Epoxy-Anhydride Vitrimers from Aminoglycidyl Resins with High Glass Transition Temperature and Efficient Stress Relaxation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Epoxy-Anhydride Vitrimers
2.2.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.3. Dynamic-Mechanical Thermal Analysis (DMTA)
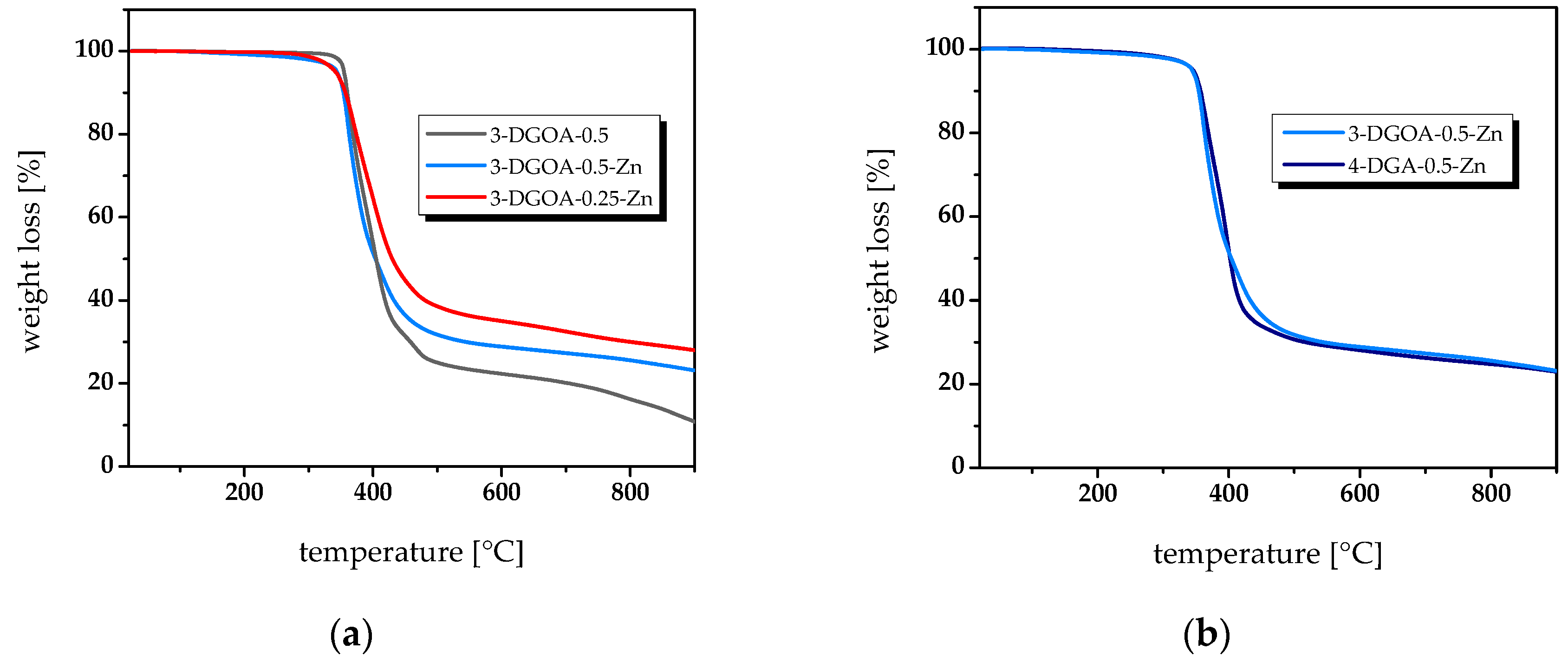
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Stress Relaxation
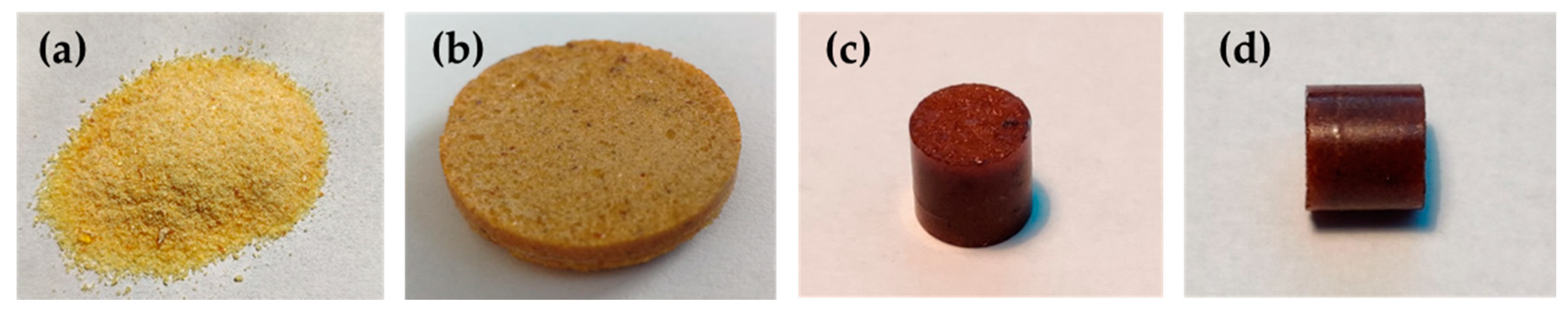
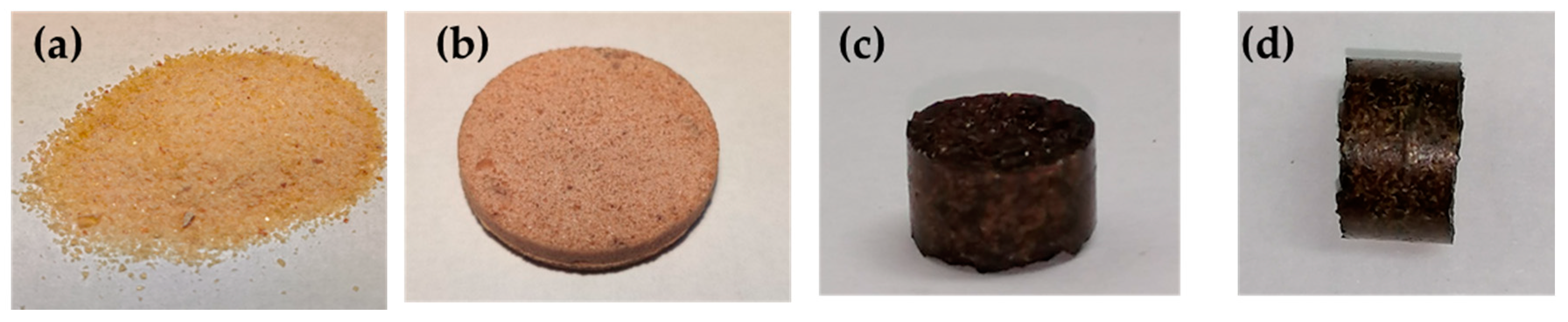
2.2.6. Recycling of Vitrimers
3. Results and Discussion
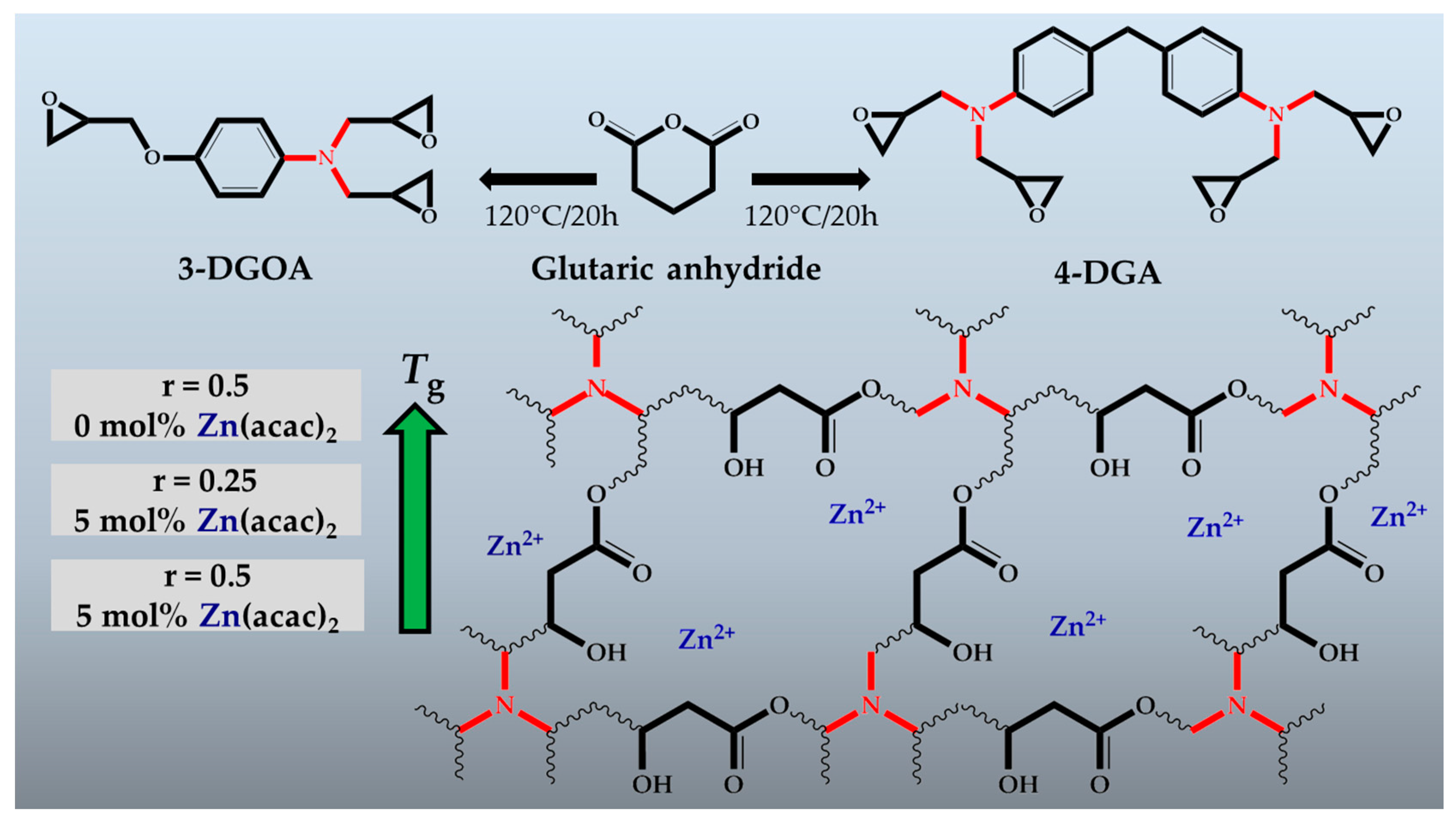
3.1. Design and Curing of High-Tg Epoxy-Anhydride Vitrimers
3.2. Thermal and Thermo-Mechanical Properties of High-Tg Epoxy-Anhydride Vitrimers
3.3. Thermally Adaptable Properties and Reprocessability of High-Tg Epoxy-Anhydride Vitrimers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, R.H.; Redmann, A.L.; Pei, Z.; Ji, Y.; Terentjev, E.M. Vitrification and plastic flow in transient elastomer networks. Polymer 2016, 95, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic control of the vitrimer glass transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winne, J.M.; Leibler, L.; Du Prez, F. Dynamic Covalent Chemistry in Polymer Networks: A Mechanistic Perspective. Polym. Chem. 2019, 10, 6091–6108. [Google Scholar] [CrossRef]
- Lu, L.; Fan, J.; Li, G. Intrinsic healable and recyclable thermoset epoxy based on shape memory effect and transesterification reaction. Polymer 2016, 105, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Reprocessing and recycling of thermosetting polymers based on bond exchange reactions. RSC Adv. 2014, 4, 10108–10117. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Kaiser, S.; Wurzer, S.; Pilz, G.; Kern, W.; Schlögl, S. Stress relaxation and thermally adaptable properties in vitrimer-like elastomers from HXNBR rubber with covalent bonds. Soft Matter. 2019, 15. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; Guo, B.; Zhang, L. Malleable, Mechanically Strong, and Adaptive Elastomers Enabled by Interfacial Exchangeable Bonds. Macromolecules 2017, 50, 7584–7592. [Google Scholar] [CrossRef]
- Cao, L.; Fan, J.; Huang, J.; Chen, Y. A robust and stretchable cross-linked rubber network with recyclable and self-healable capabilities based on dynamic covalent bonds. J. Mater. Chem. A 2019, 7, 4922–4933. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Wang, T. Dual-Triggered and Thermally Reconfigurable Shape Memory Graphene-Vitrimer Composites. ACS Appl. Mater. Interfaces 2016, 8, 21691–21699. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yano, R. Fair Investigation of Cross-Link Density Effects on the Bond-Exchange Properties for Trans-Esterification-Based Vitrimers with Identical Concentrations of Reactive Groups. Macromolecules 2020, 53, 182–189. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Christophe, D.; Sebastien-jun, M.; Tournilhac Francois-genes; Ludwik, L. Titanium-Based Catalyst for Vitrimer Resins of Epoxy/Anhydride Type. U.S. Patent 20170044307, 16 February 2017. [Google Scholar]
- Stukenbroeker, T.; Wang, W.; Winne, J.M.; Du Prez, F.E.; Nicolaÿ, R.; Leibler, L. Polydimethylsiloxane quenchable vitrimers. Polym. Chem. 2017, 8, 6590–6593. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Lewis, C.L.; Dell, E.M. A review of shape memory polymers bearing reversible binding groups. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1340–1364. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Zhang, S.; Yang, X.; Wang, L.; Han, J.; Li, Y.; Xin, J.; Zhang, J. A Self-Healable High Glass Transition Temperature Bioepoxy Material Based on Vitrimer Chemistry. Macromolecules 2018, 51, 5577–5585. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Yu, R.; Zhao, X.-J.; Zhang, Y.; Huang, W. A facile access to stiff epoxy vitrimers with excellent mechanical properties via siloxane equilibration. J. Mater. Chem. A 2018, 6, 10184–10188. [Google Scholar] [CrossRef]
- Dizman, C.; Tasdelen, M.A.; Yagci, Y. Recent advances in the preparation of functionalized polysulfones. Polym. Int. 2013, 62, 991–1007. [Google Scholar] [CrossRef]
- Rocks, J.; George, G.A.; Vohwinkel, F. Curing kinetics and thermomechanical behaviour of co-anhydride cured aminoglycidyl epoxy resins. Polym. Int. 2003, 52, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Mauri, A.N.; Riccardi, C.C. The effect of epoxy excess on the kinetics of an epoxy-anhydride system. J. Appl. Polym. Sci. 2002, 85, 2342–2349. [Google Scholar] [CrossRef]
- Varma, I.K.; Gupta, V.B. Thermosetting Resin—Properties. In Comprehensive Composite Materials; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1–56. [Google Scholar]
- Paramarta, A.; Webster, D.C. Curing kinetics of bio-based epoxy-anhydride thermosets with zinc catalyst. J. Therm. Anal. Calorim. 2017, 130, 2133–2144. [Google Scholar] [CrossRef]
- May, C. Epoxy Resins: Chemistry and Technology, 2nd ed.; CRC Press: Cleveland, OH, USA, 1987; ISBN 9780824776909. [Google Scholar]
- Chen, J.; Soucek, M.D. Ultraviolet curing kinetics of cycloaliphatic epoxide with real-time fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 2003, 90, 2485–2499. [Google Scholar] [CrossRef]
- Trappe, V.; Burchard, W.; Steinmann, B. Anhydride-cured epoxies via chain reaction. 1. The phenyl glycidyl ether/phthalic acid anhydride system. Macromolecules 1991, 24, 4738–4744. [Google Scholar] [CrossRef]
- Leukel, J.; Burchard, W.; Krüger, R.-P.; Much, H.; Schulz, G. Mechanism of the anionic copolymerization of anhydride-cured epoxies—Analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Macromol. Rapid Commun. 1996, 17, 359–366. [Google Scholar] [CrossRef]
- Barabanova, A.I.; Lokshin, B.V.; Kharitonova, E.P.; Afanasyev, E.S.; Askadskii, A.A.; Philippova, O.E. Curing cycloaliphatic epoxy resin with 4-methylhexahydrophthalic anhydride: Catalyzed vs. uncatalyzed reaction. Polymer 2019, 178, 121590. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Mondragon, I.; Riccardi, C.C.; Williams, R.J.J. Polymer networks derived from the anhydride curing of tetraepoxides. J. Appl. Polym. Sci. 1997, 64, 157–166. [Google Scholar] [CrossRef]
- Couture, G.; Granado, L.; Fanget, F.; Boutevin, B.; Caillol, S. Limonene-based epoxy: Anhydride thermoset reaction study. Molecules 2018, 23, 2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musto, P.; Abbate, M.; Ragosta, G.; Scarinzi, G. A study by Raman, near-infrared and dynamic-mechanical spectroscopies on the curing behaviour, molecular structure and viscoelastic properties of epoxy/anhydride networks. Polymer 2007, 48, 3703–3716. [Google Scholar] [CrossRef]
- Naar, N.; Lamouri, S.; Jeacomine, I.; Pron, A.; Rinaudo, M. A Comprehensive Study and Characterization of Colloidal Emeraldine-Base. J. Macromol. Sci. Part A 2012, 49, 897–905. [Google Scholar] [CrossRef]



| System | 3-DGOA (g) | 4-DGA (g) | Anhydride (g) | Zn(acac)2-H2O (mg) | r |
|---|---|---|---|---|---|
| 3-DGOA-0.5-Zn | 5 | - | 5.7 | 658 | 0.5 |
| 3-DGOA-0.25-Zn | 5 | - | 2.9 | 658 | 0.25 |
| 3-DGOA-0.5 | 5 | - | 5.7 | - | 0.5 |
| 4-DGA-0.5-Zn | - | 5.7 | 5.7 | 658 | 0.5 |
| 4-DGA-0.25-Zn | - | 5.7 | 2.9 | 658 | 0.25 |
| 4-DGA-0.5 | - | 5.7 | 5.7 | - | 0.5 |
| System | Tg (°C) | Tv (°C) | Ea (kJ/mol) | R2 |
|---|---|---|---|---|
| 3-DGOA-0.25-Zn | 120 | 113 | 53 | 0.97 |
| 3-DGOA-0.5-Zn | 112 | 190 | 87 | 0.95 |
| 3-DGOA-0.5 | 125 | 241 | 154 | 0.99 |
| 4-DGA-0.25-Zn | 122 | 188 | 111 | 0.99 |
| 4-DGA-0.5-Zn | 130 | 227 | 158 | 0.96 |
| 4-DGA-0.5 | 140 | 238 | 161 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebler, M.; Sperling, C.; Kaiser, S.; Duretek, I.; Schlögl, S. Epoxy-Anhydride Vitrimers from Aminoglycidyl Resins with High Glass Transition Temperature and Efficient Stress Relaxation. Polymers 2020, 12, 1148. https://doi.org/10.3390/polym12051148
Giebler M, Sperling C, Kaiser S, Duretek I, Schlögl S. Epoxy-Anhydride Vitrimers from Aminoglycidyl Resins with High Glass Transition Temperature and Efficient Stress Relaxation. Polymers. 2020; 12(5):1148. https://doi.org/10.3390/polym12051148
Chicago/Turabian StyleGiebler, Michael, Clemens Sperling, Simon Kaiser, Ivica Duretek, and Sandra Schlögl. 2020. "Epoxy-Anhydride Vitrimers from Aminoglycidyl Resins with High Glass Transition Temperature and Efficient Stress Relaxation" Polymers 12, no. 5: 1148. https://doi.org/10.3390/polym12051148






