Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biocomposites
2.3. Characterization Methods
3. Results and Discussion
3.1. Structural Characterization
3.2. Thermal Stability
3.3. Mechanical and Thermomechanical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of a bisphenol a based phthalonitrile resin. J. Polym. Sci. A 2005, 43, 4136–4143. [Google Scholar] [CrossRef]
- Derradji, M.; Ramdani, N.; Zhang, T.; Wang, J.; Gong, L.D.; Xu, X.D.; Lin, Z.W.; Henniche, A.; Rahoma, H.K.S.; Liu, W.B. Effect of silane surface modified titania nanoparticles on the thermal, mechanical, and corrosion protective properties of a bisphenol-A based phthalonitrile resin. Prog. Org. Coat. 2016, 90, 34–43. [Google Scholar] [CrossRef]
- Zhang, D.; Jia, D. Toughness and strength improvement of diglycidyl ether of bisphenol-A by low viscosity liquid hyperbranched epoxy resin. J. Appl. Polym. Sci. 2006, 101, 2504–2511. [Google Scholar] [CrossRef]
- Iyer, S.; Schiraldi, D.A. Role of Specific Interactions and Solubility in the Reinforcement of Bisphenol A Polymers with Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2007, 40, 4942–4952. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Liu, D.W. Ethoxylated bisphenol dimethacrylate-based amorphous calcium phosphate composites. Acta Biomater. 2006, 2, 85–94. [Google Scholar] [CrossRef]
- Imai, Y.; Terahara, A.; Hakuta, Y.; Matsui, K.; Hayashi, H.; Ueno, N. Transparent poly(bisphenol A carbonate)-based nanocomposites with high refractive index nanoparticles. Eur. Polym. J. 2009, 45, 630–638. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Na, H.; Wu, Y.; Zheng, W.; Zhu, J. How a bio-based epoxy monomer enhanced the properties of diglycidyl ether of bisphenol A (DGEBA)/graphene composites. J. Mater. Chem. A 2013, 1, 5081–5088. [Google Scholar] [CrossRef]
- Jang, L.W.; Lee, D.C. Polystyrene/bisphenol A polycarbonate molecular composite by in situ polymerization. I. Preparation and characterization. Polymer 2000, 41, 1749–1756. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Gu, H.; Colorado, A.; Wei, S.; Guo, Z. Flame-retardant electrical conductive nanopolymers based on bisphenol F epoxy resin reinforced with nano polyanilines. ACS Appl. Mater. Interfaces 2013, 5, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lee, H.T.; Chen, J.K. Preparation and properties of bisphenol-F based boron-phenolic resin/modified silicon nitride composites and their usage as binders for grinding wheels. Appl. Surf. Sci. 2015, 330, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Sun, J. Flame retardant epoxy resins from bisphenol-A epoxy cured with hyperbranched polyphosphate ester. J. Polym. Res. 2011, 18, 2229–2237. [Google Scholar] [CrossRef]
- Guzel, G.; Deveci, H. Properties of polymer composites based on bisphenol A epoxy resins with original/modified steel slag. Polym. Compos. 2018, 39, 513–521. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, M.; Fang, J.; Wang, C. Silicon–aluminum synergistic mechanism in flame retardancy of epoxy resin. Polym. Compos. 2014, 35, 1553–1558. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, S. Mechanical, thermal, and dielectric properties of aluminum nitride/glass fiber/epoxy resin composites. Polym. Compos. 2014, 35, 381–385. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Cardona, F.; Rogers, D.; Davey, S.; Van Erp, G. Investigation of the Effect of Styrene Content on the Ultimate Curing of Vinylester Resins by TGA-FTIR. J. Compos. Mater. 2007, 41, 137–152. [Google Scholar] [CrossRef]
- Jaikumar, V.; Kumar, D. New UV-Curable Prepolymer: Synthesis, Characterization, and Kinetics Analysis. Int. J. Polym. Anal. Charact. 2015, 20, 481–490. [Google Scholar] [CrossRef]
- Koelewijn, S.F.; Van Den Bosch, S.; Renders, T.; Schutyser, W.; Lagrain, B.; Smet, M.; Thomas, J.; Dehaen, W.; Van Puyvelde, P.; Witters, H.; et al. Sustainable bisphenols from renewable softwood lignin feedstock for polycarbonates and cyanate ester resins. Green Chem. 2017, 19, 2561–2570. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Madrawska, M.; Jesionowski, T. Preparation and characterisation of hydrated silica/lignin biocomposites. Physicochem. Probl. Miner. Process. 2012, 48, 463–473. [Google Scholar] [CrossRef]
- Salasinska, K.; Barczewski, M.; Górny, R.; Kloziński, A. Evaluation of highly filled epoxy composites modified with walnut shell waste filler. Polym. Bull. 2018, 75, 2511–2528. [Google Scholar] [CrossRef]
- Barczewski, M.; Sałasińska, K.; Szulc, J. Application of sunflower husk, hazelnut shell and walnut shell as waste agricultural fillers for epoxy-based composites: A study into mechanical behavior related to structural and rheological properties. Polym. Test. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Ojha, S.; Raghavendra, G.; Acharya, S.K. A comparative investigation of bio waste filler (wood apple-coconut) reinforced polymer composites. Polym. Compos. 2014, 5, 180–185. [Google Scholar] [CrossRef]
- Heriyanto, F.; Pahlevani, V.; Sahajwalla, V. Effect of different waste filler and silane coupling agent on the mechanical properties of powder-resin composite. J. Clean. Prod. 2019, 224, 940–956. [Google Scholar] [CrossRef]
- Harahap, H.; Sukardi, A.; Rusli, I.; Taslim, I.; Surya, I. Effect of Microcrystalline Cellulose from Cassava Peel Waste Filler Loading on Natural Rubber Latex Products. J. Polym. Mater. 2016, 33, 213–221. [Google Scholar]
- Salasinska, K.; Polka, M.; Gloc, M.; Ryszkowska, J. Composites with pistachio shell and sunflower husk: The effect of filler content and chemical constitution on the dimensional and fire stability. Polimery 2016, 61, 255–265. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial lignins: Analysis, properties, and applications. In Bioenergy Research: Advances and Application; Vijai, G., Maria Tuohy, G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. [Google Scholar]
- Settle, M.; Lange, H.; Crestini, C. Quantitative HSQC analyses of lignin: A practical comparison. Comput. Struct. Biotechnol. J. 2013, 6, e201303016. [Google Scholar] [CrossRef]
- Mattsson, C.; Andersson, S.I.; Belkheiri, T.; Åmand, L.E.; Olausson, L.; Vamling, L.; Theliander, H. Using 2D NMR to characterize the structure of the low and high molecular weight fractions of bio-oil obtained from LignoBoost™ kraft lignin depolymerized in subcritical water. Biomass Bioenergy 2016, 95, 364–377. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Crestini, C.; Argyropoulos, D.S. Structural Analysis of Wheat Straw Lignin by Quantitative 31P and 2D NMR Spectroscopy. The Occurrence of Ester Bonds and α-O-4 Substructures. J. Agric. Food Chem. 1997, 45, 1212–1219. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. In Biopolymers. Advances in Polymer Science; Abe, A., Dusek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 232, pp. 1–63. [Google Scholar]
- Adler, E. Structural Elements of Lignin. Ind. Eng. Chem. 1957, 49, 1377–1383. [Google Scholar] [CrossRef]
- Onnerud, H.; Gellerstedt, G. Inhomogeneities in the chemical 696 structure of spruce lignin. Holzforschung 2003, 57, 165–170. [Google Scholar] [CrossRef]
- Rencoret, J.; Kim, H.; Evaristo, A.B.; Gutiérrez, A.; Ralph, J.; Del Río, J.C. Variability in Lignin Composition and Structure in Cell Walls of Different Parts of Macaúba (Acrocomia aculeata) Palm Fruit. J. Agric. Food Chem. 2018, 66, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Svärd, A.; Sevastyanova, O.; Dobele, G.; Jurkjane, V.; Brännvall, E. COST Action FP1105: Effect of raw materials and pulping conditions on the characteristics of dissolved kraft lignins. Holzforschung 2016, 70, 1105–1114. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Chang, H.M. Recent advances in the isolation and analysis of lignins and lignin-carbohydrate complexes. In Characterization of Lignocellulosic Materials; Hu, T.Q., Ed.; Blackwell: Oxford, UK, 2008; pp. 148–170. [Google Scholar]
- Hatakeyama, H.; Hatakeyama, T. Lignin structure, properties, and applications. Adv. Polym. Sci. 2010, 232, 1–63. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of lignin filler in stabilization of natural rubber–based composites. J. Appl. Polym. Sci. 2007, 103, 1226–1231. [Google Scholar] [CrossRef]
- Strzemiecka, B.; Klapiszewski, Ł.; Jamrozik, A.; Szalaty, T.; Matykiewicz, D.; Sterzynski, T.; Voelkel, A.; Jesionowski, T. Physicochemical Characterization of Functional Lignin-Silica Hybrid Fillers for Potential Application in Abrasive Tools. Materials 2016, 9, 517. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Yeo, J.C.C.; Yuan, D.; Li, H.; Stubbs, L.P.; He, C. Lignin Epoxy Composites: Preparation, Morphology, and Mechanical Properties. Macromol. Mater. Eng. 2016, 301, 328–336. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B.; Sevastyanova, O.; Fila, K.; Chabros, A.; Pączkowski, P. Investigation of accelerated aging of lignin-containing polymer materials. Int. J. Biol. Macromol. 2019, 123, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.M.; Coles, S.R.; Maggs, S.; Meredith, J.; Kirwan, K. Use of lignin as a compatibiliser in hemp/epoxy composites. Compos. Sci. Technol. 2011, 71, 1804–1810. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal Properties of Lignin in Copolymers, Blends, and Composites; A Review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Xu, C.C.; Ragogna, P.J. Ultraviolet Curable Coatings of Modified Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14685–14694. [Google Scholar] [CrossRef]
- Podkościelna, B.; Goliszek, M.; Sevastyanova, O. New approach in the application of lignin for the synthesis of hybrid materials. Pure Appl. Chem. 2017, 89, 161–171. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B. Synthesis and characterization of polymer biocomposites with lignin. Physicochem. Probl. Miner. 2019, 55, 1375–1381. [Google Scholar] [CrossRef]
- Srinivasa, R.Y.; Kollipara, P. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants, J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Svobodova, A.; Psotova, J.; Walterova, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Garcıa, A.; Toledano, A.; Andres, M.A.; Labidi, J. Study of the antioxidant capacity of Miscanthus sinensis lignins, Proc. Biochem. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Aminzadeh, S.; Lauberts, M.; Dobele, G.; Ponomarenko, J.; Mattsson, T.; Lindström, M.E.; Sevastyanova, O. Membrane filtration of kraft lignin: Structural charactristics and antioxidant activity of the low-molecular-weight fraction. Ind. Crop. Prod. 2018, 112, 200–209. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Lauberts, M.; Sevastyanova, O.; Ponomarenko, J.; Dizhbite, T.; Dobele, G.; Volperts, A.; Lauberte, L.; Telysheva, G. Fractionation of technical lignin with ionic liquids as a method for improving purity and antioxidant activity. Ind. Crop. Prod. 2017, 95, 512–520. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent fractionation of softwood and hardwood kraft lignins for more efficient uses: Compositional, structural, thermal, antioxidant and adsorption properties. Ind. Crop. Prod. 2019, 129, 123–134. [Google Scholar] [CrossRef]
- Hamedani, G.; Ebrahimi, M.; Ghaffarian, S. Synthesis and Kinetics Study of Vinyl Ester Resin in the Presence of Triethylamine. Iran. Polym. J. 2006, 15, 871–878. [Google Scholar]
- Lee, Y.R.; Kim, S.C.; Lee, H.; Jeong, H.M.; Raghu, A.V.; Reddy, K.R.; Kim, B.K. Graphite oxides as effective fire retardants of epoxy resin. Macromol. Res. 2011, 19, 66–71. [Google Scholar] [CrossRef]
- Kawasaki, A.; Furukawa, J.; Tsuruta, T.; Wasai, G.; Makimoto, T. Infrared spectra of poly(butyl acrylates). Macromol. Chem. Phys. 1961, 49, 76–111. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assessment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification. Ind. Crop. Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Sobiesiak, M.; Podkościelna, B.; Sevastyanova, O. Thermal degradation behavior of lignin-modified porous styrene-divinylbenzene and styrene-bisphenol A glycerolate diacrylate copolymer microspheres. J. Anal. Appl. Pyrol. 2017, 123, 364–375. [Google Scholar] [CrossRef]
- Brill, R.P.; Palmese, G.R. An investigation of vinyl–ester-styrene bulk copolymerization cure kinetics using Fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 2000, 76, 1572–1582. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Maciejewska, M.; Rogulska, M. Insight into functionalized DMN-co-GMA copolymers. J. Therm. Anal. Calorim. 2019, 138, 4485–4495. [Google Scholar] [CrossRef]
- Tudorachi, N.; Mustata, F. Curing and thermal degradation of diglycidyl ether of bisphenol A epoxy resin crosslinked with natural hydroxy acids as environmentally friendly hardeners. Arab. J. Chem. 2017, 13, 671–682. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Podkościelna, B. The influence of oxidation number of sulfur on the polymerization and themo- mechanical properties of dimethacrylate copolymers. J. Therm. Anal. Calorim. 2013, 111, 1553–1560. [Google Scholar] [CrossRef]
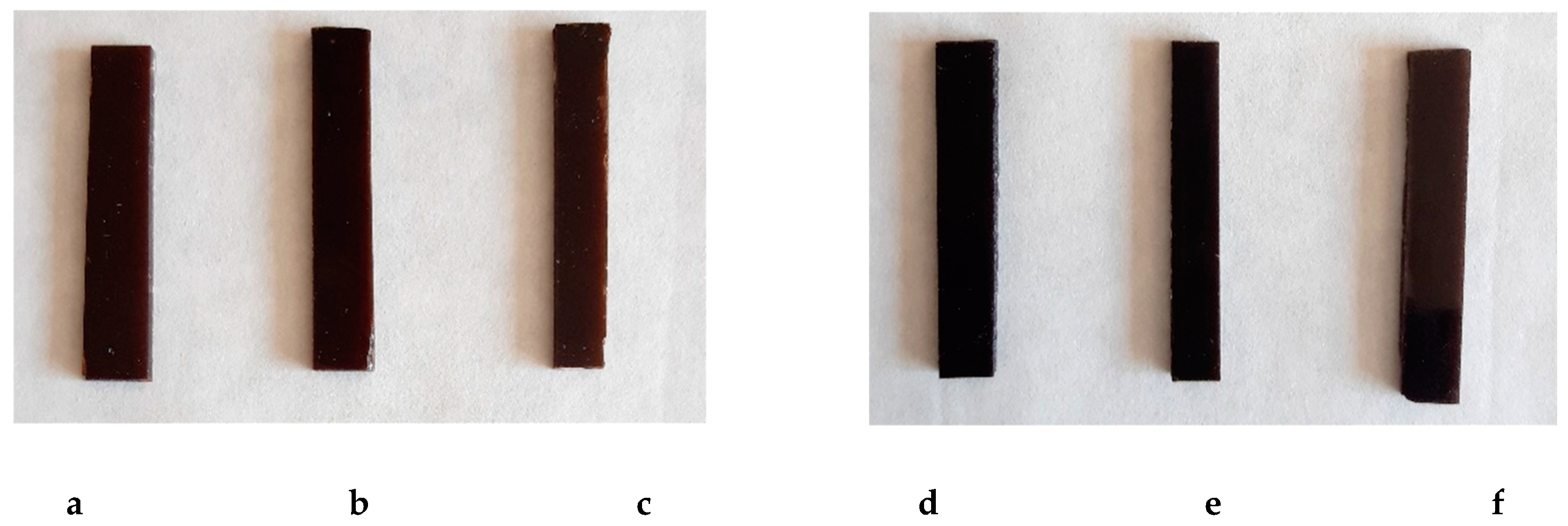







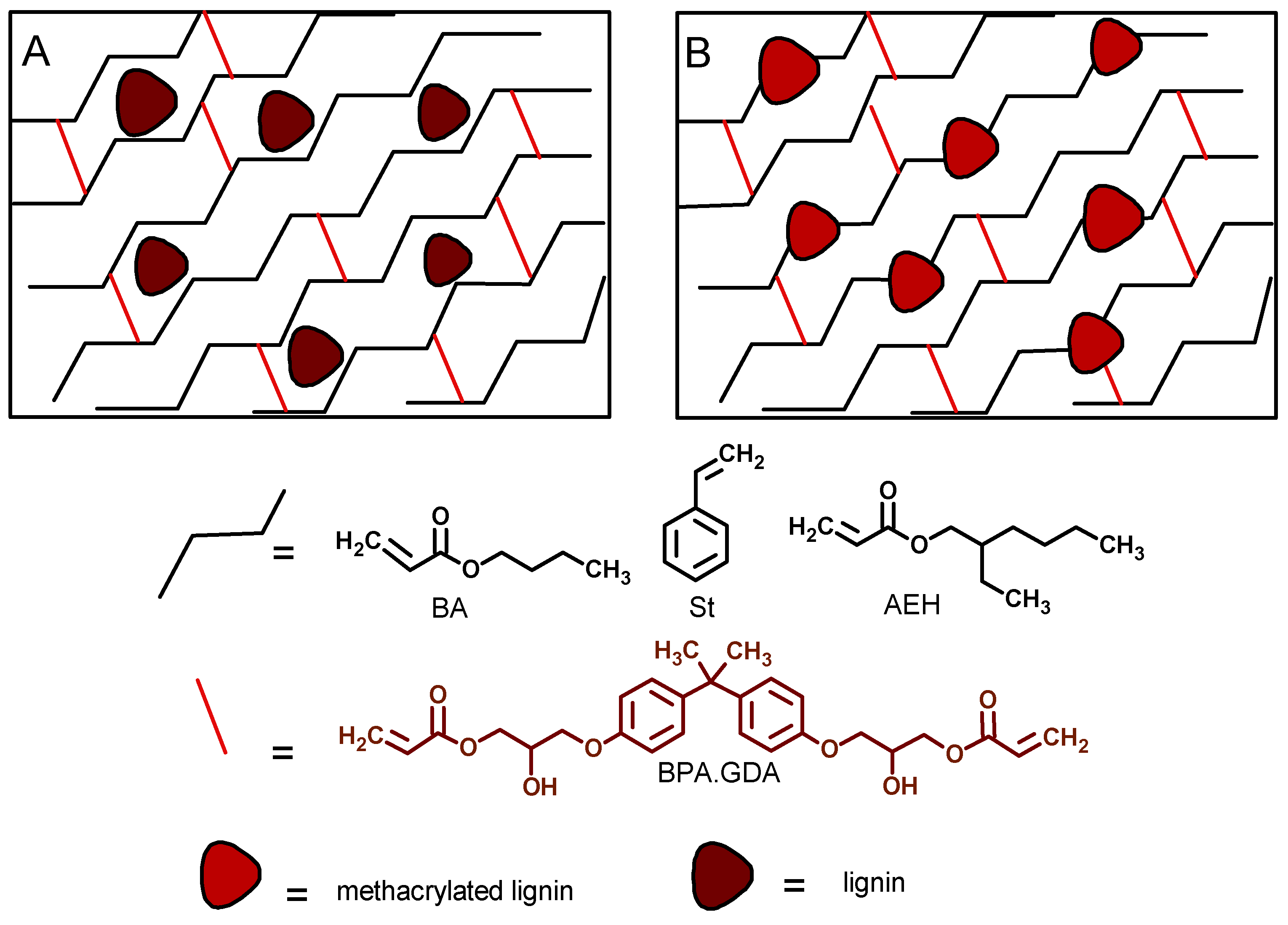
| Composite | BPA.GDA (g) | BA/EHA/St (g) | L/L-M (wt %) | L/L-M (g) |
|---|---|---|---|---|
| BA | 4.652 | 1.396 | 0 | 0 |
| BA + 5% L-M | 4.421 | 1.326 | 5 | 0.287 |
| BA + 5% L | 4.365 | 1.310 | 5 | 0.284 |
| BA + 10% L-M | 4.287 | 1.286 | 10 | 0.557 |
| BA + 10% L | 4.438 | 1.331 | 10 | 0.577 |
| EHA | 4.588 | 1.376 | 0 | 0 |
| EHA + 5% L-M | 4.498 | 1.349 | 5 | 0.292 |
| EHA + 5% L | 4.423 | 1.327 | 5 | 0.287 |
| EHA + 10% L-M | 4.364 | 1.309 | 10 | 0.567 |
| EHA + 10% L | 4.219 | 1.266 | 10 | 0.548 |
| St | 4.579 | 1.374 | 0 | 0 |
| St + 5% L-M | 4.387 | 1.316 | 5 | 0.285 |
| St + 5% L | 4.441 | 1.332 | 5 | 0.289 |
| St + 10% L-M | 4.497 | 1.349 | 10 | 0.585 |
| St + 10% L | 4.499 | 1.350 | 10 | 0.585 |
| Composite | T5% (°C) | T50% (°C) | Tmax1 (°C) | Tmax2 (°C) | mloss1 (%) | mloss2 (%) | RM (%) |
|---|---|---|---|---|---|---|---|
| BA | 349 | 418 | - | 418 | - | 92.81 | 7.19 |
| BA + 10% L-M | 182 | 413 | 176 | 418 | 8.01 | 83.14 | 8.85 |
| BA + 10% L | 290 | 409 | - | 410 | - | 85.21 | 14.79 |
| EHA | 362 | 425 | - | 425 | - | 93.65 | 6.35 |
| EHA + 10% L-M | 178 | 411 | 191 | 417 | 11.47 | 80.26 | 8.27 |
| EHA + 10% L | 185 | 406 | 186 | 411 | 8.64 | 78.08 | 13.28 |
| St | 344 | 418 | - | 422 | - | 96.20 | 3.80 |
| St + 10% L-M | 220 | 415 | 164 | 423 | 5.87 | 86.60 | 7.53 |
| St + 10% L | 269 | 414 | - | 419 | - | 90.18 | 9.82 |
| Composite | Shore Hardness Mean (°Sh) |
|---|---|
| BA | 66.3 ± 2.1 |
| BA + 5% L-M | 80.3 ± 4.5 |
| BA + 5% L | 77.3 ± 3.8 |
| BA + 10% L-M | 74.0 ± 5.9 |
| BA + 10% L | 69.2 ± 5.1 |
| EHA | 68.7 ± 2.0 |
| EHA + 5% L-M | 81.0 ± 4.2 |
| EHA + 5% L | 75.5 ± 3.5 |
| EHA + 10% L-M | 77.2 ± 4.8 |
| EHA + 10% L | 70.0 ± 4.0 |
| St | 78.1 ± 1.0 |
| St + 5% L-M | 97.3 ± 2.2 |
| St + 5% L | 90.5 ± 3.1 |
| St + 10% L-M | 88.4 ± 3.8 |
| St + 10% L | 80.2 ± 3.3 |
| Composite | Stress at Break (MPa) | Relative Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| Tensile tests | |||
| St | 46.2 | 3.1 | 1890 |
| St + 10% L | 21.1 | 1.7 | 1590 |
| St + 10% L-M | 7.1 | 0.8 | 964 |
| EHA | 31.8 | 3.2 | 1620 |
| EHA + 10% L | 9.7 | 1.3 | 599 |
| EHA + 10% L-M | 8.3 | 1 | 1120 |
| BA | 49.9 | 2.8 | 2160 |
| BA + 10% L | 18.5 | 1.9 | 1300 |
| BA + 10% L-M | 9.2 | 1.2 | 1215 |
| Bending tests | |||
| St | 78.2 | 1.7 | 4210 |
| St + 10% L | 51.3 | 2.1 | 3680 |
| St + 10% L-M | 20.1 | 1.5 | 1430 |
| EHA | 62.5 | 2.2 | 2590 |
| EHA + 10% L | 32.2 | 2.2 | 1520 |
| EHA + 10% L-M | 26.2 | 1.8 | 1488 |
| BA | 83.2 | 2.7 | 2901 |
| BA + 10% L | 53.9 | 1.9 | 2840 |
| BA + 10% L-M | 29.9 | 1.8 | 1544 |
| Properties | Composites | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BA | BA + 10% L | BA +10% L-M | EHA | EHA + 10% L | EHA + 10% L-M | St | St + 10% L | St + 10% L-M | |
| E’(25 °C)/GPa | 2.82 | 2.65 | 1.77 | 2.49 | 1.70 | 1.45 | 3.39 | 3.00 | 1.0 |
| Tg/°C a | 70.3 | 55.6 | 44.3 | 65.2 | 32.5 | 32.3 | 78.0 | 61.9 | 24.5/62.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goliszek, M.; Podkościelna, B.; Klepka, T.; Sevastyanova, O. Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers 2020, 12, 1159. https://doi.org/10.3390/polym12051159
Goliszek M, Podkościelna B, Klepka T, Sevastyanova O. Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers. 2020; 12(5):1159. https://doi.org/10.3390/polym12051159
Chicago/Turabian StyleGoliszek, Marta, Beata Podkościelna, Tomasz Klepka, and Olena Sevastyanova. 2020. "Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin" Polymers 12, no. 5: 1159. https://doi.org/10.3390/polym12051159
APA StyleGoliszek, M., Podkościelna, B., Klepka, T., & Sevastyanova, O. (2020). Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers, 12(5), 1159. https://doi.org/10.3390/polym12051159







