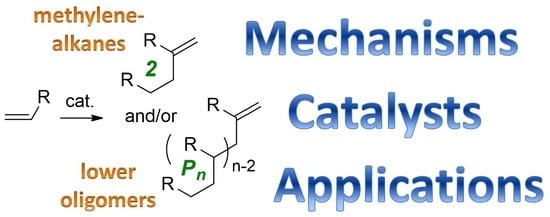
Fair Look at Coordination Oligomerization of Higher ?-Olefins
Abstract
:1. Introduction
2. Coordination Dimerization of α-Olefins
2.1. Group 4 Metallocene-Catalyzed Synthesis of Methylenealkanes
2.2. Dimerization of α-Olefins Catalyzed by Other Complexes of Transition and Rare-Earth Metals
3. Coordination Oligomerization of α-Olefins
3.1. Common Aspects of the Coordination Oligomerization of α-Olefins
3.2. Metallocene-Catalyzed Oligomerization of α-Olefins and Activation by 102–103 eq. MAO
3.3. Zirconocene-Catalyzed Oligomerization of α-Olefins at Low AlMAO/Zr Ratios
3.4. Zirconocene-Catalyzed Oligomerization of α-Olefins, Activated by Perfluoroaryl Borates
3.5. Post-Metallocene Catalysts in the Oligomerization of α-Olefins
4. The Use of Methylenealkanes
4.1. Free Radical Addition to Methylenealkanes
4.2. Free Radical Polymerization of Methylenealkanes
4.3. Epoxydation and Related Reactions
4.4. Methylenealkanes as Alkylating Reagents
4.5. Catalytic Transformations of Methylenealkanes
5. Oils and Lubricants Based on Coordination Oligomers of α-Olefins
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qiao, J.; Guo, M.; Wang, L.; Liu, D.; Zhang, X.; Yu, L.; Song, W.; Liu, Y. Recent advances in polyolefin technology. Polym. Chem. 2011, 2, 1611–1623. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 metal complexes for homogeneous olefin polymerisation: A short tutorial review. Appl. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Busico, V. Metal-catalysed olefin polymerisation into the new millennium: A perspective outlook. Dalton Trans. 2009, 41, 8794–8802. [Google Scholar] [CrossRef]
- Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Benda, R.; Bullen, J.; Plomer, A. Synthetics basics: Polyalphaolefins—Base fluids for high-performance lubricants. J. Synth. Lubric. 1996, 13, 41–57. [Google Scholar] [CrossRef]
- Nicholas, C.P. Applications of light olefin oligomerization to the production of fuels and chemicals. Appl. Catal. A Gen. 2017, 543, 82–97. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E.; Tavtorkin, A.V. Polyolefin Drag Reducing Agents (Review). Petrol. Chem. 2016, 56, 775–787. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Shlyakhtin, A.V.; Tavtorkin, A.N.; Korchagina, S.A.; Chinova, M.S.; Vinogradov, A.A.; Vinogradov, A.A.; Roznyatovsky, V.A.; Khaidapova, D.D.; Ivchenko, P.V. The synthesis of ultra-high molecular weight poly(1-hexene)s by low-temperature Ziegler-Natta precipitation polymerization in fluorous reaction media. Polymer 2018, 139, 98–106. [Google Scholar] [CrossRef]
- Grumel, V.; Brull, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Poly(pent-1-ene) synthesized with the syndiospecific catalyst i-Pr(Cp)(9-Flu)ZrCl2/MAO. Macromol. Mater. Eng. 2002, 287, 559–564. [Google Scholar] [CrossRef]
- Scupinska, J. Oligomerization of a-Olefins to Higher Oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Ray, S.; Rao, P.V.C.; Choudary, N.V. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubric. Sci. 2012, 24, 23–44. [Google Scholar] [CrossRef]
- Busca, G. Acid Catalysts in Industrial Hydrocarbon Chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Shubkin, R.L.; Baylerian, M.S.; Maler, A.R. Olefin Oligomer Synthetic Lubricants: Structure and Mechanism of Formation. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 15–19. [Google Scholar] [CrossRef]
- Gee, J.C.; Small, B.L.; Hope, K.D. Behavior of protonated cyclopropyl intermediates during polyalphaolefin synthesis: Mechanism and predicted product distribution. J. Phys. Org. Chem. 2012, 25, 1409–1417. [Google Scholar] [CrossRef]
- Brennan, J.A. Wide-temperature range synthetic hydrocarbon fluids. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 2–6. [Google Scholar] [CrossRef]
- Onopchenko, A.; Cupples, B.L.; Kresge, A.N. Boron fluoride-catalyzed oligomerization of alkenes: Structures, mechanisms, and properties. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 182–191. [Google Scholar] [CrossRef]
- Sun, H.; Shen, B.; Wu, D.; Guo, X.; Li, D. Supported Al–Ti bimetallic catalysts for 1-decene oligomerization: Activity, stability and deactivation mechanism. J. Catal. 2016, 339, 84–92. [Google Scholar] [CrossRef]
- Hogg, J.M.; Coleman, F.; Ferrer-Ugalde, A.; Atkins, M.P.; Swadźba-Kwaśny, M. Liquid coordination complexes: A new class of Lewis acids as safer alternatives to BF3 in synthesis of polyalphaolefins. Green Chem. 2015, 17, 1831–1841. [Google Scholar] [CrossRef]
- Antunes, B.M.; Rodrigues, A.E.; Lin, Z.; Portugal, I.; Silva, C.M. Alkenes oligomerization with resin catalysts. Fuel Proc. Technol. 2015, 138, 86–99. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.; Shen, Y.; Xia, S.; Xu, J. Synthesis of high viscosity index base stock and study on the lubricating properties. Ind. Lubricat. Tribol. 2016, 68, 52–56. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Paris, C.; Martínez-Franco, R.; Moliner, M.; Martínez, C.; Corma, A. Efficient Oligomerization of Pentene into Liquid Fuels on Nanocrystalline Beta Zeolites. ACS Catal. 2017, 7, 6170–6178. [Google Scholar] [CrossRef]
- Nicholas, C.P.; Laipert, L.; Prabhakar, S. Oligomerization of Light Olefins to Gasoline: An Advanced NMR Characterization of Liquid Products. Ind. Eng. Chem. Res. 2016, 55, 9140–9146. [Google Scholar] [CrossRef]
- Echaroj, S.; Santikunaporn, M.; Chavadej, S. Transformation of Bioderived 1-Decanol to Diesel-like Fuel and Biobased Oil via Dehydration and Oligomerization Reactions. Energy Fuels 2017, 31, 9465–9476. [Google Scholar] [CrossRef]
- Silva, M.S.A.F.; Fernandes, A.; Neves, P.; Antunes, M.M.; Rocha, S.M.; Ribeiro, M.F.; Silva, C.M.; Valente, A.A. Mesostructured Catalysts Based on the BEA Topology for Olefin Oligomerisation. ChemCatChem 2018, 10, 2741–2754. [Google Scholar] [CrossRef]
- Kwon, M.-H.; Chae, H.-J.; Park, M.B. Oligomerization of 1-hexene over designed SBA-15 acid catalysts. J. Ind. Eng. Chem. 2018, 65, 397–405. [Google Scholar] [CrossRef]
- Fehér, C.; Tomasek, S.; Hancsók, J.; Skoda-Földes, R. Oligomerization of light olefins in the presence of a supported Brønsted acidic ionic liquid catalyst. Appl. Catal. B Environ. 2018, 239, 52–60. [Google Scholar] [CrossRef]
- Liu, P.; Redekop, E.; Gao, X.; Liu, W.-C.; Olsbye, U.; Somorjai, G.A. Oligomerization of Light Olefins Catalyzed by Brønsted-Acidic Metal–Organic Framework-808. J. Am. Chem. Soc. 2019, 141, 11557–11564. [Google Scholar] [CrossRef]
- Silva, A.F.; Fernandes, A.; Antunes, M.M.; Ribeiro, M.F.; Silva, C.M.; Valente, A.A. Olefin oligomerisation over nanocrystalline MFI-based micro/mesoporous zeotypes synthesised via bottom-up approaches. Renew. Energy 2019, 138, 820–832. [Google Scholar] [CrossRef]
- Zhang, L.; Ke, M.; Song, Z.; Liu, Y.; Shan, W.; Wang, Q.; Xia, C.; Li, C.; He, C. Improvement of the Catalytic Efficiency of Butene Oligomerization Using Alkali Metal Hydroxide-Modified Hierarchical ZSM-5 Catalysts. Catalysts 2018, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Chae, H.-J.; Park, M.B. Oligomerization of light olefins over ZSM-5 and beta zeolite catalysts by modifying textural properties. Appl. Catal. A Gen. 2018, 553, 15–23. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Park, Y.-K.; Jeon, J.-K. Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst. Catalysts 2018, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Janiak, C.; Blank, F. Metallocene Catalysts for Olefin Oligomerization. Macromol. Symp. 2006, 236, 14–22. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Belov, G.P. Selective Dimerization, Oligomerization, Homopolymerization and Copolymerization of Olefins with Complex Organometallic Catalysts. Russ. J. Appl. Chem. 2008, 81, 1655–1666. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4658078, 14 April 1987. [Google Scholar]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1)—A catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Thiele, S.; Erker, G. Adjusting the Features of Active Metallocene Ziegler Systems for Their Potential Use as Carbon-Carbon Coupling Catalysts in Organic Synthesis. Chem. Ber. 1997, 130, 201–207. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Ivchenko, P.V. Synthesis of zirconium(III) complex by reduction of O[SiMe2(η-C5H4)]ZrCl2 and its selectivity in catalytic dimerization of hex-1-ene. Mendeleev Commun. 2018, 28, 467–469. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. The Method of the Syntheiss of Vinylidene Olefins. RU Patent 2652118, 25 April 2018. [Google Scholar]
- Janiak, C.; Lange, K.C.H.; Marquardt, P. α-Olefin oligomers with narrow molar mass distributions from zirconocene/methylaluminoxane catalysts—An examination of the structure-reactivity relationship. Macromol. Rapid Commun. 1995, 16, 643–650. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P.; Krüger, R.-P.; Hanselmann, R. Analyses of Propene and 1-Hexene Oligomers from Zirconocene/MAO Catalysts—Mechanistic Implications by NMR, SEC, and MALDI-TOF MS. Macromol. Chem. Phys. 2002, 203, 129–138. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P. Alkyl-substituted cyclopentadienyl- and phospholyl-zirconium/MAO catalysts for propene and 1-hexene oligomerization. J. Mol. Catal. A Chem. 2002, 180, 43–58. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Schwab, F.C. Post-Oligomerization of alpha-Olefin Oligomers: A Route to Single-Component and Multicomponent Synthetic Lubricating Oils. J. Appl. Polym. Sci. 2009, 111, 273–280. [Google Scholar] [CrossRef]
- Boccia, A.C.; Costabile, C.; Pragliola, S.; Longo, P. Selective Dimerization of γ-Branched α-Olefins in the Presence of C2 Group-4 Metallocene-Based Catalysts. Macromol. Chem. Phys. 2004, 205, 1320–1326. [Google Scholar] [CrossRef]
- Bagheri, V.; Eisenberg, D.C.; Ratliff, K.S.; Benda, R.; Lanier, C.V. Oligomer oils and their manufacture. U.S. Patent 6548724, 15 April 2003. [Google Scholar]
- Song, W.; Heilman, W.J. Synthesis of Poly-Alpha Olefin and Use Thereof. U.S. Patent Application 2003055184, 20 March 2003. [Google Scholar]
- Fujikawa, S.; Yokota, K.; Okano, M.; Tsuji, M. Method for Producing α-Olefin Oligomers and Lubricating Oil Compositions. U.S. Patent Application 2011207977, 25 August 2011. [Google Scholar]
- Martin, R.W.; Deckman, D.E.; Kelly, K.J.; Emett, C.J.; Hagemeister, M.P.; Harrington, B.A.; Lin, C.-Y.; Matsunaga, P.T.; Ruff, C.J.; Stavens, K.B. Low Viscosity Engine Oil Compositions. U.S. Patent 9234150, 12 January 2016. [Google Scholar]
- Emett, C.J.; Hagemeister, M.P.; Harrington, B.A.; Lin, C.Y.; Matsunaga, P.T.; Ruff, C.J.; Stavens, K.B. Process to Produce Improved Poly Alpha Olefin Composition. U.S. Patent 9365788, 14 June 2016. [Google Scholar]
- van der Heijden, H.; Hessen, B.; Orpen, A.G. A Zwitterionic Zirconocene Alkyl Complex as a Single-Component α-Olefin Dimerization Catalyst. J. Am. Chem. Soc. 1998, 120, 1112–1113. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Fujikawa, S. Base Oil for Oil Drilling Fluid and Oil Drilling Fluid Composition. U.S. Patent Application 2011251445, 13 October 2011. [Google Scholar]
- Fujikawa, S.; Okamoto, T.; Yokota, K. Process for Producing Unsaturated Hydrocarbon Compound. U.S. Patent 8119850, 21 February 2012. [Google Scholar]
- Marks, T.J.; Yang, X. Homogeneous Alpha-Olefin Dimerization Catalysts. U.S. Patent 5500398, 19 March 1996. [Google Scholar]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-Complexed Zirconocene Hydrides: Identification of Hydride-Bridged Species by NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [Green Version]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr, Al-hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. I. Simulation of Intermediate Formation in Reaction of HAlBui2 with Cp2ZrCl2. Organometallics 2009, 28, 968–977. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT and Ab Initio Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. II. Olefin Interaction with Catalytically Active Centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanton, M.J.; Daubney, L.; Lebl, T.; Polas, S.; Smith, D.M.; Willemse, A. Selective dimerisation of α-olefins using tungsten-based initiators. Dalton Trans. 2010, 7025–7037. [Google Scholar] [CrossRef]
- Gunasekara, T.; Preston, A.Z.; Zeng, M.; Abu-Omar, M.M. Highly Regioselective α-Olefin Dimerization Using Zirconium and Hafnium Amine Bis(phenolate) Complexes. Organometallics 2017, 36, 2934–2939. [Google Scholar] [CrossRef]
- Leitch, D.C.; Lam, Y.C.; Labinger, J.A.; Bercaw, J.E. Upgrading Light Hydrocarbons via Tandem Catalysis: A Dual Homogeneous Ta/Ir System for Alkane/Alkene Coupling. J. Am. Chem. Soc. 2013, 135, 10302–10305. [Google Scholar] [CrossRef] [Green Version]
- Leitch, D.C.; Labinger, J.A.; Bercaw, J.E. Scope and Mechanism of Homogeneous Tantalum/Iridium Tandem Catalytic Alkane/Alkene Upgrading using Sacrificial Hydrogen Acceptors. Organometallics 2014, 33, 3353–3365. [Google Scholar] [CrossRef] [Green Version]
- Broene, R.D.; Brookhart, M.; Lamanna, W.M.; Volpe, A.F. Cobalt-Catalyzed Dimerization of α-Olefins to Give Linear α-Olefin Products. J. Am. Chem. Soc. 2005, 127, 17194–17195. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Troyanov, S.I.; Meetsma, A.; Hessen, B.; Teuben, J.H. Regioselective Homo- and Codimerization of α-Olefins Catalyzed by Bis(2,4,7-trimethylindenyl)yttrium Hydride. Organometallics 1998, 17, 284–286. [Google Scholar] [CrossRef]
- Piers, W.E.; Shapiro, P.J.; Bunel, E.E.; Bercaw, J.E. Coping with extreme Lewis acidity: Strategies for the synthesis of stable, mononuclear organometallic derivatives of scandium. Synlett 1990, 74–84. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Ma, L.; Fu, Z.; Yang, W. Synthesis and characterization of oligomer from 1-decene catalyzed by supported Ziegler–Natta catalysts. Eur. Polym. J. 2005, 41, 2909–2915. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Sheng, Y.; Ma, L.; Fu, Z.; Yang, W. Synthesis and characterization of oligomer from 1-decene catalyzed by AlCl3/TiCl4/SiO2/Et2AlCl. J. Appl. Polym. Sci. 2006, 101, 584–590. [Google Scholar] [CrossRef]
- Kawahara, N.; Saito, J.; Matsuo, S.; Kaneko, H.; Matsugi, T.; Toda, Y.; Kashiwa, N. Study on unsaturated structures of polyhexene, poly(4-methylpentene) and poly(3-methylpentene) prepared with metallocene catalysts. Polymer 2007, 48, 425–428. [Google Scholar] [CrossRef]
- Brant, P.; Jiang, P.; Lovell, J.; Crowther, D. Termination events in sterically hindered metallocene-catalyzed olefin oligomerizations: Vinyl chain ends in oligooctenes. Organometallics 2016, 35, 2836–2839. [Google Scholar] [CrossRef]
- Shao, H.; Wang, R.; Li, H.; Guo, X.; Jiang, T. Synthesis of low-molecular-weight poly-α-olefins using silicon-bridged zirconocene catalyst for lubricant basestock. Arab. J. Chem. 2020, 13, 2715–2721. [Google Scholar] [CrossRef]
- Kim, I.; Zhou, J.-M.; Chung, H. Higher α-olefin polymerizations catalyzed by rac-Me2Si(1-C5H2-2-CH3-4-tBu)2Zr(NMe2)2/Al(iBu)3/[Ph3C][B(C6F5)4]. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1687–1697. [Google Scholar] [CrossRef]
- Zhao, X.; Odian, G.; Rossi, A. Kinetics, polymer molecular weights, and microstructure in zirconocene-catalyzed 1-hexene polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3802–3811. [Google Scholar] [CrossRef]
- Babu, G.N.; Newmark, R.A.; Chien, J.C.W. Microstructure of Poly(1-hexene) Produced by ansa-Zirconocenium Catalysis. Macromolecules 1994, 27, 3383–3388. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of Higher 1-Olefins with Metallocene/MAO Catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.H. Zirconium Allyl Complexes as Participants in Zirconocene-Catalyzed alpha-Olefin Polymerizations. Angew. Chem. Int. Ed. 2014, 53, 9645–9649. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The catalytic behavior of heterocenes activated by TIBA and MMAO under a low Al/Zr ratios in 1-octene polymerization. Appl. Catal. A: General 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Semikolenova, N.V.; Yudaev, D.V.; Zakharov, V.A.; Brintzinger, H.H.; Ystenes, M.; Rytter, E.; Talsi, E.P. 1H-, 13C-NMR and ethylene polymerization studies of zirconocene/MAO catalysts: Effect of the ligand structure on the formation of active intermediates and polymerization kinetics. J. Organomet. Chem. 2003, 683, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Tritto, I.; Donetti, R.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. Dimethylzirconocene−Methylaluminoxane Catalyst for Olefin Polymerization: NMR Study of Reaction Equilibria. Macromolecules 1997, 30, 1247–1252. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Ehm, C.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V. Role of TMA in polymerization. Dalton Trans. 2016, 45, 6847–6855. [Google Scholar] [CrossRef] [PubMed]
- Trefz, T.K.; Henderson, M.A.; Linnolahti, M.; Collins, S.; McIndoe, J.S. Mass Spectrometric Characterization of Methylaluminoxane-Activated Metallocene Complexes. Chem. Eur. J. 2015, 21, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Talarico, G.; Budzelaar, P.H.M. Variability of Chain Transfer to Monomer Step in Olefin Polymerization. Organometallics 2008, 27, 4098–4107. [Google Scholar] [CrossRef]
- Lieber, S.; Brintzinger, H.-H. Propene Polymerization with Catalyst Mixtures Containing Different ansa-Zirconocenes: Chain Transfer to Alkylaluminum Cocatalysts and Formation of Stereoblock Polymers. Macromolecules 2000, 33, 9192–9199. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.H. Reactive intermediates formed during olefin polymerization by methylalumoxane-activated ansa-zirconocene catalysts: Identification of a chain-carrying intermediate by NMR methods. J. Am. Chem. Soc. 2010, 132, 452–453. [Google Scholar] [CrossRef] [Green Version]
- Adamu, S.; Atiqullah, M.; Malaibari, Z.O.; Al-Harthi, M.A.; Emwas, A.-H.M.; Ul-Hamid, A. Metallocene-catalyzed ethylene−α-olefin isomeric copolymerization: A perspective from hydrodynamic boundary layer mass transfer and design of MAO anion. J. Taiwan Ins. Chem. Eng. 2016, 60, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene ‘dimer’. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E.; Vinogradov, A.A.; Krut’ko, D.P.; Shandryuk, G.A. Crosslinked α-olefin-diene copolymers prepared using a metallocene catalyst deposited on the surface of SiO2-modified Fe3O4: Ferromagnetic oil sponges. Polym. Sci. Ser. B 2017, 59, 83–90. [Google Scholar] [CrossRef]
- DiMaio, A.J.; Baranski, J.R.; Budworth, J.G.; Gillis, D.J. Process for Producing Liquid Polyalphaolefin Polymer, Metallocene Catalyst therefor, the Resulting Polymer and Lubricant Containing Same. U.S. Patent 6858767, 22 February 2005. [Google Scholar]
- Londaitsbehere, A.; Cuenca, T.; Mosquera, M.E.G.; Cano, J.; Milione, S.; Grassi, A. 1,3-Double Siloxo-Bridged Zirconium Metallocene for Propene and 1-Hexene Regioselective Oligomerization. Organometallics 2012, 31, 2108–2111. [Google Scholar] [CrossRef]
- Welle, A.; Wassenaar, J.; Slawinski, M. Use of a Metallocene Catalyst to Produce a Polyalpha-Olefin. U.S. Patent 9688792, 27 June 2017. [Google Scholar]
- Wu, M.M.-S.; Baugh, L.S.; Canich, J.A.; Chee, C.S.; Hagemeister, M.P.; Jackson, A.; Jiang, P.; Lee, G.H.; Lo, F.Y.-K.; Rucker, S.P.; et al. Polyalpha-Olefin Compositions and Processes to Produce the Same. U.S. Patent Application 2009005279, 01 January 2009. [Google Scholar]
- Small, B.L.; Hope, K.D.; Masino, A.P.; McDaniel, M.P.; Buck, R.M.; Beaulieu, W.B.; Yang, Q.; Baralt, E.J.; Netemeyer, E.J.; Kreischer, B. Oligomerization of Alpha Olefins Using Metallocene-SSA Catalyst Systems and Use of the Resultant Polyalphaolefins to Prepare Lubricant Blends. U.S. Patent 8536391, 17 September 2013. [Google Scholar]
- Di Maio, A.J. Process for the Oligmerization of Alpha-Olefins Having Low Unsaturation. U.S. Patent 7129306, 31 October 2006. [Google Scholar]
- Minami, H.; Egawa, T. Process for Producing a Polymer of an Alpha-Olefin and Lubricant. U.S. Patent Application 2002143113, 03 October 2002. [Google Scholar]
- Iimure, Y.; Hirano, H.; Tohi, Y.; Upakawa, N. Synthetic Lubricating Oil and Lubricating Oil Composition. U.S. Patent 7795194, 14 September 2010. [Google Scholar]
- Shao, H.; Li, H.; Lin, J.; Jiang, T.; Guo, X.; Li, J. Metallocene-catalyzed oligomerizations of 1-butene and α-olefins: Toward synthetic lubricants. Eur. Polym. J. 2014, 59, 208–217. [Google Scholar] [CrossRef]
- Kissin, Y.V. Detailed Kinetics of 1-Hexene Oligomerization Reaction with (n-Bu-Cp)2ZrCl2–MAO Catalyst. Macromol. Chem. Phys. 2009, 210, 1241–1246. [Google Scholar] [CrossRef]
- Kissin, Y.V. Oligomerization reactions of 1-hexene with metallocene catalysts: Detailed data on reaction chemistry and kinetics. Mol. Catal. 2019, 463, 87–93. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Di Capua, A.; Resconi, L.; Guidotti, S.; Camurati, I.; Nifant’ev, I.E.; Laishevtsev, I.P. Structure-Property Correlations in Polypropylene from Metallocene Catalysts: Stereodefective, Regioregular Isotactic Polypropylene. J. Am. Chem. Soc. 2004, 126, 17040–17049. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Laishevtsev, I.P.; Ivchenko, P.V.; Kashulin, I.A.; Guidotti, S.; Piemontesi, F.; Camurati, I.; Resconi, L.; Klusener, P.A.A.; Rijsemus, J.J.H.; et al. C1-Symmetric Heterocyclic Zirconocenes as Catalysts for Propylene Polymerization, 1. ansa-Zirconocenes with Linked Dithienocyclopentadienyl-Substituted Cyclopentadienyl Ligands. Macromol. Chem. Phys. 2004, 205, 2275–2291. [Google Scholar] [CrossRef]
- Resconi, L.; Guidotti, S.; Camurati, I.; Frabetti, R.; Focante, F.; Nifant’ev, I.E.; Laishevtsev, I.P. C1-Symmetric Heterocyclic Zirconocenes as Catalysts for Propylene Polymerization, 2. ansa-Zirconocenes with Linked Dithienocyclopentadienyl-Substituted Indenyl Ligands. Macromol. Chem. Phys. 2005, 206, 1405–1438. [Google Scholar] [CrossRef]
- Ryabov, A.N.; Gribkov, D.V.; Izmer, V.V.; Voskoboynikov, A.Z. Zirconium Complexes with Cyclopentadienyl Ligands Involving Fused a Thiophene Fragment. Organometallics 2002, 21, 2842–2855. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J.; Cationic Metallocene Polymerization Catalysts. Synthesis and Properities of the Cationic Metallocene Polymerization Catalysts. Synthesis and Properities of the First Base-Free Zirconocene Hydride. Angew. Chem. Int. Ed. 1992, 31, 1375–1377. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Roberts, J.A.S.; Seyam, A.M.; Li, L.; Zuccaccia, C.; Stahl, N.G.; Marks, T.J. Diversity in Weakly Coordinating Anions. Mono- and Polynuclear Halo(perfluoroaryl)metalates as Cocatalysts for Stereospecific Olefin Polymerization: Synthesis, Structure, and Reactivity. Organometallics 2006, 25, 2833–2850. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, Y.E.; Jeon, J.Y.; Go, M.J.; Lee, J.; Kim, S.K.; Lee, S.-I.; Lee, B.Y. Preparation of ansa-metallocenes for production of poly(α-olefin) lubricants. Dalton Trans. 2014, 43, 10132–10138. [Google Scholar] [CrossRef]
- Wu, M.M.; Coker, C.L.; Walzer, J.F.; Jiang, P. Process to Produce Low Viscosity Poly-Alfa-Olefins. U.S. Patent 8207390, 26 June 2012. [Google Scholar]
- Wu, M.M.; Coker, C.L.; Walzer, J.F.; Jiang, P.; Rucker, S.P. Process to Produce High Viscosity Fluids. U.S. Patent 7989670, 02 August 2011. [Google Scholar]
- Wu, M.M.; Pafford, B.J.; Stavens, K.B. Polyalphaolefins by Oligomerization and Isomerization. U.S. Patent Application 2014323665, 30 October 2014. [Google Scholar]
- Hagemeister, M.P.; Jiang, P.; Wu, M.M.; Yang, N. Production of Shear-Stable High Viscosity PAO. U.S. Patent 9365663, 14 June 2016. [Google Scholar]
- Wu, M.M.; Hagemeister, M.P.; Yang, N. Process to Produce Polyalphaolefins. U.S. Patent 8513478, 20 August 2013. [Google Scholar]
- Jiang, H.; Yu, K. Catalytic polymerization of 1-decene using a silicon-bridged metallocene system. Petrol. Sci. Technol. 2017, 35, 1451–1456. [Google Scholar] [CrossRef]
- Katayama, K.; Noda, H.; Shimizu, H.; Okano, M. Alpha-Olefin (co)Polymer, Hydrogenated α-Olefin (co)Polymer and Lubricating Oil Composition Containing Them. U.S. Patent Application 2012302481, 29 November 2012. [Google Scholar]
- Shimizu, H.; Katayama, K.; Noda, H.; Okano, M. 1-Octene, 1-Decene, 1-Dodecene Ternary Copolymer and Lubricants Therewith. U.S. Patent Application 2014256997, 11 September 2014. [Google Scholar]
- Shimizu, H.; Katayama, K.; Noda, H.; Okano, M. 1-Octene/1-Decene Copolymer and Libricating-Oil Composition Containing Same. U.S. Patent Application 2014309151, 16 October 2014. [Google Scholar]
- Patil, A.O.; Bodige, S.; Luo, S.; Chu, J.W.; Stavens, K.; Harrrington, B.A. Ultra High Viscosity Synthetic Base Stocks and Process for Preparing Same. U.S. Patent Application 2014213834, 31 July 2014. [Google Scholar]
- Patil, A.O.; Bodige, S. Synthetic Libricant Basestocks and Methods of Preparation Thereof. U.S. Patent 9422497, 23 August 2016. [Google Scholar]
- Wu, M.M.; Rucker, S.P.; Canich, A.M. Process for Producing Novel Synthetic Basestocks. U.S. Patent 9701595, 11 July 2017. [Google Scholar]
- Courtiade, M.; Sanson, J.; Welle, A.; Slawinski, M.; Wassenaar, J. Method for Preparing Low-Viscosity Lubricating Polyolefins. U.S. Patent Application 2017226441, 10 August 2017. [Google Scholar]
- Gibson, V.C.; Spitzmesser, S.K. Advances in Non-Metallocene Olefin Polymerization Catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Budagumpi, S.; Kim, K.-H.; Kim, I. Catalytic and coordination facets of single-site non-metallocene organometallic catalysts with N-heterocyclic scaffolds employed in olefin polymerization. Coord. Chem. Rev. 2011, 255, 2785–2809. [Google Scholar] [CrossRef]
- Sydora, O.L.; Jones, T.C.; Small, B.L.; Nett, A.J.; Fischer, A.A.; Carney, M.J. Selective Ethylene Tri-/Tetramerization Catalysts. ACS Catal. 2012, 2, 2452–2455. [Google Scholar] [CrossRef]
- Agapie, T. Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 2011, 255, 861–880. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M.; Clément, N.D.; Tschan, M.J.-L. New processes for the selective production of 1-octene. Coord. Chem. Rev. 2011, 255, 1499–1517. [Google Scholar] [CrossRef]
- Small, B.L. Discovery and Development of Pyridine-bis(imine) and Related Catalysts for Olefin Polymerization and Oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Alferov, K.A.; Belov, G.P.; Meng, Y. Chromium catalysts for selective ethylene oligomerization to 1-hexene and 1-octene: Recent results. Appl. Catal. A Gen. 2017, 542, 71–124. [Google Scholar] [CrossRef]
- Raucoules, R.; de Bruin, T.; Raybaud, P.; Adamo, C. Theoretical Unraveling of Selective 1-Butene Oligomerization Catalyzed by Iron−Bis(arylimino)pyridine. Organometallics 2009, 28, 5358–5367. [Google Scholar] [CrossRef]
- Majoumo-Mbe, F.; Lönnecke, P.; Volkis, V.; Sharma, M.; Eisen, M.S.; Hey-Hawkins, E. Oligomerization of α-olefins by the dimeric nickel bisamido complex [Ni{1-N(PMes2)-2-N(μ-PMes2)C6H4-κ3N,N′,P,-κ1P′}]2 activated by methylalumoxane (MAO). J. Organomet. Chem. 2008, 693, 2603–2609. [Google Scholar] [CrossRef]
- Dagorne, S.; Bellemin-Laponnaz, S.; Romain, C. Neutral and Cationic N-Heterocyclic Carbene Zirconium and Hafnium Benzyl Complexes: Highly Regioselective Oligomerization of 1-Hexene with a Preference for Trimer Formation. Organometallics 2013, 32, 2736–2743. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Takase, M.K.; Henling, L.M.; Labinger, J.A.; Bercaw, J.E. Mechanistic Insights on the Controlled Switch from Oligomerization to Polymerization of 1-Hexene Catalyzed by an NHC-Zirconium Complex. Organometallics 2015, 34, 4707–4716. [Google Scholar] [CrossRef] [Green Version]
- Nakata, N.; Nakamura, K.; Ishii, A. Highly efficient and 1,2-regioselective method for the oligomerization of 1-hexene promoted by zirconium precatalysts with [OSSO]-type bis(phenolate) ligands. Organometallics 2018, 37, 2640–2644. [Google Scholar] [CrossRef]
- Nakata, N.; Nakamura, K.; Nagaoka, S.; Ishii, A. Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins. Catalysts 2019, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Manyik, R.M.; Walker, W.E.; Wilson, T.P. A soluble chromium-based catalyst for ethylene trimerization and polymerization. J. Catal. 1977, 47, 197–209. [Google Scholar] [CrossRef]
- Köhn, R.D.; Haufe, M.; Kociok- Köhn, G.; Grimm, S.; Wasserscheid, P.; Keim, W. Selective Trimerization of α-Olefins with Triazacyclohexane Complexes of Chromium as Catalysts. Angew. Chem. Int. Ed. 2000, 39, 4337–4339. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Grimm, S.; Köhn, R.D.; Haufe, M. Synthesis of Synthetic Lubricants by Trimerization of 1-Decene and 1-Dodecene with Homogeneous Chromium Catalysts. Adv. Synth. Catal. 2001, 343, 814–818. [Google Scholar] [CrossRef]
- Coxon, A.G.N.; Köhn, R.D. Efficient 1-Hexene Trimerization with Triazacyclohexane Chromium Catalysts and Detailed Product Analysis by 13C NMR. ACS Catal. 2016, 6, 3008–3016. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Patil, A.O. Processes for Preparing Low Viscosity Lubricating Oil Base Stocks. U.S. Patent 8889931, 18 November 2014. [Google Scholar]
- Sattler, A.; Labinger, J.A.; Bercaw, J.E. Highly Selective Olefin Trimerization Catalysis by a Borane-Activated Titanium Trimethyl Complex. Organometallics 2013, 32, 6899–6902. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.P.; Shirase, S.; Yamamoto, K.; Andersen, R.A.; Eisenstein, O.; Copéret, C. NMR chemical shift analysis decodes olefin oligo- and polymerization activity of d0 group 4 metal complexes. Proc. Natl. Acad. Sci. USA 2018, 115, E5867–E5876. [Google Scholar] [CrossRef] [Green Version]
- 148 Speiser, F.; Braunstein, P.; Saussine, L. Catalytic Ethylene Dimerization and Oligomerization: Recent Developments with Nickel Complexes Containing P,N-Chelating Ligands. Acc. Chem. Res. 2005, 38, 784–793. [Google Scholar] [CrossRef]
- 149 Yao, Z.-J.; Deng, W. Half-sandwich late transition metal complexes based on functionalized carborane ligands. Coord. Chem. Rev. 2016, 309, 21–35. [Google Scholar] [CrossRef]
- 150 Yao, Z.-J.; Li, P.; Li, K.; Deng, W. Synthesis, structure and catalytic polymerization activity of half-sandwich cyclometallated iridium complexes. Appl. Organomet. Chem. 2018, 32, e4239. [Google Scholar] [CrossRef]
- 151 Antonov, A.A.; Semikolenova, N.V.; Zakharov, V.A.; Zhang, W.; Wang, Y.; Sun, W.-H.; Talsi, E.P.; Bryliakov, K.P. Vinyl Polymerization of Norbornene on Nickel Complexes with Bis(imino)pyridine Ligands Containing Electron-Withdrawing Groups. Organometallics 2012, 31, 1143–1149. [Google Scholar] [CrossRef]
- 152 Mlinar, A.N.; Keitz, B.K.; Gygi, D.; Bloch, E.D.; Long, J.R.; Bell, A.T. Selective Propene Oligomerization with Nickel(II)-Based Metal−Organic Frameworks. ACS Catal. 2014, 4, 717–721. [Google Scholar] [CrossRef]
- 153 Behr, A.; Bayrak, Z.; Peitz, S.; Stochniol, G.; Maschmeyer, D. Oligomerization of 1-butene with a homogeneous catalyst system based on allylic nickel complexes. RSC Adv. 2015, 5, 41372–41376. [Google Scholar] [CrossRef]
- 154 Forget, S.; Olivier-Bourbigou, H.; Delcroix, D. Homogeneous and Heterogeneous Nickel-Catalyzed Olefin Oligomerization: Experimental Investigation for a Common Mechanistic Proposition and Catalyst Optimization. ChemCatChem 2017, 9, 2408–2417. [Google Scholar] [CrossRef]
- 155 Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef] [Green Version]
- 156 Hu, H.; Gao, H.; Chen, D.; Li, G.; Tan, Y.; Liang, G.; Zhu, F.; Wu, Q. Ligand-Directed Regioselectivity in Amine–Imine Nickel-Catalyzed 1-Hexene Polymerization. ACS Catal. 2015, 5, 122–128. [Google Scholar] [CrossRef]
- 157 O’Connor, K.S.; Lamb, J.R.; Vaidya, T.; Keresztes, I.; Klimovica, K.; LaPointe, A.M.; Daugulis, O.; Coates, G.W. Understanding the Insertion Pathways and Chain Walking Mechanisms of α-Diimine Nickel Catalysts for α-Olefin Polymerization: A 13C NMR Spectroscopic Investigation. Macromolecules 2017, 50, 7010–7027. [Google Scholar] [CrossRef]
- 158 Ojwach, S.O.; Darkwa, J. Perspective and future prospects of tandem olefin oligomerization and Friedel–Crafts alkylation reactions catalyzed by iron, cobalt, nickel and palladium complexes. Catal. Sci. Technol. 2019, 9, 2078–2096. [Google Scholar] [CrossRef]
- Aruleswaran, N.R.; Sharko, P.T.; Crump, J.G. Vinylidene Dimer Derivatives. U.S. Patent Application 2015284350, 08 October 2015. [Google Scholar]
- Kerbal, A. Synthesis and Glutathione S-Transferase Structure−Affinity Relationships of Nonpeptide and Peptidase-Stable Glutathione Analogues. J. Med. Chem. 1998, 41, 2278–2288. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Minyaev, M.E.; Tavtorkin, A.N.; Vinogradov, A.A.; Ivchenko, P.V. Branched alkylphosphinic and disubstituted phosphinic and phosphonic acids: Effective synthesis based on α-olefin dimers and applications in lanthanide extraction and separation. RSC Adv. 2017, 7, 24122–24128. [Google Scholar] [CrossRef] [Green Version]
- Nifant’ev, I.E.; Lyadov, A.S.; Tavtorkin, A.N.; Vinogradov, A.A.; Kochubeev, A.A.; Ivchenko, P.V. Branched alkylphosphinic acids demonstrate explicit anti-wear effect. Mendeleev Commun. 2019, 29, 558–560. [Google Scholar] [CrossRef]
- Mishima, E.; Tamura, T.; Yamago, S. Controlled Copolymerization of Acrylate and 6-Methyleneundecane by Organotellurium-Mediated Living Radical Polymerization (TERP). Macromolecules 2012, 45, 2989–2994. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Bondarenko, G.N.; Korchagina, S.A.; Shlyakhtin, A.V.; Roznyatovsky, V.A.; Ivchenko, P.V. Copolymers of Maleic Anhydride and Methylene Alkanes: Synthesis, Modification, and Pour Point Depressant Properties. Polym. Sci. Ser. B 2018, 60, 469–480. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Ivchenko, P. DFT Modeling of the Alternating Radical Copolymerization and Alder-Ene Reaction between Maleic Anhydride and Olefins. Polymers 2020, 12, 744. [Google Scholar] [CrossRef] [Green Version]
- Shanker, S.K.; Shiga, M.; Fuchi, M.; Nakagawa, T. Lubricating Oil Composition and Method for Operating Automatic Transmission. U.S. Patent Application 2013029890, 31 January 2013. [Google Scholar]
- Sato, H.; Okamoto, T. Dioxolane Derivative and Method for Producing Same. EP Patent 1792900, 06 June 2007. [Google Scholar]
- Sato, H.; Kashiwamura, T.; Ikeda, Y. Process for Producing Aldehyde with 2-Postiion Branched Long-Chain Alkyl. EP Patent 1908746, 09 April 2008. [Google Scholar]
- Sato, H.; Shishikura, A. Alkyl Acetal Compound, Process for Producing the Same, and Lubricatiog Oil Composition. U.S. Patent Application 2008234152, 25 September 2008. [Google Scholar]
- Harvey, B.G.; Meylemansa, H.A.; Quintana, R.L. Synthesis of renewable plasticizer alcohols by formal anti-Markovnikov hydration of terminal branched chain alkenes via a borane-free oxidation/reduction sequence. Green Chem. 2012, 14, 2450–2456. [Google Scholar] [CrossRef]
- Sato, H.; Kamimura, H. Process for Producing Saturated Aliphatic Hydrocarbon Compound, and Lubricant Composition. U.S. Patent 8373011, 12 February 2013. [Google Scholar]
- Patil, A.O. Diphenylamine Functionalization of Poly-Alpha-Olefins. U.S. Patent 7847030, 07 December 2009. [Google Scholar]
- Sato, H.; Kashiwamura, T.; Okamoto, T.; Yokota, K. Carbonyl Compound Containing Long-Chain Branched Alkyl Group. U.S. Patent 7402610, 22 July 2008. [Google Scholar]
- De Kraker, A.R. Olefin Oligomer Composition. U.S. Patent 8383869, 26 February 2013. [Google Scholar]
- Patil, A.; Lewis, K.G.; Bodige, S.; Zushma, S. Ester Compounds, Lubricating Oil Composition Containing Same and Process for Making Same. U.S. Patent Application 2019062663, 28 February 2019. [Google Scholar]
- Nafant’ev, I.E.; Sevostyanova, N.T.; Batashev, S.A.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Ivchenko, P.V. Synthesis of methyl β-alkylcarboxylates by Pd/diphosphine-catalyzed methoxycarbonylation of methylenealkanes RCH2CH2C(R)=CH2. Appl. Catal. A Gen. 2019, 581, 123–132. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Bagrov, V.; Vinogradov, A.; Vinogradov, A.; Ilyin, S.; Sevostyanova, N.; Batashev, S.; Ivchenko, P. Methylenealkane-based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants 2020, 8, 50. [Google Scholar] [CrossRef]
- Patil, A.O.; Bodige, S. Low Viscosity, Low Volatility Lubricating Oil Basestocks. U.S. Patent 9822326, 21 November 2017. [Google Scholar]
- Denis, J. The relationships between structure and rheological properties of hydrocarbons and oxygenated compounds used as base stocks. J. Synth. Lubr. 1984, 1, 201–238. [Google Scholar] [CrossRef]
- Lahtela, M.; Pakkanen, T.A.; Nissfolk, F. Molecular Modeling of Poly-a-olefin Synthetic Oils. J. Phys. Chem. 1995, 99, 10267–10271. [Google Scholar] [CrossRef]


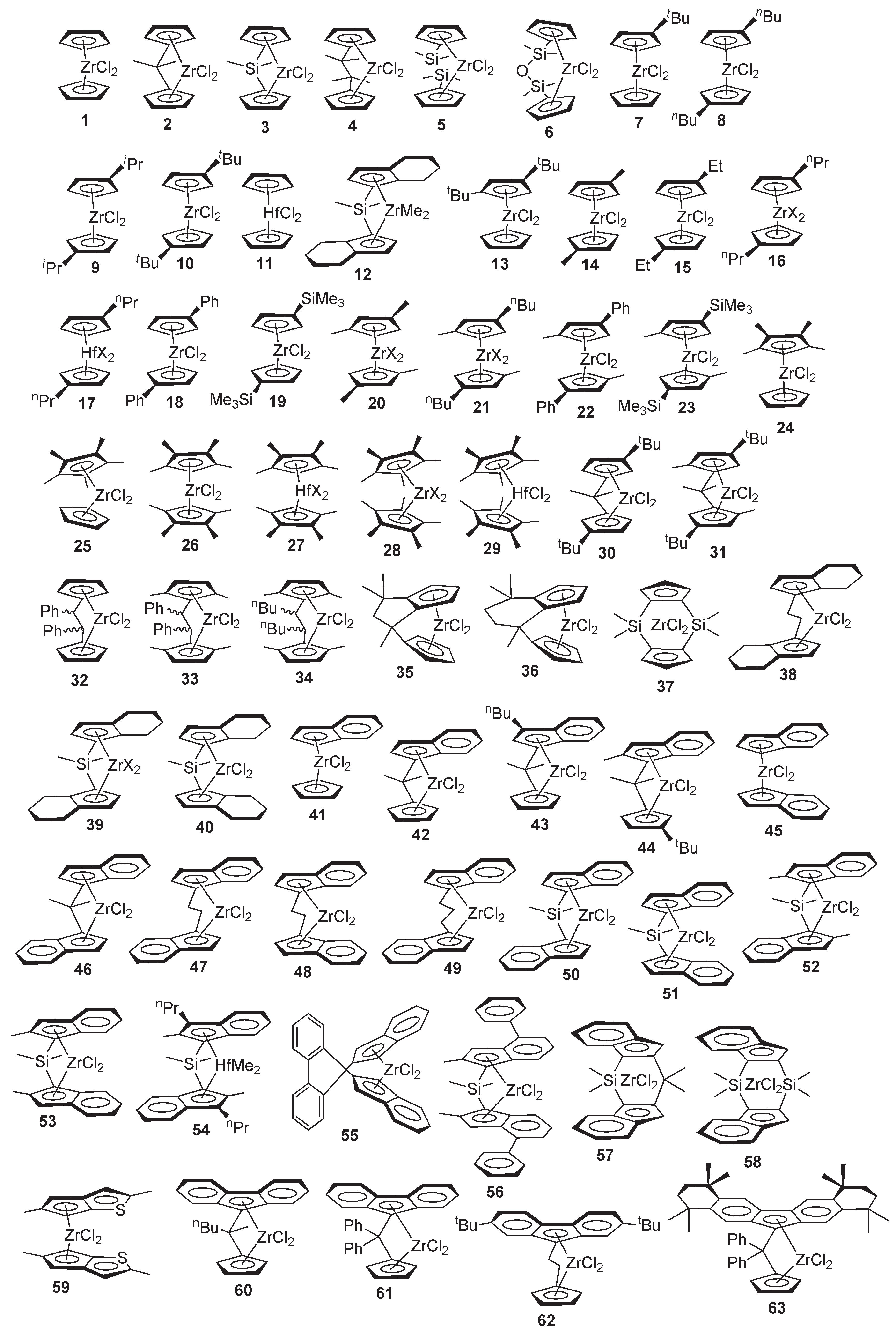
















| Cat. | Mon. | [Mon]/[Zr] | [Al]/ [Zr] | T, °C | H2, bar | TOF, h−1 | DPn | KV100 1 | VI2 | Additional Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| or Oligomer Distribution for DPn 2,3,4,5 | |||||||||||
| 1 | C6 | 1.3 × 105 | 4000 | 50 | – | 5.9 × 104 | 4.9 | – | – | – | [45,46] |
| C6 | 2 × 103 | 200 | 60 | – | – | % olig. 45 (2):20 (3–5), res. 21 3 | [91] | ||||
| C8 | 2 × 103 | 200 | 60 | – | – | % olig. 45 (2):25 (3–5), res. 15 | [91] | ||||
| C10 | 1.0 × 105 | 1000 | 40 | – | 1.1 × 104 | – | 41 | 196 | – | [93] | |
| C10 | 1.0 × 105 | 1000 | 80 | – | 3.2 × 104 | – | 2.5 | 181 | – | [93] | |
| C10 | 3.0 × 104 | 30 | 50 | 1 | 2.9 × 104 | % olig. 42:11:17:5, res. 35 | [51] | ||||
| C10 | 5.0 × 104 | 300 | 70 | – | 9.2 × 103 | – | 17 | 167 | 24% of dimer | [49] | |
| C10 | 5.0 × 104 | 300 | 110 | – | 3.7 × 103 | – | 5.9 | 152 | 55% of dimer | [49] | |
| C14 | 1.6 × 103 | 530 | 40 | – | 1.1 × 103 | % olig. 42:25:16:8, res. 6 | [50] | ||||
| C14 | 1.6 × 103 | 530 | 60 | – | >2 × 103 | % olig. 60:23:8:4, res. 5 | [50] | ||||
| 3 | C10 | 1.0 × 105 | 1000 | 40 | – | 1.1 × 104 | – | 2460 | 344 | – | [93] |
| 7 | C6 | 1.3 × 105 | 4000 | 50 | – | 9.4 × 104 | 5.7 | – | – | – | [45,46] |
| 8 | C6 | 1.3 × 105 | 4000 | 50 | – | 2.0 × 104 | 3.9 | – | – | [45,46] | |
| C6 | 2 × 103 | 200 | 60 | – | - | % olig. 15 (2):30 (3–5), res. 41 | [91] | ||||
| C8 | 500 | 200 | 60 | – | - | % olig. 31 (2):41 (3–5), res. 16 | [91] | ||||
| C10 | 1.0 × 105 | 1000 | 90 | – | 6.3 × 104 | – | 2.3 | 163 | [93] | ||
| C10 | 3.0 × 104 | 30 | 50 | 1 | 2.7 × 104 | % olig. 47:26:11:4, res. 12 | [51] | ||||
| C10 | 3.0 × 104 | 100 | 50 | 1 | >3 × 104 | % olig. 44:27:11:5, res. 13 | [51] | ||||
| C10 | 3.0 × 104 | 300 | 50 | 1 | 2.8 × 104 | % olig. 54:28:9:3, res. 6 | [51] | ||||
| C10 | 3.0 × 104 | 600 | 50 | 1 | 2.9 × 104 | % olig. 27:28:16:9, res. 20 | [51] | ||||
| C10 | 5.0 × 104 | 300 | 110 | – | 6.0 × 103 | – | 6.7 | 156 | 49% of dimer | [49] | |
| C10 | 5.0 × 104 | 300 | 110 | – | 6.0 × 103 | – | 6.7 | 156 | 49% of dimer | [49] | |
| 9 | C10 | 2.7 × 103 | 340 | 50 | – | >3 × 103 | % olig. 43:24:11:5, res. 17 | [50] | |||
| C10 | 3.0 × 104 | 30 | 50 | 1 | 1.6 × 104 | % olig. 28:12:8:6, res. 46 | [51] | ||||
| C10 | 5.0 × 104 | 300 | 110 | – | 6.0 × 103 | – | 5.7 | 152 | 50% of dimer | [49] | |
| 13 | C6 | 1.3 × 105 | 4000 | 50 | – | 3.2 × 104 | 14 | - | - | [45,46] | |
| 14 | C10 | 5.0 × 104 | 300 | 110 | – | 6.1 × 103 | – | 8.2 | 159 | 40% of dimer | [49] |
| 19 | C10 | 3 × 104 | 30 | 50 | 1 | 1.5 × 104 | % olig. 25:24:10:6, res. 25 | [51] | |||
| 20 | C10 | 5.0 × 104 | 300 | 70 | – | 1.1 × 104 | – | 61 | 190 | 6% of dimer | [49] |
| C10 | 5.0 × 104 | 300 | 110 | – | 9.8 × 103 | – | 17 | 162 | 15% of dimer | [49] | |
| 23 | C10 | 3.0 × 104 | 30 | 50 | 1 | 8 × 103 | % olig. 13:5:4:3, res. 75 | [51] | |||
| 24 | C6 | 1.3 × 105 | 4000 | 50 | – | 7.5 × 104 | 14 | – | – | [45,46] | |
| 25 | C6 | 1.3 × 105 | 4000 | 50 | – | 3.9 × 104 | 45 | – | – | [45,46] | |
| 26 | C6 | 1.3 × 105 | 4000 | 50 | – | 6.5 × 104 | 83 | – | – | [45,46] | |
| C10 | 5.0 × 104 | 300 | 70 | – | 1.2 × 104 | - | 154 | – | 1% of dimer | [49] | |
| 28, X = Cl | C6 | 1.3 × 105 | 4000 | 50 | – | 3.4 × 104 | 19 | – | – | [45,46] | |
| C10 | 5.0 × 104 | 300 | 70 | – | 2.3 × 103 | – | 115 | 224 | 5% of dimer | [49] | |
| 37 | C6 | 1.0 × 103 | 500 | 25 | – | 630 | ~3 | 49% of dimer | [94] | ||
| 38 | C10 | 1.5 × 105 | 1000 | 80 | – | 8.3 × 104 | 26 | 101 | – | [95] | |
| C10 | 1.5 × 105 | 1000 | 80 | 5 | 8.2 × 104 | 14 | 28 | – | [95] | ||
| C10 | 1.5 × 105 | 1000 | 80 | 15 | 1.3 × 105 | 12 | 32 | – | [95] | ||
| 41 | C10 | 1.0 × 105 | 250 | 100 | – | 1.1 × 104 | 72 | – | – | [96] | |
| 42 | C8 | 1.7 × 105 | 1000 | 25 | 40 | 63 | 831 | – | [97] | ||
| 44 | C10 | 1.5 × 105 | 1000 | 80 | – | 7.4 × 104 | 27 | 143 | – | [95] | |
| C10 | 1.5 × 105 | 1000 | 80 | 5 | 1.2 × 105 | 17 | 71 | – | [95] | ||
| C10 | 1.5 × 105 | 1000 | 80 | 15 | 1.9 × 105 | 19 | 86 | – | [95] | ||
| 47 | C10 | 1.0 × 105 | 1000 | 40 | – | 1.0 × 105 | – | 702 | 296 | [93] | |
| 48 | C10 | 2.6 × 105 | 600 | 80 | 6.9 | 611 | – | 11 | 211 | [98] | |
| 51 | C10 | 1.3 × 105 | 500 | 80 | 13.8 | 8.3 × 104 | – | 112 | 208 | [98] | |
| 52 | C10 | 1.0 × 105 | 1000 | 40 | – | 5.0 × 104 | – | 2460 | 344 | [93] | |
| C10 | 2.6 × 105 | 200 | 100 | 13.8 | 8.9 × 104 | 21 | 116 | 214 | [98] | ||
| 53 | C10 | 2.6 × 105 | 250 | 100 | 6.9 | 2.6 × 105 | 19 | 94 | 213 | [98] | |
| C10 | 2.6 × 105 | 250 | 90 | – | 5.6 × 104 | 133 | 1227 | – | [98] | ||
| C10 | 2.6 × 105 | 250 | 90 | 1.7 | 1.0 × 105 | 57 | 453 | – | [98] | ||
| C10 | 2.6 × 105 | 250 | 90 | 3.4 | 2.6 × 105 | 40 | 333 | – | [98] | ||
| C10 | 2.6 × 105 | 250 | 90 | 6.9 | 2.6 × 105 | 19 | 94 | – | [98] | ||
| C10 | 2.6 × 105 | 250 | 90 | 13.8 | 2.1 × 105 | 12 | 39 | 179 | [98] | ||
| 56 | C10 | 4.2 × 105 | 260 | 80 | 13.8 | 1.4 × 105 | 21 | 25 | 183 | [98] | |
| 57 | C8 | 2.5 × 105 | 1000 | 65 | – | 1.4 × 105 | 23 | 139 | 192 | [99] | |
| C8 | 2.5 × 105 | 1000 | 65 | 8 | 2.2 × 105 | 18 | 43 | 168 | [99] | ||
| 60 | C8 | 2.0 × 105 | 1000 | 25 | – | 1.3 × 105 | 83 | 1119 | – | [97] | |
| 61 | C10 | 1.0 × 105 | 1000 | 40 | – | 1.0 × 105 | – | 2460 | 344 | [93] | |
| C10 | 3.0 × 105 | 1000 | 70 | – | 2.4 × 105 | 64 | 635 | 282 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 150 | – | 1.6 × 105 | – | 58 | 195 | [93] | ||
| C10 | 3.0 × 105 | 500 | 70 | – | 2.3 × 105 | – | 1134 | 307 | [93] | ||
| C10 | 3.0 × 105 | 250 | 70 | – | 1.7 × 105 | – | 1308 | 314 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 70 | 0.5 | 1.5 × 105 | – | 1074 | 308 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 70 | 1 | 2.1 × 105 | – | 863 | 296 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 70 | 2 | 2.0 × 105 | – | 722 | 288 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 70 | 13 | 2.4 × 105 | – | 512 | 271 | [93] | ||
| C6 | 3.0 × 105 | 1000 | 90 | – | 1.8 × 105 | – | 2862 | 251 | [93] | ||
| C8 | 3.0 × 105 | 1000 | 90 | – | 2.0 × 105 | – | 888 | 276 | [93] | ||
| C10 | 3.0 × 105 | 1000 | 90 | – | 1.6 × 105 | – | 515 | 272 | [93] | ||
| C12 | 3.0 × 105 | 1000 | 90 | – | 1.3 × 105 | – | 402 | 264 | [93] | ||
| 62 | C10 | 7.0 × 105 | 1500 | 63 | 8 | 1.6 × 105 | 15 | 37 | 177 | [100] | |
| Cat. | Mon. | [Mon]/[Zr] | AlTIBA/Zr | AlMAO /Zr | T, °C | H2, bar | Conv. (h) | DPn | Additional Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| or Oligomer Distribution | ||||||||||
| 1 | C6 | 2 × 103 | 20 | 10 | 60 | – | 97 (4) | % olig. 85 (2):12 (3–5), res. 1 1 | [91] | |
| 8 | C6 | 2 × 103 | 20 | 10 | 60 | – | 85 (4) | % olig. 35 (2):36 (3–5), res. 20 | [91] | |
| C6 | 2 × 103 | 20 | 10 | 60 | 1 | 99 (4) | % olig. 50 (2):31 (3–5), res. 11 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 60 | – | 83 (4) | % olig. 40 (2):40 (3–5), res. 11 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 60 | – | 72 (4) | % olig. 38 (2):35 (3–5), res. 17 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 100 | – | 69 (4) | % olig. 40 (2):36 (3–5), res. 10 | [91] | ||
| 10 | C6 | 2 × 103 | 20 | 10 | 60 | – | 16 (4) | % olig. 40 (2):30 (3–5), res. 19 | [91] | |
| 18 | C6 | 2 × 103 | 20 | 10 | 60 | – | 92 (4) | % olig. 35 (2):31 (3–5), res. 31 | [91] | |
| 22 | C6 | 2 × 103 | 20 | 10 | 60 | – | 72 (4) | % olig. 8 (2):34 (3–5), res. 55 | [91] | |
| 26 | C6 | 4 × 103 | 80 | 40 | 60 | – | 90 (4) | 38 | [92] | |
| 30 | C6 | 2 × 103 | 20 | 10 | 60 | – | 89 (4) | 44 | [91] | |
| 31 | C6 | 4 × 103 | 80 | 40 | 60 | – | 75 (4) | 190 | [92] | |
| 35 | C6 | 2 × 103 | 20 | 10 | 60 | – | 98 (4) | % olig. 41 (2):28 (3–5), res. 24 | [91] | |
| C8 | 2 ×·103 | 20 | 10 | 60 | – | 98 (4) | % olig. 36 (2):32 (3–5), res. 29 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 60 | – | 98 (4) | % olig. 40 (2):33 (3–5), res. 35 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 100 | – | 98 (4) | % olig. 41 (2):34 (3–5), res. 19 | [91] | ||
| 36 | C6 | 2·× 103 | 20 | 10 | 60 | – | 98 (4) | % olig. 40 (2):30 (3–5), res. 11 | [90] | |
| 41 | C6 | 2 × 103 | 20 | 10 | 60 | – | 95 (4) | % olig. 22 (2):32 (3–5), res. 39 | [91] | |
| 42 | C6 | 2 × 103 | 20 | 10 | 60 | – | 94 (4) | % olig. 20 (2):31 (3–5), res. 40 | [91] | |
| 45 | C6 | 2·× 103 | 20 | 10 | 60 | – | 88 (4) | 25 | [91] | |
| C6 | 4 × 103 | 80 | 40 | 60 | – | 76 (4) | 25 | [92] | ||
| 46 | C6 | 2 × 103 | 20 | 10 | 60 | – | 94 (4) | 108 | [91] | |
| 47 | C6 | 2 × 103 | 20 | 10 | 60 | – | 92 (4) | 37 | [91] | |
| C6 | 4 × 103 | 80 | 40 | 60 | – | 94 (4) | 35 | [92] | ||
| 50 | C6 | 4 × 103 | 80 | 40 | 60 | – | 95 (4) | 108 | [92] | |
| 55 | C6 | 4 × 103 | 80 | 40 | 60 | – | 95 (4) | 68 | [92] | |
| 59 | C6 | 2 × 103 | 20 | 10 | 60 | – | 100 (4) | % olig. 9 (2):17 (3–5), res. 72 | [91] | |
| C6 | 2 × 103 | 20 | 10 | 60 | 1 | 100 (4) | % olig. 13 (2):24 (3–5), res. 57 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 60 | – | 100 (4) | % olig. 23 (2):30 (3–5), res. 45 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 80 | – | 100 (4) | % olig. 28 (2):26 (3–5), res. 44 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 100 | – | 100 (4) | % olig. 37 (2):34 (3–5), res. 27 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 120 | – | 100 (4) | % olig. 44 (2):32 (3–5), res. 21 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 100 | 1 | 100 (4) | % olig. 47 (2):34 (3–5), res. 8 | [91] | ||
| C8 | 2 × 103 | 20 | 10 | 120 | 1 | 100 (4) | % olig. 51 (2):40 (3–5), res. 10 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 100 | – | 93 (4) | % olig. 44 (2):45 (3–5), res. 7 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 100 | 1 | 99 (4) | % olig. 45 (2):41 (3–5), res. 6 | [91] | ||
| C10 | 2 × 103 | 20 | 10 | 120 | 1 | 99 (4) | % olig. 51 (2):33 (3–5), res. 10 | [91] | ||
| Cat. | Mon. | [Mon] /[Zr] | Al/Zr | T, °C | H2, bar | TOF, h−1 | DPn | KV100 | VI | AlR3, Additional Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| or Oligomer Distribution for DPn 2,3,4,5+ | |||||||||||
| 11, X = Me | C10 | 5.2 × 105 | 50 | 100 | – | 1.0·× 103 | 27 | 103 | – | Al(n-Oct)3, NB 1 | [113] |
| C10 | 5.2·× 105 | 50 | 100 | 3 | 3.6·× 105 | 23 | 11.3 | – | Al(n-Oct)3, NB | [113] | |
| 16, X = Me | C10 | 5.2·× 105 | 50 | 100 | – | 5.2·× 104 | 10.6 | 4.5 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | – | 3.1·× 104 | 8.8 | 3.4 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 100 | 3.7 | 7.8·× 104 | 9.5 | 3.7 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 8.0·× 104 | 8.3 | 3.1 | – | Al(n-Oct)3, NB | [113] | |
| 17, X = Me | C10 | 5.2·× 105 | 50 | 100 | – | 8.4·× 103 | 18 | 10.2 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | – | 1.2·× 104 | 11 | 4.9 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 5.9·× 104 | 10.6 | 4.5 | – | Al(n-Oct)3, NB | [113] | |
| 8 | C10 | 5.2·× 105 | 50 | 100 | – | 6.3 × 104 | 9.3 | 4.0 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | – | 3.9 × 104 | 7.9 | 3.1 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 100 | 3 | 7.4·× 104 | 9.0 | 3.8 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 8.8·× 104 | 7.9 | 3.1 | – | Al(n-Oct)3, NB | [113] | |
| 20 | C10 | 5.2·× 105 | 50 | 100 | 3 | 8.7·× 104 | 7.9 | 3.0 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 8.8·× 104 | 7.5 | 2.8 | – | Al(n-Oct)3, NB | [113] | |
| 21, X = Me | C10 | 8.5·× 104 | 4 | 50 | – | 1.3·× 104 | - | 96 | 199 | Al(n-Oct)3, NB | [114] |
| C10 | 8.5·× 104 | 4 | 60 | 1 | 1.6·× 104 | - | 44 | 180 | Al(n-Oct)3, NB | [114] | |
| C10 | 8.5·× 104 | 4 | 70 | 1 | 1.8·× 104 | - | 20 | 179 | Al(n-Oct)3, NB | [114] | |
| C10 | 8.5·× 104 | 4 | 80 | 1 | 2.0·× 104 | - | 11.0 | 163 | Al(n-Oct)3, NB | [114] | |
| C10 | 8.5·× 104 | 4 | 100 | 1 | 1.7·× 104 | - | 8.0 | 161 | Al(n-Oct)3, NB | [114] | |
| C10 | 8.5·× 104 | 4 | 120 | 1 | 2.1·× 104 | - | 5.0 | 151 | Al(n-Oct)3, NB | [114] | |
| C10 | 5.2·× 105 | 50 | 80 | – | 2.2·× 104 | 13 | 5.9 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 100 | – | 3.3 ×·104 | 10 | 4.1 | 148 | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 120 | – | 3.3·× 104 | 8.5 | 3.3 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 100 | 3 | 1.2·× 105 | 8.6 | 3.3 | – | Al(n-Oct)3, NB | [113] | |
| 26 | C10 | 1.2·× 105 | 100 | 110 | – | – | – | 3.9 | 144 | TIBA, NB | [115] |
| 27, X = Me | C10 | 5.2·× 105 | 50 | 100 | – | 7.2·× 103 | 10 | 4.1 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | – | 1.8·× 104 | 8.3 | 3.1 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 100 | 3 | 1.1·× 105 | 9.3 | 3.6 | – | Al(n-Oct)3, NB | [113] | |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 3.1·× 104 | 8.0 | 3.0 | – | Al(n-Oct)3, NB | [113] | |
| 28, X = Me | C10 | 5.2·× 105 | 50 | 100 | 3 | 2.0·× 104 | 7.6 | 2.8 | – | Al(n-Oct)3, NB | [113] |
| C10 | 5.2·× 105 | 50 | 120 | 3 | 1.4·× 104 | 7.9 | 3.2 | – | Al(n-Oct)3, NB | [113] | |
| 32 | C10 | 2.6·× 105 | 200 | 120 | – | 4.7·× 104 | 9.6/7.0/4.0/2.6 + 64 | TIBA, CB 2 | [112] | ||
| 33 | C8 | 2.6·× 105 | 200 | 120 | – | 1.9·× 105 | 13.1/14.1/11.8/33.4 | TIBA, CB | [112] | ||
| C10 | 2.6·× 105 | 200 | 120 | – | 1.5·× 105 | 13.3/14.3/11.3/34.7 | TIBA, CB | [112] | |||
| 34 | C10 | 2.6·× 105 | 200 | 120 | – | 9.1·× 104 | 17.0/18.4/13.9/24.6 | TIBA, CB | [112] | ||
| 38 | C10 | 1.5·× 105 | 200 | 120 | + | 1.2·× 105 | 6.6 | 5.5 | 164 | TIBA, NB | [95] |
| C10 | 1.5·× 105 | 200 | 140 | + | 1.2·× 105 | 4.4 | 2.6 | 140 | TIBA, NB | [95] | |
| 39, X = Me | C10 | 2.0·× 105 | 20 | 120 | – | ~2·× 105 | ~3 | C30 3.4 C40 9.3 | 128 158 | Al(n-Oct)3, NB | [52,53] |
| C10 | 8.9·× 104 | 0.01 | 60 | – | 7.3·× 104 | 32 | 169 | 225 | Al(n-Oct)3, NB | [116] | |
| C10 | 8.9·× 104 | 0.01 | 80 | – | 8.4·× 104 | 14 | 26.5 | 169 | Al(n-Oct)3, NB | [116] | |
| C10 | 8.9·× 104 | 0.01 | 100 | – | 8.8·× 104 | 9.3 | 11.2 | 150 | Al(n-Oct)3, NB | [116] | |
| 40 | C10 | 3.6·× 105 | >100 | 70 | – | 3.1·× 105 | - | 18.5 | 164 | TIBA, CB | [117] |
| C10 | 3.6·× 105 | >100 | 100 | – | 2.0·× 105 | - | 8.9 | 153 | TIBA, NB | [117] | |
| C10 | 3.6·× 105 | >100 | 100 | – | 2.5·× 105 | - | 14.6 | 158 | TIBA, CB | [117] | |
| 48 | C10 | 3.5·× 105 | >100 | 50 | – | 1.5·× 105 | 226 | 3715 | 379 | TIBA, NB | [117] |
| C10 | 3.5·× 105 | >100 | 84 | – | 2.9·× 105 | 80 | 724 | 291 | TIBA, NB | [117] | |
| C10 | 3.5·× 105 | >100 | 100 | – | 2.3·× 105 | 64 | 363 | 250 | TIBA, NB | [117] | |
| 50 | C10 | 2–4·× 105 | 20-200 | 60–100 | 1–10 | 1.0·× 105 | – | 253–586 | 247–281 | TIBA, NB | [118] |
| 58 | C8/C12 | 1.0·× 105 | 10 | 100 | 0.5 | ~1·× 105 | 15–35 | 39–140 | 175–212 | TIBA, NB | [119] |
| C8/C10/C12 | 1.0·× 105 | 10 | 100 | 0.5 | ~1·× 105 | 15–30 | 40–120 | 179–205 | TIBA, NB | [120] | |
| C8/C10 | 1.0·× 105 | 10 | 100 | 0.5 | ~1·× 105 | 19–32 | 45–109 | 175–204 | TIBA, NB | [121] | |
| 61 | C10 | 4.4·× 105 | 10 | 80 | 2 | 3.6·× 105 | - | 834 | 304 | TIBA, NB | [122] |
| C10 | 4.4·× 105 | 10 | 105 | 2 | 2.4·× 105 | 76 | 622 | 289 | TIBA, NB | [123,124] | |
| C10 | 4.4·× 105 | 10 | 110 | 2 | 3.2·× 105 | 71 | 558 | 280 | TIBA, NB | [122] | |
| C10 | 4.4·× 105 | 10 | 120 | 2 | 2.4·× 105 | 63 | 434 | 270 | TIBA, NB | [122] | |
| C10 | 4.4·× 105 | 10 | 130 | 2 | 2.4·× 105 | 57 | 377 | 266 | TIBA, NB | [122] | |
| Cat. | [Mon]/[Zr] | T, °C | TOF, h–1 | KV100 | VI |
|---|---|---|---|---|---|
| 1 | 1.1 × 105 | 90 | 3.1 × 103 | 8.9 | 211 |
| 8 | 5.6 × 105 | 105 | 4.1 × 104 | 45 | 175 |
| 9 | 3.1 × 105 | 110 | 3.4 × 104 | 62 | 186 |
| 15 | 1.5 × 106 | 70 | 1.3 × 105 | 130 | 222 |
| 29 | 5.5 × 105 | 90 | 2.5 × 104 | 8.3 | 157 |
| 42 | 4.3 × 105 | 120 | 3.1 × 104 | 159 | 214 |
| 43 | 4.4 × 105 | 120 | 3.6 × 104 | 132 | 200 |
| 45 | 1.1 × 105 | 90 | 9.0 × 103 | 23 | 169 |
| 47 | 4.2 × 105 | 115 | 4.3 × 104 | 136 | 210 |
| 49 | 1.0 × 105 | 100 | 5.2 × 103 | 10.3 | 194 |

| Cat. | Regioisomer Abundance (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | K | L | M | |
| 83 | 39.9 | 22.4 | 11.8 | 16.5 | 3.6 | 2.0 | 0.9 | 0.4 | 0.2 | 1.2 | 0.4 | 0.7 |
| 84 | 38.5 | 19.2 | 15..5 | 15.6 | 3.7 | 1.9 | 0.8 | 0.6 | 0.2 | 1.5 | 0.4 | 1.4 |
| 85 | 46.9 | 15.3 | 3.0 | 19.7 | 8.3 | 3.1 | 1.5 | 0.3 | 0.2 | 0.7 | 0.3 | 0.7 |
| 86 | 36.9 | 28.8 | 1.6 | 19.8 | 9.1 | 2.2 | 0.7 | 0.1 | <0.1 | 0.4 | <0.1 | 0.3 |
| No. of C Atoms | Oligomer | KV−40, sSt | KV40, sSt | KV100, sSt | VI | PP, °C | Ref. |
|---|---|---|---|---|---|---|---|
| 12 | H2H | 9.00 | 1.28 | – | – | −73 | [91] |
| 16 | O2 | – | 2.6 | – | – | [91] | |
| 16 | O2H | 53.1 | 2.82 | – | – | −43 | [91] |
| 18 | H3 | 3.12 | [91] | ||||
| 18 | H3h | 167.2 | 3.57 | – | – | −94 | [91] |
| 18 | H3h (BF3 catalyst) | 165 | 3.8 | 1.4 | – | <−55 | [16] |
| 20 | D2 | 4.55 | 1.7 | 14 | [91] | ||
| 20 | D2h | 5.30 | – | 14 | −7 | [91] | |
| 24 | H4 | 7.55 | 2.10 | 62 | [91] | ||
| 24 | H4h | 1335 | 8.93 | 2.28 | 46 | −79 | [91] |
| 24 | H4h (BF3 catalyst) | 1780 | 9.8 | 2.6 | 94 | [16] | |
| 24 | H22h | 3030 | 12.4 | 2.72 | 27 | −71 | [91] |
| 24 | O3 | 6.5 | 2.06 | 114 | [91] | ||
| 24 | O3h | 552.1 | 7.56 | 2.20 | 92 | −86 | [91] |
| 24 | O3H (BF3 catalyst) | 580 | 8.0 | 2.3 | 92 | <−55 | [16] |
| 30 | H5 | 17.55 | 3.6 | 76 | [91] | ||
| 30 | H5h | 6798 | 19.2 | 3.8 | 76 | −67 | [91] |
| 30 | H5h (BF3 catalyst) | 7850 | 18.1 | 3.8 | 96 | [16] | |
| 30 | D3 | 14.61 | 3.65 | 140 | [91] | ||
| 30 | D3h (catalyst 79) | 12.2 | 3.2 | 126 | [143] | ||
| 30 | D3h (catalyst 80) | 13.0 | 3.3 | 131 | [143] | ||
| 30 | D3h (catalyst 82) | 12.1 | 3.2 | 137 | [143] | ||
| 30 | D3h | 1897 | 15.05 | 3.70 | 137 | −75 | [91] |
| 30 | D3h [EBTHI]Zr - borate | 13.5 | 3.39 | 128 | [52] | ||
| 30 | D3h (BF3 catalyst) | 2070 | 15.6 | 3.7 | 122 | <−55 | [16] |
| 32 | O4 | 13.94 | 3.44 | 125 | [91] | ||
| 32 | O4h | 3135 | 18.4 | 4.0 | 115 | −74 | [91] |
| 32 | O4h (BF3 catalyst) | 4750 | 20.0 | 4.1 | 106 | <−55 | [16] |
| 32 | O22 | 28.1 | 5.4 | 130 | [91] | ||
| 32 | O22h | 6374 | 29.4 | 5.4 | 119 | −68 | [91] |
| 40 | O5 | 32.9 | 6.05 | 132 | [91] | ||
| 40 | O5h | 11651 | 36.2 | 6.4 | 129 | −68 | [91] |
| 40 | O5h (BF3 catalyst) | 10225 | 30.9 | 5.6 | 124 | [16] | |
| 40 | D4 | 31.3 | 6.03 | 142 | [91] | ||
| 40 | D4h | 8631 | 34.5 | 6.52 | 145 | −66 | [91] |
| 30 | D4h (BF3 catalyst) | 7475 | 29.0 | 5.7 | 141 | [16] | |
| 40 | D22 | 29.43 | 5.90 | 150 | [91] | ||
| 40 | D22h | 15615 | 42.0 | 7.25 | 136 | −52 | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher ?-Olefins. Polymers 2020, 12, 1082. https://doi.org/10.3390/polym12051082
Nifant’ev I, Ivchenko P. Fair Look at Coordination Oligomerization of Higher ?-Olefins. Polymers. 2020; 12(5):1082. https://doi.org/10.3390/polym12051082
Chicago/Turabian StyleNifant’ev, Ilya, and Pavel Ivchenko. 2020. "Fair Look at Coordination Oligomerization of Higher ?-Olefins" Polymers 12, no. 5: 1082. https://doi.org/10.3390/polym12051082
APA StyleNifant’ev, I., & Ivchenko, P. (2020). Fair Look at Coordination Oligomerization of Higher ?-Olefins. Polymers, 12(5), 1082. https://doi.org/10.3390/polym12051082