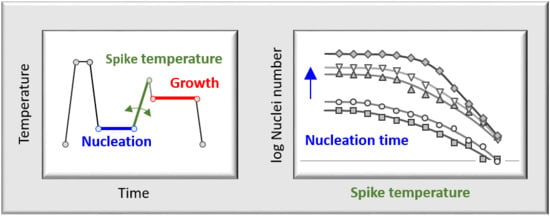
Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature
Abstract
:1. Introduction
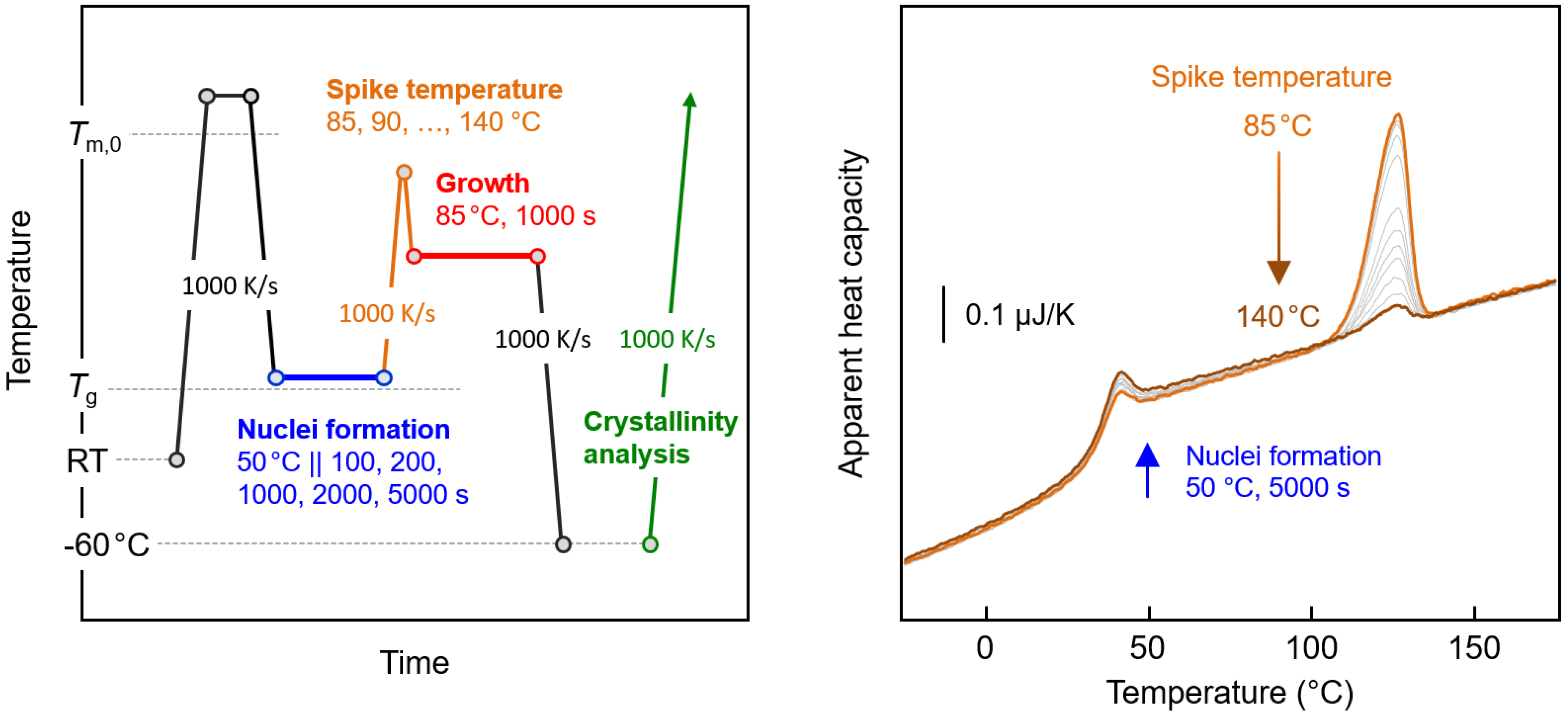
2. Materials and Methods
3. Results and Discussion
3.1. Critical Rates of Temperature-Change for Prevention of Non-Isothermal Crystal Nucleation and Crystal Growth
3.2. Thermal Stability of Crystal Nuclei Formed Near the Glass Transition Temperature
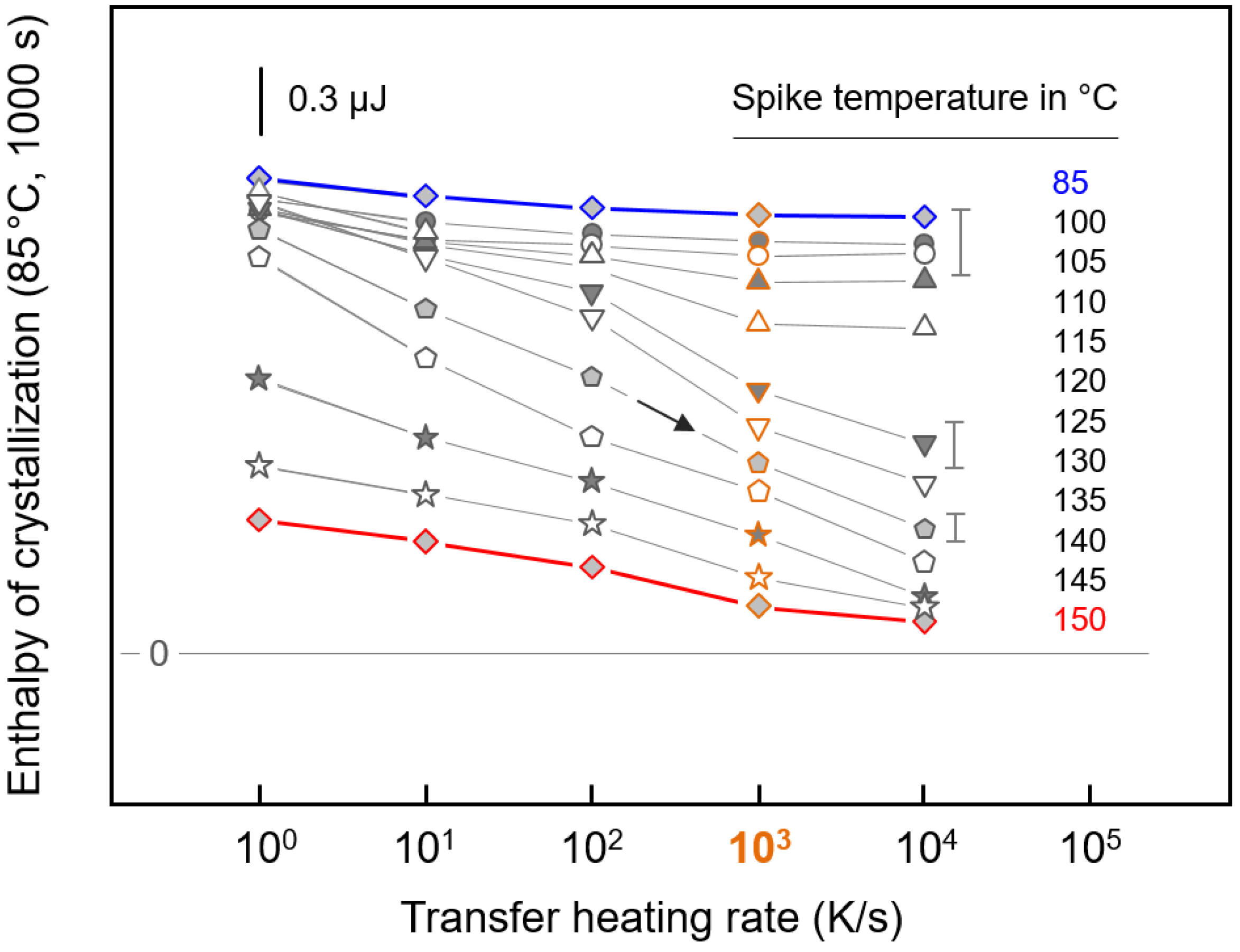
3.3. Effect of Transfer Heating Rate on Crystal Nuclei Reorganization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radusch, H.-J. Poly(butylene terephthalate). In Handbook of Thermoplastic Polyesters; Fakirov, S., Ed.; Wiley-VCH: Weinheim, Germany, 2001; Volume 1, pp. 389–419. [Google Scholar]
- Ludwig, H.J.; Eyerer, P. Influence of the processing conditions on morphology and deformation behavior of poly(butylene terephthalate) (PBT). Polym. Eng. Sci. 1988, 28, 143–146. [Google Scholar] [CrossRef]
- Shibaya, M.; Ishihara, H.; Yamashita, K.; Yoshihara, N.; Nonomura, C. Effect of mold temperature on structure and property variations of PBT injection moldings in the thickness direction. Int. Polym. Proc. 2004, 19, 303–307. [Google Scholar] [CrossRef]
- Rhoades, A.M.; Williams, J.L.; Wonderling, N.; Androsch, R.; Guo, J. Skin/core crystallinity of injection-molded poly(butylene terephthalate) as revealed by microfocus X-ray diffraction and fast scanning chip calorimetry. J. Therm. Anal. Calorim. 2017, 127, 939–946. [Google Scholar] [CrossRef]
- Illers, K.H. Heat of fusion and specific volume of poly(ethylene terephthalate) and poly (butylene terephthalate. Coll. Polym. Sci. 1980, 258, 117–124. [Google Scholar] [CrossRef]
- Conix, A.; Van Kerpel, R. Crystallization behavior and melting properties of m-phenylene group containing polyesters. J. Polym. Sci. 1959, 40, 521–532. [Google Scholar] [CrossRef]
- Runt, J.; Miley, D.M.; Zhang, X.; Gallagher, K.P.; McFeaters, K.; Fishburn, J. Crystallization of poly (butylene terephthalate) and its blends with polyarylate. Macromolecules 1992, 25, 1929–1934. [Google Scholar] [CrossRef]
- Cheng, S.Z.; Pan, R.; Wunderlich, B. Thermal analysis of poly (butylene terephthalate) for heat capacity, rigid-amorphous content, and transition behavior. Die Makromol. Chem. Macromol. Chem. Phys. 1988, 189, 2443–2458. [Google Scholar] [CrossRef]
- Pyda, M.; Nowak-Pyda, E.; Heeg, J.; Huth, H.; Minakov, A.A.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Melting and crystallization of poly(butylene terephthalate) by temperature-modulated and superfast calorimetry. J. Polym. Sci. Polym. Phys. 2006, 44, 1364–1377. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Influence of processing conditions on polymer crystallization measured by fast scanning DSC. J. Therm. Anal. Calorim. 2014, 116, 1165–1173. [Google Scholar] [CrossRef]
- Androsch, R.; Rhoades, A.M.; Stolte, I.; Schick, C. Density of heterogeneous and homogeneous crystal nuclei in poly(butylene terephthalate). Eur. Polym. J. 2015, 66, 180–189. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Crystal nucleation of polymers at high supercooling of the melt. Adv. Polym. Sci. 2015, 276, 257–288. [Google Scholar]
- Schick, C.; Androsch, R.; Schmelzer, J.W.P. Homogeneous crystal nucleation in polymers. J. Phys. Cond. Matter 2017, 29, 453002. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Hybart, F.J. Effect of chemical structure on crystallization rates and melting of polymers: 2. Aliphatic polyesters. Polymer 1974, 15, 407–412. [Google Scholar] [CrossRef]
- Castles, J.L.; Vallance, M.A.; McKenna, J.M.; Cooper, S.L. Thermal and Mechanical Properties of Short-Segment Block Copolyesters and Copolyether-Esters. J. Polym. Sci. Polym. Phys. 1985, 23, 2119–2147. [Google Scholar] [CrossRef]
- Phillips, R.A.; McKenna, J.M.; Cooper, S.L. Glass Transition and Melting Behavior of Poly(Ether-ester) Multiblock Copolymers with Poly(Tetramethylene Isophthalate) Hard Segments. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 791–802. [Google Scholar] [CrossRef]
- Finelli, L.; Lotti, N.; Munari, A. Influence of Branching on the Thermal Behavior of Poly(Butylene Isophthalate). J. Appl. Polym. Sci. 2002, 84, 2001–2010. [Google Scholar] [CrossRef]
- Righetti, M.C.; Pizzoli, M.; Lotti, N.; Munari, A. Crystallization Kinetics and Melting Behavior of Poly(Butylene Adipate), Poly(Butylene Isophthalate) and Their Copolymers. Macromol. Chem. Phys. 1998, 199, 2063–2070. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zhao, X.; Zhu, D.; You, J. Self-Segregation Behavior of n-Ethyl-Pentadecafluoro- octanamide-Terminated Polybutylene Isophthalate and Its Effects on Film Morphology and Wettability. J. Phys. Chem. B 2009, 113, 15204–15211. [Google Scholar] [CrossRef]
- Huang, X.; Guo, R.; Lan, J. Synthesis and Characterizations of Branched Poly(Butylene Isophthalate)-Co-Poly(Tetramethylene Glycol)-Co-Poly(Ethylene Glycol) Fibers with Excellent Low Temperature Elastic Recovery. Polym. Sci.-Ser. B 2015, 57, 313–321. [Google Scholar] [CrossRef]
- Gómez, F.; Quintana, R.; De Ilarduya, A.M.; Rudé, E.; Muñoz-Guerra, S. Poly(Butylene Terephthalate-Co-5-Tert-Butyl Isophthalate) Copolyesters: Synthesis, Characterization, and Properties. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, J.C.; Cooper, S.L. Microstructure and Property Changes Accompanying Hard-Segment Crystallization in Block Copoly(Ether-Ester) Elastomers. Macromolecules 1988, 21, 1309–1316. [Google Scholar] [CrossRef]
- Lotti, N.; Finelli, L.; Munari, A.; Siracusa, V. Melting Behavior and Crystallization Kinetics of Sulfonated Poly(Butylene Isophthalate). Polym. Eng. Sci. 2002, 42, 1590–1599. [Google Scholar] [CrossRef]
- Finelli, L.; Lotti, N.; Munari, A. Crystallization Kinetics and Melting Behavior of Poly(Butylene Isophthalate/Terephthalate) Random Copolyesters. Eur. Polym. J. 2001, 37, 2039–2046. [Google Scholar] [CrossRef]
- Wang, Z.; Appelhans, D.; Synytska, A.; Komber, H.; Simon, F.; Grundke, K.; Voit, B. Studies of Surface Segregation and Surface Properties of N-Pentylperfluorooctaneamide End-Capped Semicrystalline Poly(Butylene Isophthalate) Films. Macromolecules 2008, 41, 8557–8565. [Google Scholar] [CrossRef]
- Alvarez, C.; Capitan, M.J.; Lotti, N.; Munari, A.; Ezquerra, T.A. Structure−Dynamics Relationships in Random Poly (butylene isophthalate-co-butylene adipate) Copolyesters As Revealed by Dielectric Loss Spectroscopy and X-ray Scattering. Macromolecules 2003, 36, 3245–3253. [Google Scholar] [CrossRef]
- Quattrosoldi, S.; Androsch, R.; Janke, A.; Soccio, M.; Lotti, N. Enthalpy Relaxation, Crystal Nucleation and Crystal Growth of Biobased Poly (butylene Isophthalate). Polymers 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkweather, H.W.; Brooks, R.E. Effect of spherulites on the mechanical properties of nylon 66. J. Appl. Polym. Sci. 1959, 1, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Way, J.L.; Atkinson, J.R.; Nutting, J. The effect of spherulite size on the fracture morphology of polypropylene. J. Mater. Sci. 1974, 9, 293–299. [Google Scholar] [CrossRef]
- Perkins, W.G. Polymer toughness and impact resistance. Polym. Eng. Sci. 1999, 39, 2445–2460. [Google Scholar] [CrossRef]
- Zia, Q.; Androsch, R.; Radusch, H.J. Effect of the structure at the micrometer and nanometer scales on the light transmission of isotactic polypropylene. J. Appl. Polym. Sci. 2010, 117, 1013–1020. [Google Scholar] [CrossRef]
- Tammann, G. Ueber die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur. Z. für Phys. Chem. 1898, 25, 441–479. [Google Scholar] [CrossRef]
- Tammann, G.; Jenckel, E. Die Kristallisationsgeschwindigkeit und die Kernzahl des Glycerins in Abhängigkeit von der Temperatur. Z. Anorg. Allg. Chem. 1930, 193, 76–80. [Google Scholar] [CrossRef]
- Schmelzer, J.W.; Abyzov, A.S.; Fokin, V.M.; Schick, C.; Zanotto, E.D. Crystallization of glass-forming liquids: Maxima of nucleation, growth, and overall crystallization rates. J. Non-Cryst. Solids 2015, 429, 24–32. [Google Scholar] [CrossRef]
- Mikhnevich, G.L.; Browko, J.F. Stability of the crystallization centers of an organic liquid at various temperatures and conclusions to be drawn therefrom concerning Tammann’s method. Phys. Z. Sowjetunion 1938, 13, 113–122. [Google Scholar]
- Nascimento, M.L.F.; Fokin, V.M.; Zanotto, E.D.; Abyzov, A.S. Dynamic Processes in a Silicate Liquid from above Melting to below the Glass Transition. J. Chem. Phys. 2011, 135, 194703. [Google Scholar] [CrossRef] [Green Version]
- Fokin, V.M.; Zanotto, E.D.; Yuritsyn, N.S.; Schmelzer, J.W.P. Homogeneous Crystal Nucleation in Silicate Glasses: A 40 Years Perspective. J. Non-Cryst. Solids 2006, 352, 2681–2714. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Wunderlich, B.; Schick, C. Kinetics of nucleation and crystallization in poly (ɛ-caprolactone) (PCL). Polymer 2011, 52, 1983–1997. [Google Scholar] [CrossRef]
- Wurm, A.; Zhuravlev, E.; Eckstein, K.; Jehnichen, D.; Pospiech, D.; Androsch, R.; Wunderlich, B.; Schick, C. Crystallization and homogeneous nucleation kinetics of poly (ε-caprolactone) (PCL) with different molar masses. Macromolecules 2012, 45, 3816–3828. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Crystal nucleation in glassy poly (l-lactic acid). Macromolecules 2013, 46, 6048–6056. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Kinetics of crystal nucleation of poly (l-lactic acid). Polymer 2013, 54, 6882–6885. [Google Scholar] [CrossRef]
- Androsch, R.; Iqbal, H.N.; Schick, C. Non-isothermal crystal nucleation of poly (l-lactic acid). Polymer 2015, 81, 151–158. [Google Scholar] [CrossRef]
- Andrianov, R.A.; Androsch, R.; Zhang, R.; Mukhametzyanov, T.A.; Abyzov, A.S.; Schmelzer, J.W.P.; Schick, C. Growth and dissolution of crystal nuclei in poly(l-lactic acid) (PLLA) in Tammann’s development method. Polymer 2020, 196, 122453. [Google Scholar] [CrossRef]
- Stolte, I.; Androsch, R.; Di Lorenzo, M.L.; Schick, C. Effect of aging the glass of isotactic polybutene-1 on form II nucleation and cold crystallization. J. Phys. Chem. B 2013, 117, 15196–15203. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Schick, C.; Schmelzer, J.W.P. Sequence of Enthalpy Relaxation, Homogeneous Crystal Nucleation and Crystal Growth in Glassy Polyamide 6. Eur. Polym. J. 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Rhoades, A.M. Application of Tammann’s Two-Stage Crystal Nuclei Development Method for Analysis of the Thermal Stability of Homogeneous Crystal Nuclei of Poly(Ethylene Terephthalate). Macromolecules 2015, 48, 8082–8089. [Google Scholar] [CrossRef]
- Holand, W.; Beall, G.H. Glass-Ceramic Technology; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-42369-0. [Google Scholar]
- Minakov, A.A.; Mordvintsev, D.A.; Schick, C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer 2004, 45, 3755–3763. [Google Scholar] [CrossRef]
- Jariyavidyanont, K.; Androsch, R.; Schick, C. Crystal reorganization of poly (butylene terephthalate). Polymer 2017, 124, 274–283. [Google Scholar] [CrossRef]
- Furushima, Y.; Schick, C.; Toda, A. Crystallization, recrystallization, and melting of polymer crystals on heating and cooling examined with fast scanning calorimetry. Polym. Cryst. 2018, 1, e10005. [Google Scholar] [CrossRef]
- Davis, M.J. Effect of the Growth Treatment on Two-Stage Nucleation Experiments. J. Am. Ceram. Soc. 2001, 84, 492–496. [Google Scholar] [CrossRef]
- Deubener, J.; Montazerian, M.; Krüger, S.; Peitl, O.; Zanotto, E.D. Heating rate effects in time-dependent homogeneous nucleation in glasses. J. Non-Cryst. Solids 2017, 474, 1–8. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Androsch, R.; Schick, C. Experimental test of Tammann’s nuclei development approach in crystallization of macromolecules. Cryst. Growth Des. 2015, 15, 786–798. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quattrosoldi, S.; Lotti, N.; Soccio, M.; Schick, C.; Androsch, R. Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature. Polymers 2020, 12, 1099. https://doi.org/10.3390/polym12051099
Quattrosoldi S, Lotti N, Soccio M, Schick C, Androsch R. Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature. Polymers. 2020; 12(5):1099. https://doi.org/10.3390/polym12051099
Chicago/Turabian StyleQuattrosoldi, Silvia, Nadia Lotti, Michelina Soccio, Christoph Schick, and René Androsch. 2020. "Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature" Polymers 12, no. 5: 1099. https://doi.org/10.3390/polym12051099
APA StyleQuattrosoldi, S., Lotti, N., Soccio, M., Schick, C., & Androsch, R. (2020). Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature. Polymers, 12(5), 1099. https://doi.org/10.3390/polym12051099