free radical photopolymerizations: Predictions based on simulations using established data. Polym. Chem. 2018, 9, 5107–5115. [Google Scholar] [CrossRef] [Green Version]
- Ciuciu, A.I.; Cywiński, P.J. Two-photon polymerization of hydrogels-versatile solutions to fabricate well-defined 3D structures. RSC Adv. 2014, 4, 45504–45516. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Xu, C.; Wu, X.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable cinnamoyl dyes for radical visible photoinitiators. Dye. Pigment. 2020, 108350. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Weiß, T.; Hildebrand, G.; Schade, R.; Liefeith, K. Two-photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application. Eng. Life Sci. 2009, 9, 384–390. [Google Scholar]
- Tromayer, M.; Dobos, A.; Gruber, P.; Ajami, A.; Dedic, R.; Ovsianikov, A.; Liska, R. A biocompatible diazosulfonate initiator for direct encapsulation of human stem cells: Via two-photon polymerization. Polym. Chem. 2018, 9, 3108–3117. [Google Scholar] [CrossRef]
- Woo, H.Y.; Hong, J.W.; Liu, B.; Mikhailovsky, A.; Korystov, D.; Bazan, G.C. Water-soluble [2.2]paracyclophane chromophores with large two-photon action cross sections. J. Am. Chem. Soc. 2005, 127, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, B.A.; Brott, L.L.; Clarson, S.J.; Dillard, A.G.; Bhatt, J.C.; Kannan, R.; Yuan, L.; He, G.S.; Prasad, P.N. Highly Active Two-Photon Dyes: Design, Synthesis, and Characterization toward Application. Chem. Mater. 1998, 10, 1863–1874. [Google Scholar] [CrossRef]
- Wrzyszczyński, A. Two-photon initiators of polymerization. Polimery 2010, 55, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Demet, K.B.; Temel, G.; Aydin, M.; Arsu, N. Thioxanthone based water-soluble photoinitiators for acrylamide photopolymerization. Eur. Polym. J. 2010, 46, 1374–1379. [Google Scholar]
- Liska, R. Photoinitiators with functional groups. V. New water-soluble photoinitiators containing carbohydrate residues and copolymerizable derivatives thereof. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1504–1518. [Google Scholar] [CrossRef]
- Temel, G.; Arsu, N. One-pot synthesis of water-soluble polymeric photoinitiator via thioxanthonation and sulfonation process. J. Photochem. Photobiol. A Chem. 2009, 202, 63–66. [Google Scholar] [CrossRef]
- Guo, R.; Gao, Y.; Wu, M.; Wang, H. Aliphatic ketones and aldehydes as water-soluble photoinitiators for the photopolymerization of methacrylic acid. Polymer 2013, 54, 4940–4947. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, L.; Xiong, Y.; Wu, Q.; Tang, H. A facile method to prepare a polyethylene glycol modified polysilane as a waterborne photoinitiator. J. Photochem. Photobiol. A Chem. 2015, 299, 9–17. [Google Scholar] [CrossRef]
- Lougnot, D.J.; Fouassier, J.P. Comparative reactivity of water soluble photoinitiators as viewed in terms of excited states processes. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 1021–1033. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Burr, D.; Wieder, F. Water-soluble photoinitiators: Primary processes in hydroxy alkyl phenyl ketones. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1319–1327. [Google Scholar] [CrossRef]
- Knaus, S.; Gruber, H.F. Photoinitiators with functional groups. III. Water-soluble photoinitiators containing carbohydrate residues. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 929–939. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng. Part C Methods 2011, 17, 173–179. [Google Scholar] [CrossRef]
- Ma, Z.; Niu, X.; Xu, Z.; Guo, J. Synthesis of novel macrophotoinitiator for the photopolymerization of acrylate. J. Appl. Polym. Sci. 2014, 131, 40352. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, S.; Gao, Y.; Sun, F. Regulating photochemical behavior and property of imidazolium-based water soluble polysiloxane macromolecular photoinitiators by anions. J. Photochem. Photobiol. A Chem. 2018, 364, 363–372. [Google Scholar] [CrossRef]
- Goering, R.V.; Pattee, P.A. Mutants of Staphylococcus aureus with increased sensitivity to ultraviolet radiation. J. Bacteriol. 1971, 106, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Kappes, U.P.; Luo, D.; Potter, M.; Schulmeister, K.; Rünger, T.M. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J. Invest. Dermatol. 2006, 126, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, K.; Takada, T. Synthesis and characteristics of nanocomposite gels prepared by in situ photopolymerization in an aqueous system. Macromolecules 2010, 43, 4294–4299. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Wang, D.Y.; Venkatraman, S.; Seliktar, D. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012, 8, 1838–1848. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Andre, C.; Castanheira, I.; Cruz, J.M.; Pastorelli, S.; Simoneau, C.; Paseiro-Losada, P. Study of the migration of photoinitiators used in printed food-packaging materials into food simulants. J. Agric. Food Chem. 2009, 57, 9516–9523. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Marin, M.C.; Edge, M.; Davies, D.W.; Garrett, J.; Jones, F.; Navaratnam, S.; Parsons, B.J. Photochemistry and photoinduced chemical crosslinking activity of type I & II co-reactive photoinitiators in acrylated prepolymers. J. Photochem. Photobiol. A Chem. 1999, 126, 135–149. [Google Scholar]
- Bahney, C.S.; Lujan, T.J.; Hsu, C.W.; Bottlang, M.; West, J.L.; Johnstone, B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cells Mater. 2011, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; Zellner, L.; Oliveira, C.; Engelse, M.; Koning, E.d.; Mano, J.K.; Apeldoorn, J.; Moroni, L. Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization. J. Mater. Sci. Mater. Med. 2017, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yeo, D.C.; Wiraja, C.; Tey, H.L.; Mrksich, M.; Xu, C. Peptide delivery with poly(ethylene glycol) diacrylate microneedles through swelling effect. Bioeng. Transl. Med. 2017, 2, 258–267. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Machen, A.J.; Anseth, K.S. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials 2009, 30, 6048–6054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buwalda, S.J.; Perez, L.B.; Teixeira, S.; Calucci, L.; Forte, C.; Feijen, J.; Dijkstra, P.J. Self-assembly and photo-cross-linking of eight-armed PEG-PTMC star block copolymers. Biomacromolecules 2011, 12, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gao, Y.; Jiang, S.; Nie, J.; Sun, F. Design of photoinitiator-functionalized hydrophilic nanogels with uniform size and excellent biocompatibility. Polym. Chem. 2019, 10, 2812–2821. [Google Scholar] [CrossRef]
- Assmann, A.; Vegh, A.; Ghasemi-Rad, M.; Bagherifard, S.; Cheng, G.; Sani, E.S.; Ruiz-Esparza, G.U.; Noshadi, I.; Lassaletta, A.D.; Gangadharan, S.; et al. A highly adhesive and naturally derived sealant. Biomaterials 2017, 140, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X.; et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097–4105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhang, Y.; Sun, W.; Wang, C.; Ranson, D.; Ye, Y.; Weng, Y.; Gu, Z. Internalized compartments encapsulated nanogels for targeted drug delivery. Nanoscale 2016, 8, 9178–9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Omer, A.M.; Abu-Serie, M.M.; Khattab, S.N.; Ahmed, H.M.; Elbardan, A.A. Photopolymerized PVA-g-GMA Hydrogels for Biomedical Applications: Factors Affecting Hydrogel Formation and Bioevaluation Tests. Arab. J. Sci. Eng. 2018, 43, 3565–3575. [Google Scholar] [CrossRef]
- Chen, R.T.; Marchesan, S.; Evans, R.A.; Styan, K.E.; Such, G.K.; Postma, A.; McLean, K.M.; Muir, B.W.; Caruso, F. Photoinitiated alkyne-azide click and radical cross-linking reactions for the patterning of PEG hydrogels. Biomacromolecules 2012, 13, 889–895. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mason, M.N.; Hinman, A.D.; Thorne, K.; Anseth, K.S. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J. Control. Release 2002, 83, 53–63. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005, 24, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.S.; Shah, D.N.; Walker, G.; Leinwand, L.A.; Anseth, K.S. Designing scaffolds for valvular interstitial cells: Cell adhesion and function on naturally derived materials. J. Biomed. Mater. Res. Part A 2004, 71, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Durand, K.L.; Anseth, K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. Part A 2003, 67, 1430–1436. [Google Scholar] [CrossRef]
- Bryant, S.J.; Chowdhury, T.T.; Lee, D.A.; Bader, D.L.; Anseth, K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 2004, 32, 407–417. [Google Scholar] [CrossRef]
- Masters, K.S.; Shah, D.N.; Leinwand, L.A.; Anseth, K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 2005, 26, 2517–2525. [Google Scholar] [CrossRef]
- Alhadlaq, A.; Tang, M.; Mao, J.J. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: Implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005, 11, 556–566. [Google Scholar] [CrossRef]
- Liska, R.; Knaus, S.; Gruber, H.; Wendrinsky, J. Carbohydrate modified photoinitiators. JOCCA Surf. Coatings Int. 2000, 83, 297–303. [Google Scholar] [CrossRef]
- Kojima, K.; Ito, M.; Morishita, H.; Hayashi, N. A Novel Water-Soluble Photoinitiator for the Acrylic Photopolymerization Type Resist System. Chem. Mater. 1998, 10, 3429–3433. [Google Scholar] [CrossRef]
- Atry, F.; Rentchler, E.; Alkmin, S.; Dai, B.; Li, B.; Eliceiri, K.W.; Campagnola, P.J. Parallel multiphoton excited fabrication of tissue engineering scaffolds using a diffractive optical element. Opt. Express 2020, 28, 2744. [Google Scholar] [CrossRef] [PubMed]
- Serien, D.; Kawano, H.; Miyawaki, A.; Midorikawa, K.; Sugioka, K. Femtosecond Laser Direct Write Integration of Multi-Protein Patterns and 3D Microstructures into 3D Glass Microfluidic Devices. Appl. Sci. 2018, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Serien, D.; Sugioka, K. Three-Dimensional Printing of Pure Proteinaceous Microstructures by Femtosecond Laser Multiphoton Cross-Linking. ACS Biomater. Sci. Eng. 2020, 6, 1279–1287. [Google Scholar] [CrossRef]
- Ullrich, G.; Ganster, B.; Salz, U.; Moszner, N.; Liska, R. Photoinitiators with functional groups. IX. Hydrophilic bisacylphosphine oxides for acidic aqueous formulations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1686–1700. [Google Scholar] [CrossRef]
- Sodré, C.S.; Albuquerque, P.P.A.C.; Isolan, C.P.; Moraes, R.R.; Schneider, L.F. Relative photon absorption determination and the influence of photoinitiator system and water content on C=C conversion, water sorption/solubility of experimental self-etch adhesives. Int. J. Adhes. Adhes. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Majima, T.; Schnabel, W.; Weber, W. Phenyl-2,4,6-trimethylbenzoylphosphinates as water-soluble photoinitiators. Generation and reactivity of O∙Ṗ(C6H5)(O−) radical anions. Makromol. Chemie 1991, 192, 2307–2315. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [Green Version]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3d-printed hydrogel nanocomposites. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Calderon, G.A.; Thai, P.; Hsu, C.W.; Grigoryan, B.; Gibson, S.M.; Dickinson, M.E.; Miller, J.S. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels. Biomater. Sci. 2017, 5, 1652–1660. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Lin, C.C. The influence of matrix degradation and functionality on cell survival and morphogenesis in PEG-based hydrogels. Macromol. Biosci. 2013, 13, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Müller, G.; Zalibera, M.; Gescheidt, G.; Rosenthal, A.; Santiso-Quinones, G.; Dietliker, K.; Grützmacher, H. Simple One-Pot Syntheses of Water-Soluble Bis(acyl)phosphane Oxide Photoinitiators and Their Application in Surfactant-Free Emulsion Polymerization. Macromol. Rapid Commun. 2015, 36, 553–557. [Google Scholar] [CrossRef]
- Markovic, M.; Van Hoorick, J.; Hölzl, K.; Tromayer, M.; Gruber, P.; Nürnberger, S.; Dubruel, P.; Van Vlierberghe, S.; Liska, R.; Ovsianikov, A. Hybrid Tissue Engineering Scaffolds by Combination of Three-Dimensional Printing and Cell Photoencapsulation. J. Nanotechnol. Eng. Med. 2015, 6, 0210011–0210017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Stanic, S.; Altun, A.A.; Schwentenwein, M.; Dietliker, K.; Jin, L.; Stampfl, J.; Baudis, S.; Liska, R.; Grützmacher, H. A highly efficient waterborne photoinitiator for visible-light-induced three-dimensional printing of hydrogels. Chem. Commun. 2018, 54, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.A.; Saada, G.; Cooperstein, I.; Larush, L.; Jackman, J.A.; Tabaei, S.R.; Cho, N.J.; Magdassi, S. High-performance 3D printing of hydrogels by water-dispersible photoinitiator nanoparticles. Sci. Adv. 2016, 2, e1501381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Li, S.; Hingorani, H.; Serjouei, A.; Larush, L.; Pawar, A.A.; Goh, W.H.; Sakhaei, A.H.; Hashimoto, M.; Kowsari, K.; et al. Highly stretchable hydrogels for UV curing based high-resolution multimaterial 3D printing. J. Mater. Chem. B 2018, 6, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Occhetta, P.; Sadr, N.; Piraino, F.; Redaelli, A.; Moretti, M.; Rasponi, M. Fabrication of 3D cell-laden hydrogel microstructures through photo-mold patterning. Biofabrication 2013, 5, 035002. [Google Scholar] [CrossRef] [Green Version]
- Occhetta, P.; Visone, R.; Russo, L.; Cipolla, L.; Moretti, M.; Rasponi, M. VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Res. Part A 2015, 103, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jin, X.; Dai, R.; Holzman, J.F.; Kim, K. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 2016, 6, 21099–21104. [Google Scholar] [CrossRef] [Green Version]
- Han, W.T.; Jang, T.; Chen, S.; Chong, L.S.H.; Jung, H.D.; Song, J. Improved cell viability for large-scale biofabrication with photo-crosslinkable hydrogel systems through a dual-photoinitiator approach. Biomater. Sci. 2020, 8, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Encinas, M.V.; Rufs, A.M.; Bertolotti, S.G.; Previtali, C.M. Xanthene dyes/amine as photoinitiators of radical polymerization: A comparative and photochemical study in aqueous medium. Polymer 2009, 50, 2762–2767. [Google Scholar] [CrossRef]
- Neckers, D.C.; Hassoon, S.; Klimtchuk, E. Photochemistry and photophysics of hydroxyfluorones and xanthenes. J. Photochem. Photobiol. A Chem. 1996, 95, 33–39. [Google Scholar] [CrossRef]
- Cruise, G.M.; Hegre, O.D.; Scharp, D.S.; Hubbell, J.A. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol. Bioeng. 1998, 57, 655–665. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Chesneau, E. Polymérisation induite sous irradiation laser visible, 4. Le système éosine/photoamorceur ultra-violet/amine. Die Makromol. Chemie 1991, 192, 245–260. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials 1993, 14, 1008–1016. [Google Scholar] [CrossRef]
- Valdes-Aguilera, O.; Pathak, C.P.; Shi, J.; Watson, D.; Neckers, D.C. Photopolymerization Studies Using Visible Light Photoinitiators. Macromolecules 1992, 25, 541–547. [Google Scholar] [CrossRef]
- Avens, H.J.; Randle, T.J.; Bowman, C.N. Polymerization behavior and polymer properties of eosin-mediated surface modification reactions. Polymer 2008, 49, 4762–4768. [Google Scholar] [CrossRef] [Green Version]
- Desmangles, A.I.; Jordan, O.; Marquis-Weible, F. Interfacial photopolymerization of beta-cell clusters: approaches to reduce coating thickness using ionic and lipophilic dyes. Biotechnol. Bioeng. 2001, 72, 634–641. [Google Scholar] [CrossRef]
- Elbert, D.L.; Hubbell, J.A. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules 2001, 2, 430–441. [Google Scholar] [CrossRef]
- Nachlas, A.L.Y.; Li, S.; Jha, R.; Singh, M.; Xu, C.; Davis, M.E. Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells. Acta Biomater. 2018, 71, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kizilel, S.; Pérez-Luna, V.H.; Teymour, F. Photopolymerization of poly(ethylene glycol) diacrylate on eosin-functionalized surfaces. Langmuir 2004, 20, 8652–8658. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Seker, F.; Bagci-Onder, T.; Kizilel, S. Gelatin Methacryloyl Hydrogels in the Absence of a Crosslinker as 3D Glioblastoma Multiforme (GBM)-Mimetic Microenvironment. Macromol. Biosci. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Lin, C.C. Visible-light-mediated thiol-ene hydrogelation using eosin-Y as the only photoinitiator. Macromol. Rapid Commun. 2013, 34, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chu, C.C. Visible light induced dextran-methacrylate hydrogel formation using (-)-riboflavin vitamin B2 as a photoinitiator and L-arginine as a co-initiator. Fibers Polym. 2009, 10, 14–20. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Komasa, A.; Koput, J.; Ferreira, L.F.V.; Herance, J.R.; Bourdelande, J.L.; Williams, S.L.; Worrall, D.R.; Insińska-Rak, M.; et al. Spectroscopy and photophysics of flavin related compounds: Riboflavin and iso-(6,7)-riboflavin. Chem. Phys. 2005, 314, 239–247. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Bertolotti, S.G.; Previtali, C.M.; Rufs, A.M.; Encinas, M.V. Riboflavin/triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules 1999, 32, 2920–2924. [Google Scholar] [CrossRef]
- Encinas, M.V.; Rufs, A.M.; Bertolotti, S.; Previtali, C.M. Free radical polymerization photoinitiated by riboflavin/amines. Effect of the amine structure. Macromolecules 2001, 34, 2845–2847. [Google Scholar] [CrossRef]
- Orellana, B.; Rufs, A.M.; Encinas, M.V.; Previtali, C.M.; Bertolotti, S. The photoinitiation mechanism of vinyl polymerization by riboflavin/ triethanolamine in aqueous medium. Macromolecules 1999, 32, 6570–6573. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Gittard, S.D.; Koroleva, A.; Schlie, S.; Gaidukeviciute, A.; Chichkov, B.N.; Narayan, R.J. Two-photon polymerization of polyethylene glycol diacrylate scaffolds with riboflavin and triethanolamine used as a water-soluble photoinitiator. Regen. Med. 2013, 8, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chu, C.C. Fabrication of a biodegradable polysaccharide hydrogel with riboflavin, vitamin B2, as a photo-initiator and L-arginine as coinitiator upon UV irradiation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, R.R.; Kwandou, G.; Spicer, P.T.; Stenzel, M.H. Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol-ene polymerisation of PEG-based hydrogels. Polym. Chem. 2017, 8, 980–984. [Google Scholar] [CrossRef]
- Choe, E.; Huang, R.; Min, D.B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2005, 70. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Chae, K.H.; Rawls, H.R. Development of a new photoinitiation system for dental light-cure composite resins. Dent. Mater. 1999, 15, 120–127. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Menzel, H. HES-HEMA nanocomposite polymer hydrogels: Swelling behavior and characterization. J. Polym. Res. 2012, 19, 1–14. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone-amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Pande, C.S.; Bassi, D.; Jain, N.; Dhar, A.; Glass, J.D. Diketopinic acid—A novel reagent for the modification of arginine. J. Biosci. 1991, 16, 127–135. [Google Scholar] [CrossRef]
- Kamoun, E.A.; El-Betany, A.; Menzel, H.; Chen, X. Influence of photoinitiator concentration and irradiation time on the crosslinking performance of visible-light activated pullulan-HEMA hydrogels. Int. J. Biol. Macromol. 2018, 120, 1884–1892. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mason, M.N.; Anseth, K.S. In situ forming lactic acid based orthopaedic biomaterials: Influence of oligomer chemistry on osteoblast attachment and function. J. Biomater. Sci. Polym. Ed. 2001, 12, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Declercq, H.A.; Gorski, T.L.; Tielens, S.P.; Schacht, E.H.; Cornelissen, M.J. Encapsulation of osteoblast seeded microcarriers into injectable, photopolymerizable three-dimensional scaffolds based on D,L-lactide and ε-caprolactone. Biomacromolecules 2005, 6, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Yao, X.; Ye, Q. Effect of coinitiator and wafer on the photoreactivity and photopolymerization of HEMA/camphoquinone-based reactant mixtures. J. Biomed. Mater. Res. Part A 2006, 78, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application Carboxylated camphorquinone as visible-light photoinitiator. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Magoshi, T. Preparation of vinylated polysaccharides and photofabrication of tubular scaffolds as potential use in tissue engineering. Biomacromolecules 2002, 3, 942–950. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ji-Youn, K.; Nishi, S.; Ueno, H.; Matsuda, T. Development of high-performance stent: gelatinous photogel-coated stent that permits drug delivery and gene transfer. J. Biomed. Mater. Res. 2001, 57, 559–566. [Google Scholar] [CrossRef]
- Okino, H.; Nakayama, Y.; Tanaka, M.; Matsuda, T. In situ hydrogelation of photocurable gelatin and drug release. J. Biomed. Mater. Res. 2002, 59, 233–245. [Google Scholar] [CrossRef]
- Okino, H.; Manabe, T.; Tanaka, M.; Matsuda, T. Novel therapeutic strategy for prevention of malignant tumor recurrence after surgery: Local delivery and prolonged release of adenovirus immobilized in photocured, tissue-adhesive gelatinous matrix. J. Biomed. Mater. Res. Part A 2003, 66, 643–651. [Google Scholar] [CrossRef]
- Almeida, S.M.; Meereis, C.T.W.; Leal, F.B.; Carvalho, R.V.; Boeira, P.O.; Chisini, L.A.; Cuevas-Suárez, C.E.; Lima, G.S.; Piva, E. Evaluation of alternative photoinitiator systems in two-step self-etch adhesive systems. Dent. Mater. 2020, 36, e29–e37. [Google Scholar] [CrossRef]
- Davis, K.A.; Burdick, J.A.; Anseth, K.S. Photoinitiated crosslinked degradable copolymer networks for tissue engineering applications. Biomaterials 2003, 24, 2485–2495. [Google Scholar] [CrossRef]
- Poshusta, A.K.; Anseth, K.S. Photopolymerized biomaterials for application in the temporomandibular joint. Proc. Cells Tissues Organs 2001, 169, 272–278. [Google Scholar]
- Lapchak, P.A. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert Rev. Med. Devices 2012, 9, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-L.; Chen, J.-C.; Wang, W.-J. Near-infrared Absorption Property of Biological Soft Tissue Constituents. J. Med. Biol. Eng. 2001, 21, 7–13. [Google Scholar]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Miwa, M.; Juodkazis, S.; Kawakami, T.; Matsuo, S.; Misawa, H. Femtosecond two-photon stereo-lithography. Appl. Phys. A Mater. Sci. Process. 2001, 73, 561–566. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mironov, V.; Stampf, J.; Liska, R. Engineering 3D cell-culture matrices: Multiphoton processing technologies for biological and tissue engineering applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitz, J.; Plamadeala, C.; Wiesbauer, M.; Freudenthaler, P.; Wollhofen, R.; Jacak, J.; Klar, T.A.; Magnus, B.; Köstner, D.; Weth, A.; et al. Bone-forming cells with pronounced spread into the third dimension in polymer scaffolds fabricated by two-photon polymerization. J. Biomed. Mater. Res. Part A 2017, 105, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Straub, M.; Gu, M. Acrylate-Based Photopolymer for Two-Photon Microfabrication and Photonic Applications. Adv. Funct. Mater. 2005, 15, 209–216. [Google Scholar] [CrossRef]
- You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J. Mater. Chem. B 2018, 6, 2187–2197. [Google Scholar] [CrossRef]
- Li, Z.; Pucher, N.; Cicha, K.; Torgersen, J.; Ligon, S.C.; Ajami, A.; Husinsky, W.; Rosspeintner, A.; Vauthey, E.; Naumov, S.; et al. A straightforward synthesis and structure-activity relationship of highly efficient initiators for two-photon polymerization. Macromolecules 2013, 46, 352–361. [Google Scholar] [CrossRef]
- Jhaveri, S.J.; McMullen, J.D.; Sijbesma, R.; Tan, L.S.; Zipfel, W.; Ober, C.K. Direct three-dimensional microfabrication of hydrogels via two-photon lithography in aqueous solution. Chem. Mater. 2009, 21, 2003–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgersen, J.; Qin, X.-H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for Two-Photon Polymerization: A Toolbox for Mimicking the Extracellular Matrix. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Soman, P.; Meggs, K.; Qu, X.; Chen, S. Tuning the poisson’s ratio of biomaterials for investigating cellular response. Adv. Funct. Mater. 2013, 23, 3226–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, K.J.; Hales, J.M.; Balu, M.; Belfield, K.D.; Van Stryland, E.W.; Hagan, D.J. Two-photon absorption cross-sections of common photoinitiators. J. Photochem. Photobiol. A Chem. 2004, 162, 497–502. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Pflaum, M.; Wilhelmi, M.; Dubruel, P.; Chichkov, B. Laser fabrication of 3D gelatin scaffolds for the generation of bioartificial tissues. Mater. Basel 2010, 4, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Dubruel, P.; Möller, L.; Drager, G.; Chichkov, B. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Meißner, M.; Schlie-Wolter, S.; Chichkov, B.N. Water-soluble photopolymerizable chitosan hydrogels for biofabrication via two-photon polymerization. Acta Biomater. 2015, 18, 186–195. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Schlie, S.; Ngezahayo, A.; Haverich, A.; Chichkov, B.N. Two-photon polymerization technique for microfabrication of CAD-designed 3D scaffolds from commercially available photosensitive materials. J. Tissue Eng. Regen. Med. 2007, 1, 443–449. [Google Scholar] [CrossRef]
- Nava, M.M.; Di Maggio, N.; Zandrini, T.; Cerullo, G.; Osellame, R.; Martin, I.; Raimondi, M.T. Synthetic niche substrates engineered via two-photon laser polymerization for the expansion of human mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2017, 11, 2836–2845. [Google Scholar] [CrossRef] [Green Version]
- Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mageswaran, P.; Mente, P.; Modi, R.; Auyeung, R.; Chrisey, D.B.; Ovsianikov, A.; Chichkov, B. Two photon induced polymerization of organic-inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006, 2, 267–275. [Google Scholar] [CrossRef]
- Doraiswamy, A.; Ovsianikov, A.; Gittard, S.D.; Monteiro-Riviere, N.A.; Crombez, R.; Montalvo, E.; Shen, W.; Chichkov, B.N.; Narayan, R.J. Fabrication of microneedles using two photon polymerization for transdermal delivery of nanomaterials. J. Nanosci. Nanotechnol. 2010, 10, 6305–6312. [Google Scholar] [CrossRef] [PubMed]
- Farsari, M.; Filippidis, G.; Sambani, K.; Drakakis, T.S.; Fotakis, C. Two-photon polymerization of an Eosin Y-sensitized acrylate composite. J. Photochem. Photobiol. A Chem. 2006, 181, 132–135. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Delguidice, D.M.; Epling, G.A.; Hoffacker, K.D.; Howell, A.R.; Pitts, J.D.; Goodman, S.L. 3-dimensional submicron polymerization of acrylamide by multiphoton excitation of xanthene dyes. Macromolecules 2000, 33, 1511–1513. [Google Scholar] [CrossRef]
- Pitts, J.D.; Campagnola, P.J.; Epling, G.A.; Goodman, S.L. Submicron multiphoton free-form fabrication of proteins and polymers: studies of reaction efficiencies and applications in sustained release. Macromolecules 2000, 33, 1514–1523. [Google Scholar] [CrossRef]
- Cunningham, L.P.; Veilleux, M.P.; Campagnola, P.J. Freeform multiphoton excited microfabrication for biological applications using a rapid prototyping CAD-based approach. Opt. Express 2006, 14, 8613. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Hoch, E.; Borchers, K.; Meyer, W.; Krüger, H.; Tovar, G.E.M.; Gillner, A. Fabrication of 2D protein microstructures and 3D polymer-protein hybrid microstructures by two-photon polymerization. Biofabrication 2011, 3, 025003. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Rodionov, V.; Terasaki, M.; Campagnola, P.J. Multiphoton-excited microfabrication in live cells via Rose Bengal cross-linking of cytoplasmic proteins. Opt. Lett. 2005, 30, 159. [Google Scholar] [CrossRef]
- Pitts, J.D.; Howell, A.R.; Taboada, R.; Banerjee, I.; Wang, J.; Goodman, S.L.; Campagnola, P.J. New photoactivators for multiphoton excited three-dimensional submicron cross-linking of proteins: bovine serum albumin and type 1 collagen. Photochem. Photobiol. 2002, 76, 135–144. [Google Scholar] [CrossRef]
- Tromayer, M.; Gruber, P.; Markovic, M.; Rosspeintner, A.; Vauthey, E.; Redl, H.; Ovsianikov, A.; Liska, R. A biocompatible macromolecular two-photon initiator based on hyaluronan. Polym. Chem. 2017, 8, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.Y.; Liu, B.; Kohler, B.; Korystov, D.; Mikhailovsky, A.; Bazan, G.C. Solvent effects on the two-photon absorption of distyrylbenzene chromophores. J. Am. Chem. Soc. 2005, 127, 14721–14729. [Google Scholar] [CrossRef]
- Torgersen, J.; Ovsianikov, A.; Mironov, V.; Pucher, N.; Qin, X.; Li, Z.; Cicha, K.; Machacek, T.; Liska, R.; Jantsch, V.; et al. Photo-sensitive hydrogels for three-dimensional laser microfabrication in the presence of whole organisms. J. Biomed. Opt. 2012, 17, 105008. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-H.; Torgersen, J.; Saf, R.; Mühleder, S.; Pucher, N.; Ligon, S.C.; Holnthoner, W.; Redl, H.; Ovsianikov, A.; Stampfl, J.; et al. Three-dimensional microfabrication of protein hydrogels via two-photon-excited thiol-vinyl ester photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4799–4810. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Zhao, Y.; Xue, J.; Wu, F.; Fang, X. Water-soluble benzylidene cyclopentanone dye for two-photon photopolymerization. J. Photochem. Photobiol. A Chem. 2009, 202, 74–79. [Google Scholar] [CrossRef]
- Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R. Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels. RSC Adv. 2013, 3, 15939–15946. [Google Scholar] [CrossRef] [Green Version]
- Ovsianikov, A.; Mühleder, S.; Torgersen, J.; Li, Z.; Qin, X.H.; Van Vlierberghe, S.; Dubruel, P.; Holnthoner, W.; Redl, H.; Liska, R.; et al. Laser photofabrication of cell-containing hydrogel constructs. Langmuir 2014, 30, 3787–3794. [Google Scholar] [CrossRef]
- Yang, W.; Zou, Q.; Zhou, Y.; Zhao, Y.; Huang, N.; Gu, Y.; Wu, F. Carboxylate modified benzylidene cyclopentanone dyes for one- and two-photon excited photodynamic therapy. J. Photochem. Photobiol. A Chem. 2011, 222, 228–235. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, T.; Zou, Q.; Zhao, Y.; Wu, F. Cationic benzylidene cyclopentanone photosensitizers for selective photodynamic inactivation of bacteria over mammalian cells. RSC Adv. 2015, 5, 56067–56074. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhao, Y. Study on a series of water-soluble photoinitiators for fabrication of 3D hydrogels by two-photon polymerization. Dye. Pigment. 2017, 141, 413–419. [Google Scholar] [CrossRef]
- Wenz, G. Cyclodextrine als Bausteine supramolekularer Strukturen und Funktionseinheiten. Angew. Chemie 1994, 106, 851–870. [Google Scholar] [CrossRef]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Baysah, C.Z.; Zheng, M.; Xing, J. Biomaterial-based microstructures fabricated by two-photon polymerization microfabrication technology. RSC Adv. 2019, 9, 34472–34480. [Google Scholar] [CrossRef] [Green Version]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel naphthalimide-amine based photoinitiators operating under violet and blue LEDs and usable for various polymerization reactions and synthesis of hydrogels. Polym. Chem. 2016, 7, 418–429. [Google Scholar] [CrossRef]
- Balta, D.K.; Bagdatli, E.; Arsu, N.; Ocal, N.; Yagci, Y. Chemical incorporation of thioxanthone into β-cyclodextrin and its use in aqueous photopolymerization of methyl methacrylate. J. Photochem. Photobiol. A Chem. 2008, 196, 33–37. [Google Scholar] [CrossRef]
- Temel, G.; Parali, T.; Tulu, M.; Arsu, N. Photopolymerization of acrylamide with benzophenone/methylated-β-cyclodextrin inclusion complex in the presence of jeffamine based dendrimers as coinitiators in aqueous media. J. Photochem. Photobiol. A Chem. 2010, 213, 46–51. [Google Scholar] [CrossRef]
- Ayub, N.F.; Hashim, S.; Jamaluddin, J.; Adrus, N. New UV LED curing approach for polyacrylamide and poly(: N -isopropylacrylamide) hydrogels. N. J. Chem. 2017, 41, 5613–5619. [Google Scholar] [CrossRef]
- Xing, J.; Liu, J.; Zhang, T.; Zhang, L.; Zheng, M.; Duan, X. A water soluble initiator prepared through host-guest chemical interaction for microfabrication of 3D hydrogels via two-photon polymerization. J. Mater. Chem. B 2014, 2, 4318–4323. [Google Scholar] [CrossRef]
- Zuo, X.; Morlet-Savary, F.; Schmitt, M.; Le Nouën, D.; Blanchard, N.; Goddard, J.P.; Lalevée, J. Novel applications of fluorescent brighteners in aqueous visible-light photopolymerization: high performance water-based coating and LED-assisted hydrogel synthesis. Polym. Chem. 2018, 9, 3952–3958. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Zivic, N.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New violet to yellow light sensitive diketo pyrrolo-pyrrole photoinitiators: High performance systems with unusual bleaching properties and solubility in water. Polym. Chem. 2017, 8, 2028–2040. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent Hydrogel-Textile Composite Material Synthesized by Photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-photon polymerization for biological applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.H.; Ovsianikov, A.; Stampfl, J.; Liska, R. Additive manufacturing of photosensitive Hydrogels for tissue engineering applications. BioNanoMaterials 2014, 15, 49–70. [Google Scholar] [CrossRef] [Green Version]
- Kasko, A.M.; Wong, D.Y. Two-photon lithography in the future of cell-based therapeutics and regenerative medicine: A review of techniques for hydrogel patterning and controlled release. Future Med. Chem. 2010, 2, 1669–1680. [Google Scholar] [CrossRef]
- Eren, T.N.; Lalevée, J.; Avci, D. Water soluble polymeric photoinitiator for dual-curing of acrylates and methacrylates. J. Photochem. Photobiol. A Chem. 2020, 389, 112288. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Cheng, A.W.M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Fukui, S.; Sonomoto, K.; Itoh, N.; Tanaka, A. Several novel methods for immobilization of enzymes, microbial cells and organelles. Biochimie 1980, 62, 381–386. [Google Scholar] [CrossRef]
- Almeida, J.F.; Ferreira, P.; Lopes, A.; Gil, M.H. Photocrosslinkable biodegradable responsive hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Lee, W.Y.W.; Feng, Q.; Xu, L.; Wang, B.; Man, G.C.W.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res. Ther. 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masurovsky, E.B.; Peterson, E.R. Photo-reconstituted collagen gel for tissue culture substrates. Exp. Cell Res. 1973, 76, 447–448. [Google Scholar] [CrossRef]
- He, C.; Li, F.; Ahn, J.I.; Latorre, M.; Griffith, M. Photo-induced in situ forming hydrogels based on collagen and a biocompatible macromolecular photoinitiator. J. Control. Release 2011, 152 (Suppl. S1). [Google Scholar] [CrossRef]
- Anseth, K.S.; Metters, A.T.; Bryant, S.J.; Martens, P.J.; Elisseeff, J.H.; Bowman, C.N. In situ forming degradable networks and their application in tissue engineering and drug delivery. Proc. J. Control. Release 2002, 78, 199–209. [Google Scholar]
- Geng, J.; Li, W.; Zhang, Y.; Thottappillil, N.; Clavadetscher, J.; Lilienkampf, A.; Bradley, M. Radical polymerization inside living cells. Nat. Chem. 2019, 11, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, J.; Wang, X.; Li, X.; Kawazoe, N.; Chen, G. Single mammalian cell encapsulation by: In situ polymerization. J. Mater. Chem. B 2016, 4, 7662–7668. [Google Scholar] [CrossRef]
- Tetsuka, H.; Shin, S.R. Materials and Technical Innovations in 3D Printing in Biomedical Applications. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef]
- Käpylä, E.; Sedlačík, T.; Aydogan, D.B.; Viitanen, J.; Rypáček, F.; Kellomäki, M. Direct laser writing of synthetic poly(amino acid) hydrogels and poly(ethylene glycol) diacrylates by two-photon polymerization. Mater. Sci. Eng. C 2014, 43, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Seidlits, S.K.; Schmidt, C.E.; Shear, J.B. High-resolution patterning of hydrogels in three dimensions using direct-write photofabrication for cell guidance. Adv. Funct. Mater. 2009, 19, 3543–3551. [Google Scholar] [CrossRef]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Shadish, J.A.; DeForest, C.A. Photopolymers for Multiphoton Lithography in Biomaterials and Hydrogels. In Multiphoton Lithography; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 183–220. [Google Scholar]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed]
- Husar, B.; Hatzenbichler, M.; Mironov, V.; Liska, R.; Stampfl, J.; Ovsianikov, A. Photopolymerization-based additive manufacturing for the development of 3D porous scaffolds. In Biomaterials for Bone Regeneration: Novel Techniques and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 149–201. ISBN 9780857098108. [Google Scholar]
- Kizilel, S.; Sawardecker, E.; Teymour, F.; Pérez-Luna, V.H. Sequential formation of covalently bonded hydrogel multilayers through surface initiated photopolymerization. Biomaterials 2006, 27, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Xu, C. Perspectives in Micro- and Nanotechnology for Biomedical Applications; Imperial Collage Press: London, UK, 2016; ISBN 978-1-78326-960-0. [Google Scholar]












| Initiator | Derivative of | Spectroscopic Properties | Solubility [g/dm3] | Toxicity LC50 [mmol/dm3] | |
|---|---|---|---|---|---|
| λmax-ab [nm] | ε @λmax-ab [dm3·mol-1·cm-1] | ||||
| LAP | MAPO | 380.5 | 191 | 47 | 3.1 |
| TPO-Na | MAPO | 380.5 | 250 | 29 | < 0.56 |
| BAPO-OLi | BAPO | 383.5 | 197 | 54 | 2.6 |
| Bapo-ONa | BAPO | 383.5 | 256 | 60 | 2.8 |
| Type of Initiator | Name of Initiator | Structure, Together with a Simplified Scheme of Photoinduced Cleavage of Photoinitiator | Maximum Absorbance / Source of Irradiation | Key Strengths | Key Drawbacks | Ref. |
|---|---|---|---|---|---|---|
| Type I | Irgacure 2959 |  | 276 nm/ 365 nm | High initiation rate, low cytotoxicity, and immune- genicity | Low initiation efficiency, need for UV light sources, low water solubility (<5 w.%) | [104] |
| Type I | TPO |  | 267, 298, and 380 nm | Cleaves into highly reactive radicals, good thermal stability | Poor water solubility | [146] |
| Type I | LAP |  | 375 nm/ (320-390 nm) 405 nm | Good water solubility, possibility of using UV and visible light sources | Low initiation efficiency, especially when exposed to light from the visible range | [138], [142] |
| Type I | BAPO-OLi |  | 375 nm/ (320-420 nm) | Good water solubility ~54 g/l | Very low extinction coefficient in the range above 400 nm | [142,143,144] |
| Type I | VA-086 |  | 365 nm/ (365-385 nm) | Low toxicity, providing 90% cell survival, high initiation rate, good water solubility | Released nitrogen causes bubble formation | [97], [150,151,152] |
| Type II | Eosin-Y |  | 528 nm/ (400-800 nm) | Good water solubility, low cytotoxicity, wide range of absorbance, possibility to use different light sources in visible range, possibility to use low light powers | A second ingredient is needed for high initiation efficiency – the co-initiator | [158], [159,243] |
| Type II | Camphor- quinone |  | 444 nm/ (400 -500 nm) | Wide absorption range based on the visible range | Modification needed to increase solubility in water, strongly yellow after reaction | [105], [186] |
| Type II | Riboflavin | 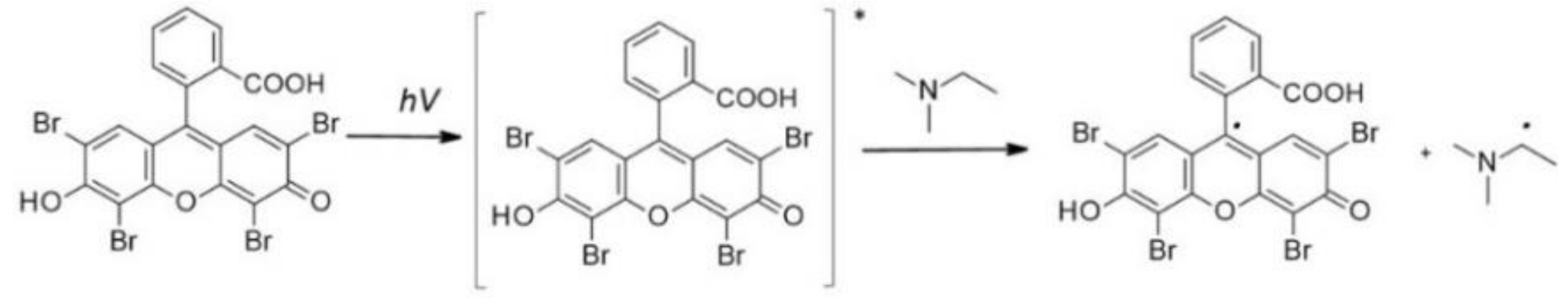 | 223, 267, 373 and 444 nm / (300-500 nm) | Excellent water solubility, wide absorption range, also in the visible area, non-toxic, beneficial to cells | Possibility of creating reactive oxygen species | [167], [177] |
| 2PP | WSPI |  | source of irradiation: laser – 800 nm | very good water solubility, excellent optical sensitivity, and resolution, no toxicity | significant limitations of speed fabrication | [207] [221] [244] |
| 2PP | BDEA |  | [224] | |||
| 2PP | P2CK |  | [225] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. https://doi.org/10.3390/polym12051073
Tomal W, Ortyl J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers. 2020; 12(5):1073. https://doi.org/10.3390/polym12051073
Chicago/Turabian StyleTomal, Wiktoria, and Joanna Ortyl. 2020. "Water-Soluble Photoinitiators in Biomedical Applications" Polymers 12, no. 5: 1073. https://doi.org/10.3390/polym12051073
APA StyleTomal, W., & Ortyl, J. (2020). Water-Soluble Photoinitiators in Biomedical Applications. Polymers, 12(5), 1073. https://doi.org/10.3390/polym12051073