Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Neem Leaves Powder
2.3. Extraction of Neem Leaves
2.4. Preparation of Seaweed-Neem Films
2.5. Characterization of Seaweed-Based Films Incorporated with Neem Leaves Extract and Irradiated
2.5.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5.2. Surface Characterization by Scanning Electron Microscope (SEM) and Atomic Force Microscope (AFM)
2.5.3. Colour and Opacity Properties
2.5.4. Moisture Content
2.5.5. Solubility
2.5.6. Water Vapour Permeability (WVP)
2.5.7. Wettability Test
2.5.8. Tensile Properties
2.5.9. Antimicrobial Activity
2.5.10. Soil Burial Test
3. Results and Discussion
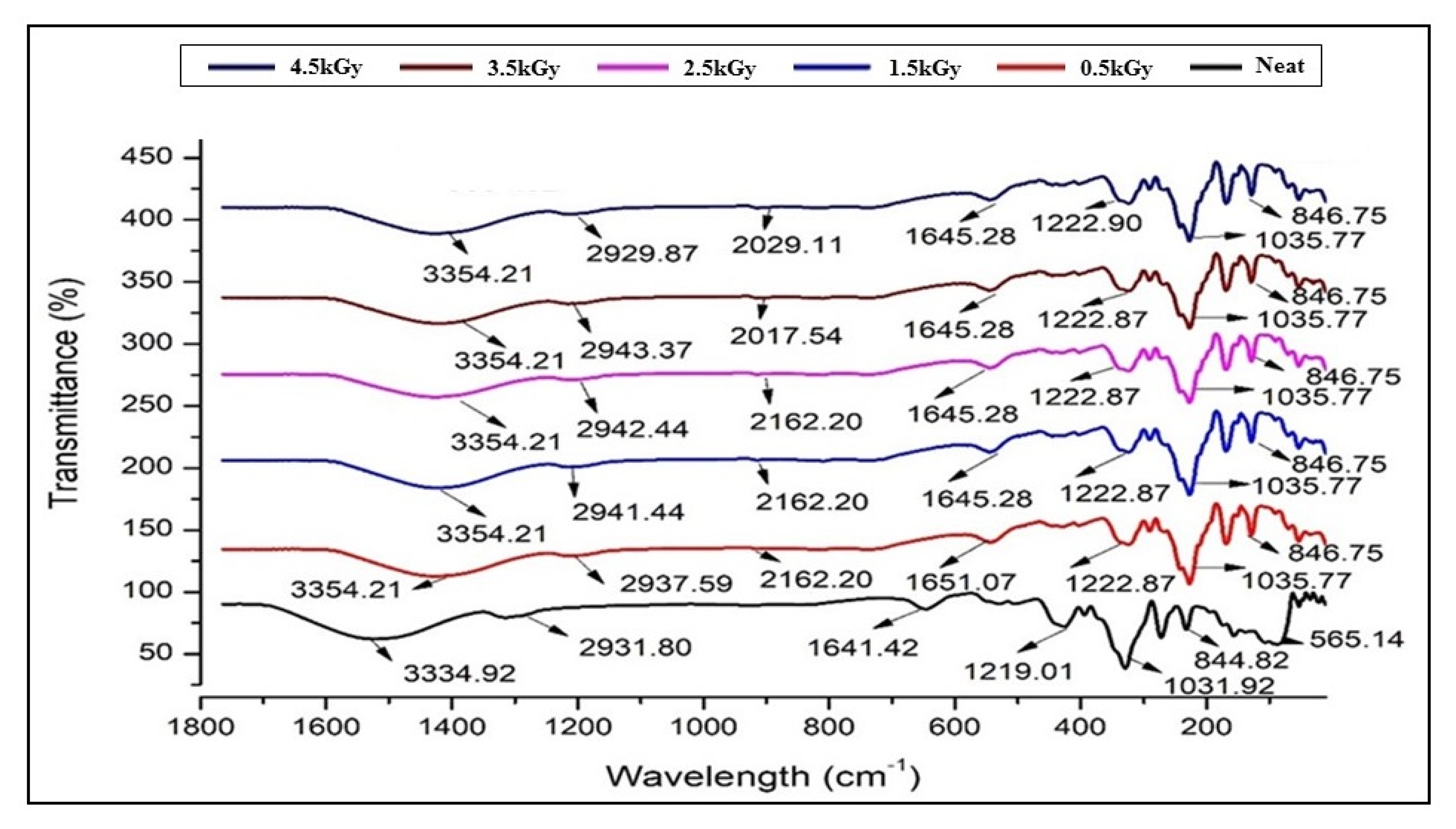
3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
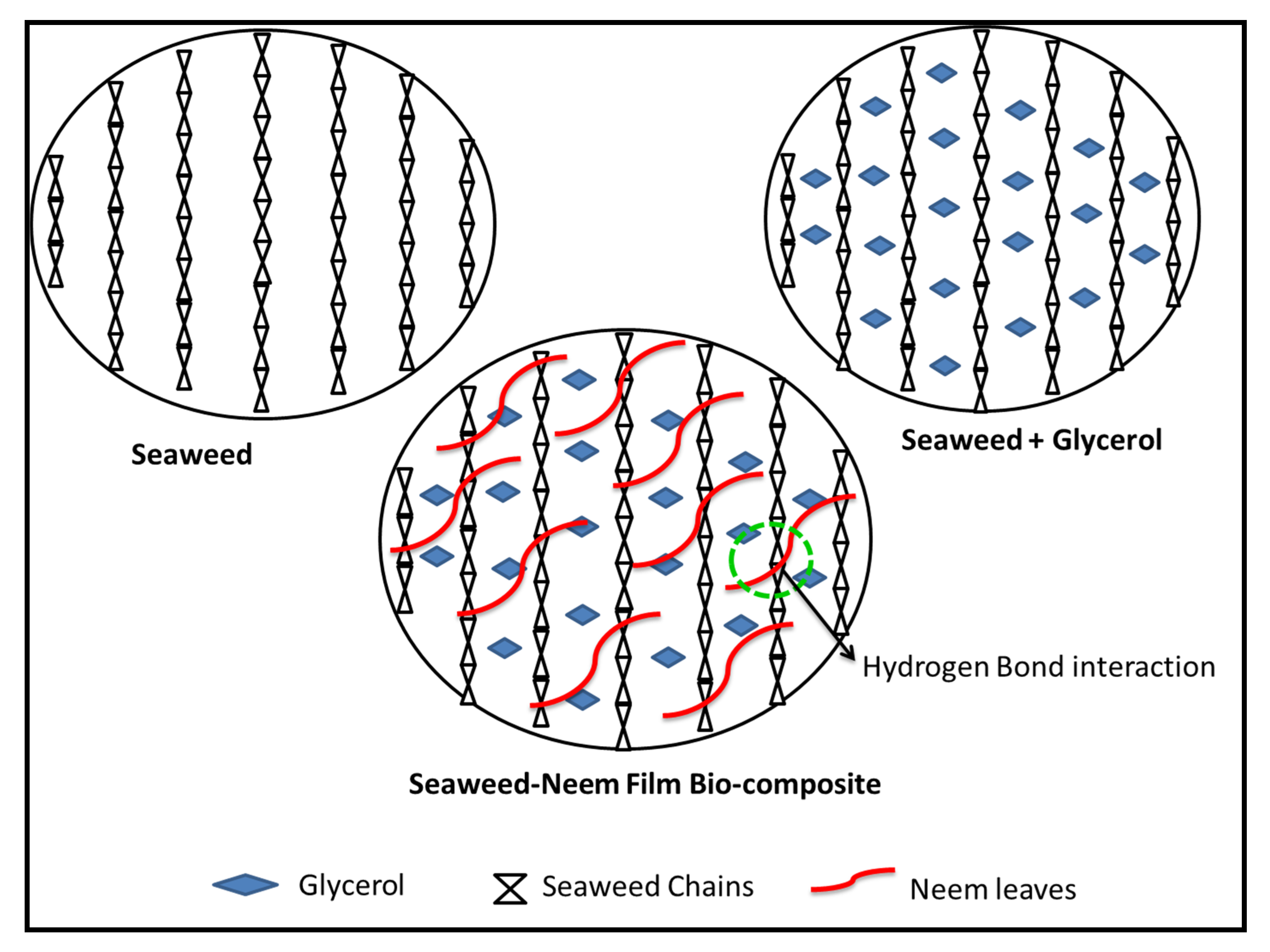
3.2. Surface Characterization of Seaweed-Neem Films
3.3. Colour and Opacity
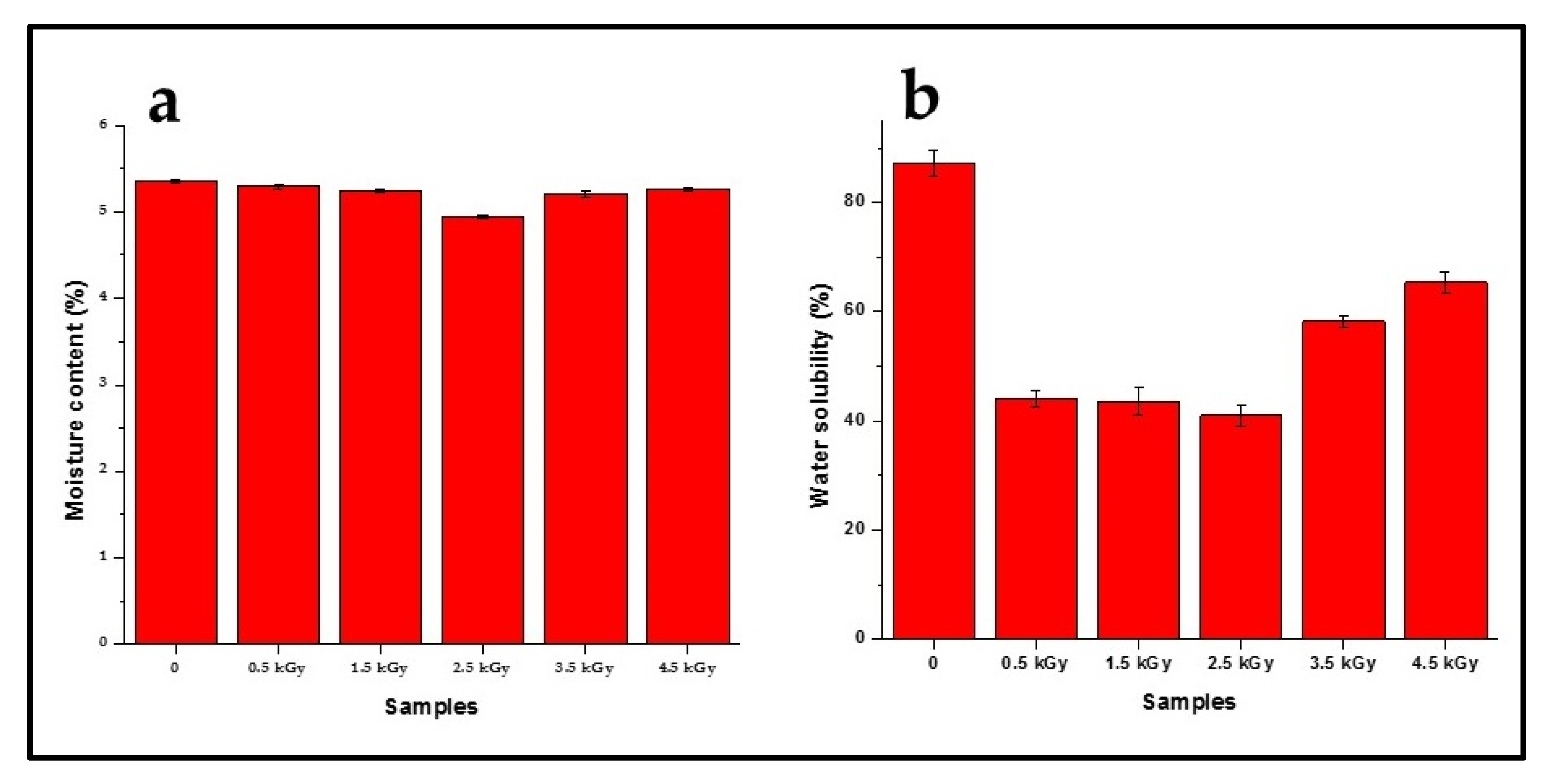
3.4. Moisture Content and Water Solubility of the Films
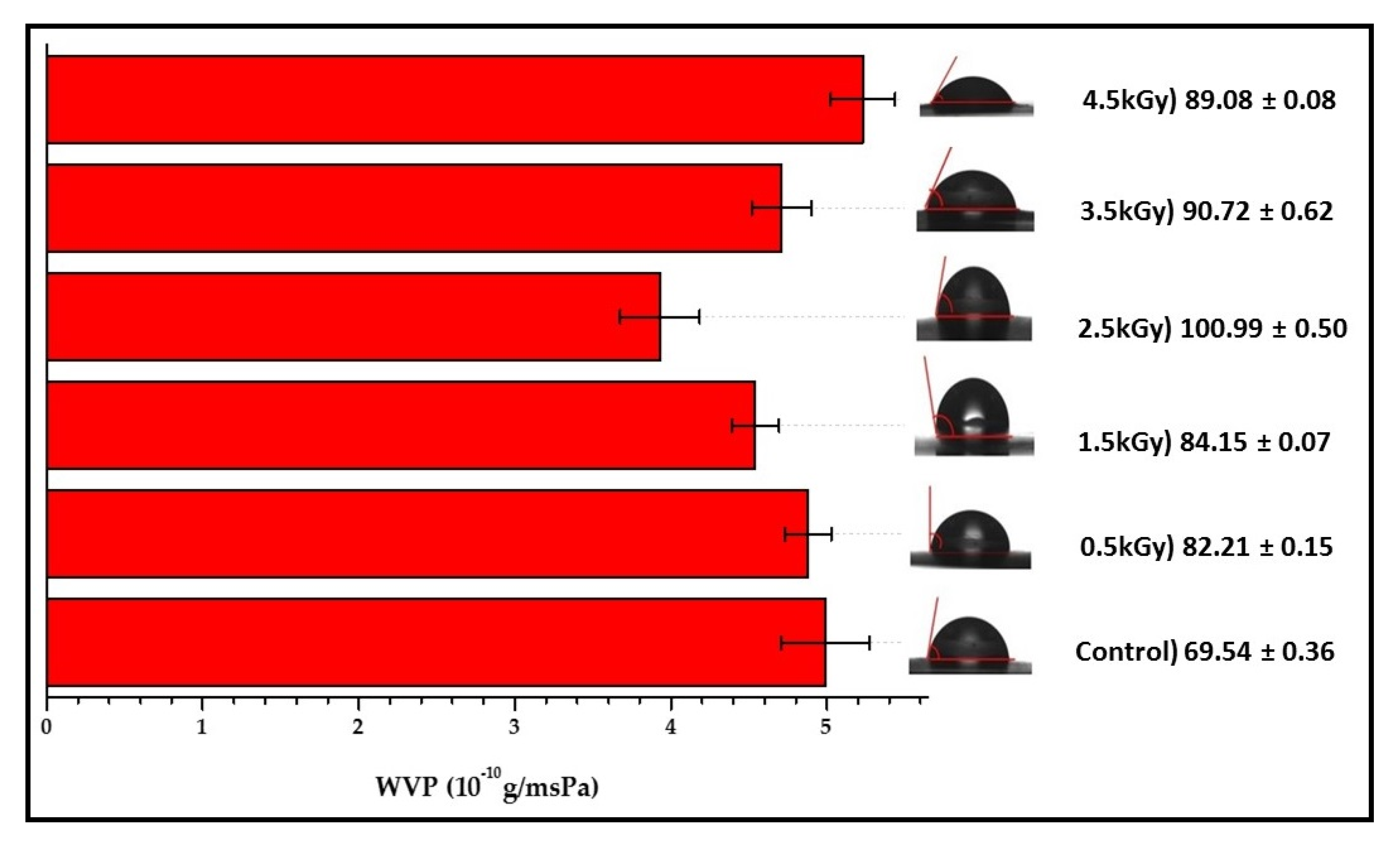
3.5. Water Vapour Permeability
3.6. Contact Angle
3.7. Mechanical Properties of Films
3.8. Biodegradability
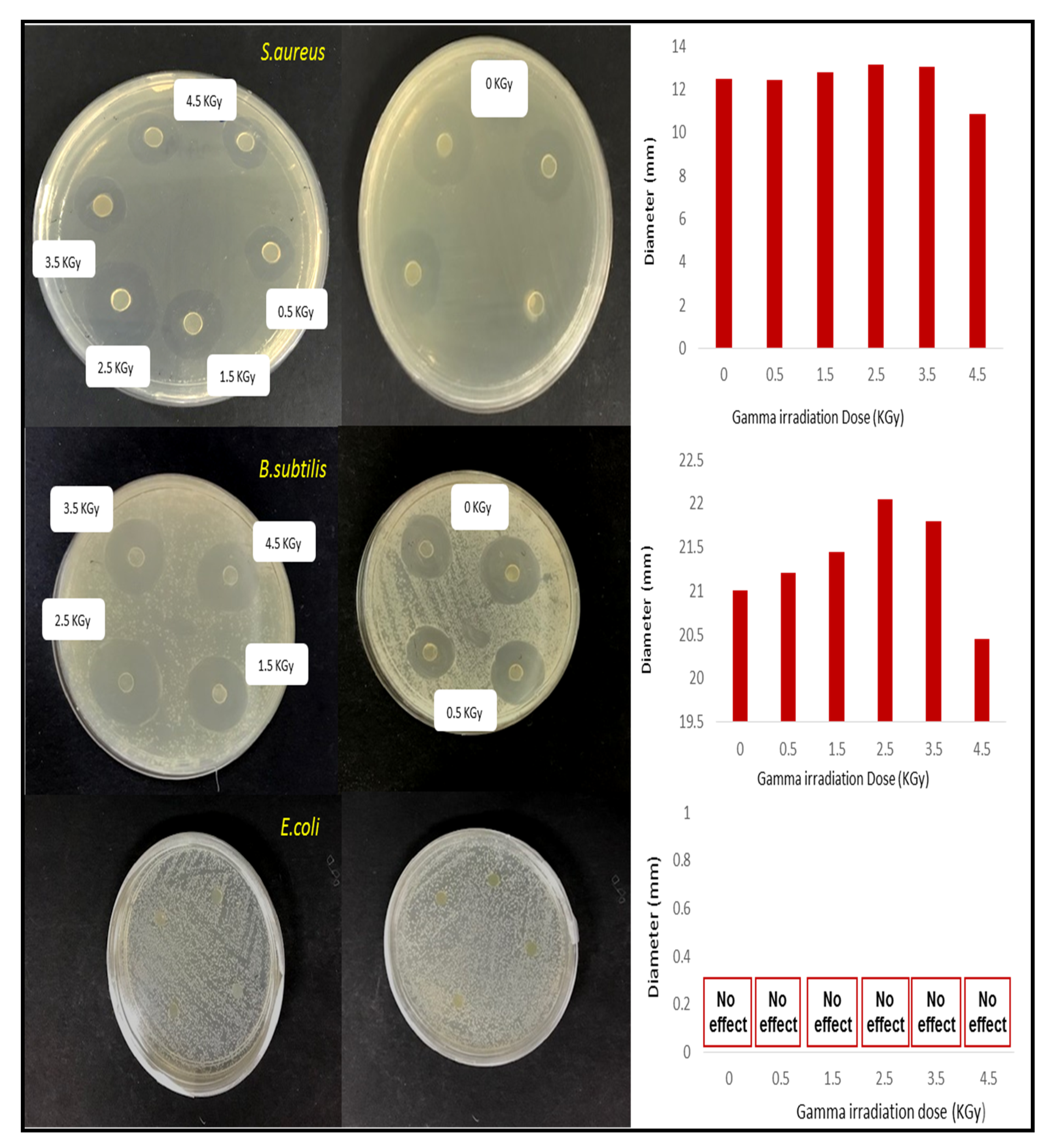
3.9. Effect of Irradiation Dose on the Antimicrobial Activity of Seaweed-Neem Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Chong, E.; Owolabi, F.; Asniza, M.; Tye, Y.; Rizal, S.; Nurul Fazita, M.; Mohamad Haafiz, M.; Nurmiati, Z.; Paridah, M. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Saurabh, C.K.; Variyar, P.S.; Sharma, A. Comparative analysis of dietary fiber activities of enzymatic and gamma depolymerized guar gum. Food Hydrocoll. 2015, 48, 149–154. [Google Scholar] [CrossRef]
- Olaiya, N.; Surya, I.; Oke, P.; Rizal, S.; Sadiku, E.; Ray, S.S.; Farayibi, P.; Hossain, M.S.; Abdul Khalil, H.P.S. Properties and Characterization of a PLA–Chitin–Starch Biodegradable Polymer Composite. Polymers 2019, 11, 1656. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Dogan, M.; Kayacier, A.; Ic, E. Rheological characteristics of some food hydrocolloids processed with gamma irradiation. Food Hydrocoll. 2007, 21, 392–396. [Google Scholar] [CrossRef]
- Kim, J.K.; Jo, C.; Park, H.J.; Byun, M.W. Effect of gamma irradiation on the physicochemical properties of a starch-based film. Food Hydrocoll. 2008, 22, 248–254. [Google Scholar]
- Sangetha, S.; Sasidharan, S.; Zuraini, Z.; Suryani, S. Antioxidant activity of methanolic extracts of Cassia surattensis. Pharmacologyonline 2008, 2, 829–838. [Google Scholar]
- Jeong, J.O.; Park, J.S.; Kim, Y.A.; Yang, S.J.; Jeong, S.I.; Lee, J.Y.; Lim, Y.M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- AlSalhi, M.S.; Prasad, S.; Devaraj, D.; Abo Mustafa, Z.S. Gamma-irradiation effects on the spectral and amplified spontaneous emission (ASE) properties of conjugated polymers in solution. Polymers 2017, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Navarro, R.; Burillo, G.; Adem, E.; Marcos-Fernández, A. Effect of ionizing radiation on the chemical structure and the physical properties of polycaprolactones of different molecular weight. Polymers 2018, 10, 397. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- E96/E96M-16, A. Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Teimouri, S.; Abbasi, S.; Sheikh, N. Effects of gamma irradiation on some physicochemical and rheological properties of Persian gum and gum tragacanth. Food Hydrocoll. 2016, 59, 9–16. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Borzacchiello, A.; Tay, F.R.; Ashtari, B.; Padil, V.V. Antimicrobial gum bio-based nanocomposites and their industrial and biomedical applications. Chem. Commun. 2019, 55, 14871–14885. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 1910021. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Jamaledin, R.; Naserzadeh, P.; Afjeh-Dana, E.; Ashtari, B.; Hosseinzadeh, M.; Vecchione, R.; Wu, A.; Tay, F.R.; Borzacchiello, A. Metal-based Nanostructures/PLGA Nanocomposites: Antimicrobial Activity, Cytotoxicity and Their Biomedical Applications. Acs Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Singh, S.P.; Bhargava, C.; Dubey, V.; Mishra, A.; Singh, Y. Silver nanoparticles: Biomedical applications, toxicity, and safety issues. Int. J. Res. Pharm. Pharm. Sci. 2017, 4, 1–10. [Google Scholar]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Harun, A.; Ab Rahim, N.E.A.; Abd Jalil, M.A.; Rosdi, A.M.; Daud, S.; Harith, S.S.; So’ad, S.Z.M.; Hassan, N.M. Comparative study of antioxidant and antimicrobial activity of root, stem and leaves of Leea indica species. Malays. J. Sci. 2017, 35, 259–274. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, P.; Anandan, R.; Nagini, S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and protective effects against H2O2-induced oxidative damage to pBR322 DNA and red blood cells. J. Agric. Food Chem. 2009, 57, 6990–6996. [Google Scholar] [CrossRef]
- Kumar, U.S.U.; Paridah, M.; Owolabi, F.T.; Gopakumar, D.A.; Rizal, S.; Amirul, A.; Rahman, A.; Alfatah, T.; Mistar, E.; Aprilia, N.S.; et al. Neem Leaves Extract Based Seaweed Bio-degradable Composite Films with Excellent Antimicrobial Activity for Sustainable Packaging Material. BioResources 2018, 14, 700–713. [Google Scholar]
- Al-Hashemi, Z.S.S.; Hossain, M.A. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac. Sci. Rev. A 2016, 18, 128–131. [Google Scholar] [CrossRef] [Green Version]
- Shellikeri, A.; Kaulgud, V.; Yaradoddi, J.; Ganachari, S.; Banapurmath, N.; Shettar, A. Development of Neem Based Bioplastic for Food Packaging Application. In IOP Conference Series: Materials Science and Engineering, Prcceedings of International Conference on Advances in Manufacturing, Materials and Energy Engineering (ICon MMEE), Mangalore Institute Of Technology & Engineering, Badaga Mijar, Moodbidri, Karnataka, India, 2–3 March 2018; IOP Publishing: Bangalore, India, 2018. [Google Scholar]
- ASTM International. ASTM D882-12, Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Rahman, N.; Dafader, N.; Banu, P. Preparation and Property Analysis of Biodegradable Packaging Film from Alginate, Starch and Citric acid. J. Polym. Sci. Technol. 2017, 2, 20–35. [Google Scholar]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Effect of γ-irradiation on the physical and mechanical properties of kefiran biopolymer film. Int. J. Biol. Macromol. 2015, 74, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Yap, S.W.; Tye, Y.Y.; Tahir, P.M.; Rizal, S.; Fazita, M.N. Effects of corn starch and Kappaphycus alvarezii seaweed blend concentration on the optical, mechanical, and water vapor barrier properties of composite films. BioResources 2018, 13, 1157–1173. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Yamashita, F.; Garcia, M.A.; Martino, M.N.; Zaritzky, N.E.; Grossmann, M.V.E. Constrained mixture design applied to the development of cassava starch–chitosan blown films. J. Food Eng. 2012, 108, 262–267. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Soliman, E.A.; Mohy Eldin, M.S.; Furuta, M. Biodegradable zein-based films: Influence of γ-irradiation on structural and functional properties. J. Agric. Food Chem. 2009, 57, 2529–2535. [Google Scholar] [CrossRef]
- Gul-E-Noor, F.; Khan, M.A.; Ghoshal, S.; Mazid, R.A.; Sarwaruddin Chowdhury, A.; Khan, R.A. Grafting of 2-ethylhexyl acrylate with urea on to gelatin film by gamma radiation. J. Macromol. Sci. Part A 2009, 46, 615–624. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.; Saurabh, C.; Leh, C.; Lai, T.; Chong, E.; Fazita, M.; Hafiidz, J.M.; Banerjee, A.; Syakir, M. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Chan, M.Y.; Koay, S.C. Biodegradation and thermal properties of crosslinked chitosan/corn cob biocomposite films by electron beam irradiation. Polym. Eng. Sci. 2019, 59, E59–E68. [Google Scholar] [CrossRef]
- Sam, S.; Nuradibah, M.; Ismail, H.; Noriman, N.; Ragunathan, S. Recent advances in polyolefins/natural polymer blends used for packaging application. Polym. Plast. Technol. Eng. 2014, 53, 631–644. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Degradation behavior of biocomposites based on cassava starch buried under indoor soil conditions. Carbohydr. Polym. 2014, 101, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Koona, S.; Budida, S. Antibacterial Potential of the Extracts of the Leaves of Azadirachta indica Linn. Not. Sci. Biol. 2011, 3, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Castell-Perez, M.; Moreira, R. The influence of electron beam irradiation of antimicrobial-coated LDPE/polyamide films on antimicrobial activity and film properties. Lwt-Food Sci. Technol. 2007, 40, 1545–1554. [Google Scholar] [CrossRef]




| Parameter | Dose (kGy) | |||||
|---|---|---|---|---|---|---|
| 0.5 | 1.5 | 2.5 | 3.5 | 4.5 | Control | |
| L* | 74.5 ± 0.02 a | 76.1 ± 0.03 b | 80.5 ± 0.01 f | 79.1 ± 0.02 e | 78.6 ± 0.06 d | 83.8 ± 0.04 g |
| a* | −0.5 ± 0.01 b,c | −0.6 ± 0.01 a,b | −0.6 ± 0.10 a | −0.4 ± 0.01 c | −0.4 ± 0.01 c | −0.4 ± 0.03 bc |
| b* | 35.1 ± 0.02 f | 33.2 ± 0.37 e | 29.1 ± 0.07 b | 30.4 ± 0.05 c | 30.6 ± 0.18 c,d | 21.5 ± 0.10 a |
| ∆E | 116.3 ± 0.21 g | 114.3 ± 0.28 f | 109.0 ± 0.05 b | 110.7 ± 0.15 c | 113.4 ± 0.08 e | 104.3 ± 0.06 a |
| Opacity | 17.0 ± 0.02 c | 16.8 ± 0.03 b | 16.6 ± 0.01 a | 16.6 ± 0.04 a | 17.5 ± 0.03 d,c | 17.0 ± 0.01 c |
| Dose (kGy) | Thickness (mm) | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation (%) |
|---|---|---|---|---|
| 0.5 | 0.1072 ± 0.0013 a | 40.57 ± 0.67 c | 316.12 ± 18.70 | 19.25 ± 0.14 f |
| 1.5 | 0.1083 ± 0.0019 a | 41.81 ± 1.76 c | 335.37 ± 13.31 | 18.70 ± 0.14 e |
| 2.5 | 0.1085 ± 0.0058 a | 43.23 ± 0.25 d | 355.90 ± 10.98 | 18.22 ± 0.08 d |
| 3.5 | 0.1088 ± 0.0096 a | 38.55 ± 0.22 b | 323.94 ± 15.79 | 17.85 ± 0.10 c |
| 4.5 | 0.1090 ± 0.0083 a | 36.79 ± 0.73 a | 314.62 ± 17.17 | 17.54 ± 0.06 b |
| Control | 0.1060 ± 0.0053 a | 39.95 ± 0.35 c,d | 309.05 ± 10.57 | 19.39 ± 0.74 a,d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthaya Kumar, U.S.; Abdulmadjid, S.N.; Olaiya, N.G.; Amirul, A.A.; Rizal, S.; Rahman, A.A.; Alfatah, T.; Mistar, E.M.; Abdul Khalil, H.P.S. Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polymers 2020, 12, 1119. https://doi.org/10.3390/polym12051119
Uthaya Kumar US, Abdulmadjid SN, Olaiya NG, Amirul AA, Rizal S, Rahman AA, Alfatah T, Mistar EM, Abdul Khalil HPS. Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polymers. 2020; 12(5):1119. https://doi.org/10.3390/polym12051119
Chicago/Turabian StyleUthaya Kumar, U. Seeta, S. N. Abdulmadjid, N. G. Olaiya, A. A. Amirul, S. Rizal, A. A. Rahman, Tata Alfatah, E. M. Mistar, and H. P. S. Abdul Khalil. 2020. "Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films" Polymers 12, no. 5: 1119. https://doi.org/10.3390/polym12051119
APA StyleUthaya Kumar, U. S., Abdulmadjid, S. N., Olaiya, N. G., Amirul, A. A., Rizal, S., Rahman, A. A., Alfatah, T., Mistar, E. M., & Abdul Khalil, H. P. S. (2020). Extracted Compounds from Neem Leaves as Antimicrobial Agent on the Physico-Chemical Properties of Seaweed-Based Biopolymer Films. Polymers, 12(5), 1119. https://doi.org/10.3390/polym12051119









