Non-Invasive Approaches for the Evaluation of the Functionalization of Melamine Foams with In-Situ Synthesized Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of the ME Foams
2.3. Experimental Techniques
3. Results and Discussion
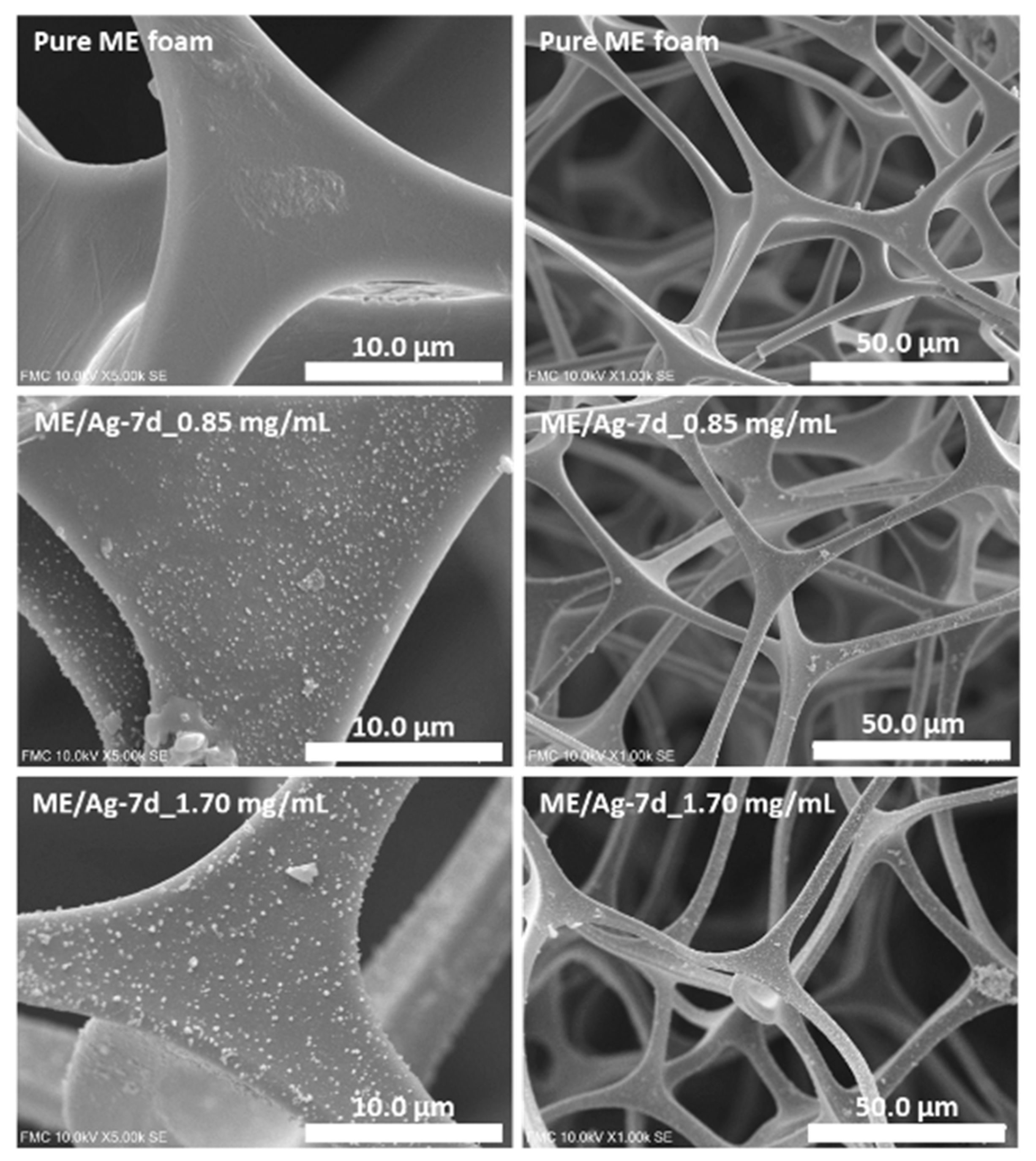
3.1. Characterization of the ME and ME/Ag Foams
3.2. TGA Study of ME/Ag Foams
3.3. Colorimetry Study of ME/Ag Foams
3.4. X-ray Study of the ME/Ag Foams
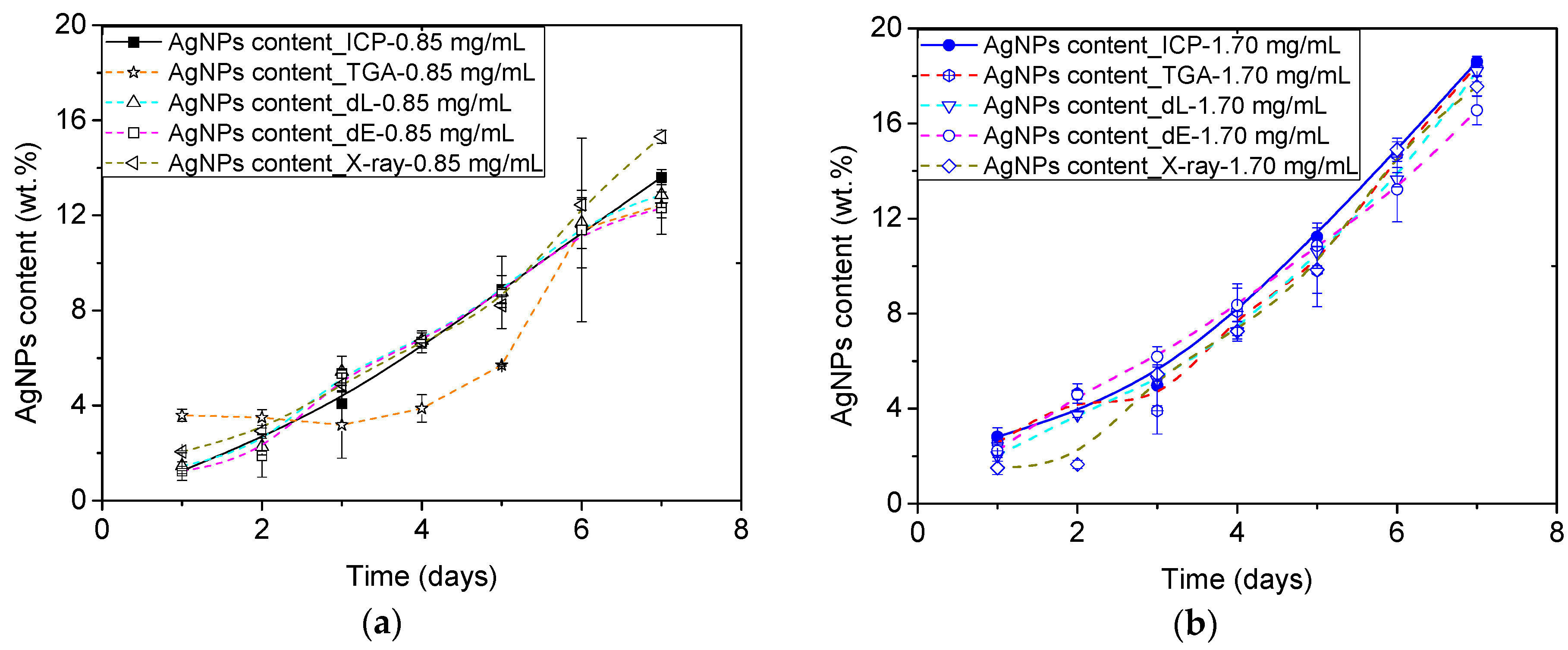
3.5. Accuracy of the Proposed Approaches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Merillas, B.; Cimavilla-Román, P.; Rodriguez-Perez, M.A.; Pinto, J. Enhanced nitrates-polluted water remediation by polyurethane/sepiolite cellular nanocomposites. J. Clean. Prod. 2020, 254. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Mele, E.; Ruffilli, R.; Athanassiou, A. Polymeric foams with functional nanocomposite cells. RSC Adv. 2014, 4, 19177–19182. [Google Scholar] [CrossRef]
- Tamayo, L.; Palza, H.; Bejarano, J.; Zapata, P.A. Polymer Composites With Metal Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128140642. [Google Scholar]
- Cataldi, P.; Ceseracciu, L.; Athanassiou, A.; Bayer, I.S. Healable Cotton-Graphene Nanocomposite Conductor for Wearable Electronics. ACS Appl. Mater. Interfaces 2017, 9, 13825–13830. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zhang, P.; Zeng, G.M.; Song, B.; Liu, H.Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef]
- Taghavimehr, M.; Navid Famili, M.H.; Shirsavar, M.A. Effect of nanoparticle network formation on electromagnetic properties and cell morphology of microcellular polymer nanocomposite foams. Polym. Test. 2020, 86, 106469. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, G.; Zhou, X.; Jiang, W.; Wang, T.; Zhang, N.; Yu, L. One-pot synthesis of robust superhydrophobic, functionalized graphene/polyurethane sponge for effective continuous oil-water separation. Chem. Eng. J. 2016, 302, 155–162. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Pérez-Tamarit, S.; Cimavilla-Román, P.; Blasco, V.; Ruiz, C.; París, R.; Villafañe, F.; Rodríguez-Pérez, M.Á. X-ray radioscopy validation of a polyol functionalized with graphene oxide for producing rigid polyurethane foams with improved cellular structures. Eur. Polym. J. 2019, 118, 404–411. [Google Scholar] [CrossRef]
- Pinto, J.; Magrì, D.; Valentini, P.; Palazon, F.; Heredia-Guerrero, J.A.; Lauciello, S.; Barroso-Solares, S.; Ceseracciu, L.; Pompa, P.P.; Athanassiou, A.; et al. Antibacterial Melamine Foams Decorated with in Situ Synthesized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 16095–16104. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Ouyang, Y.; Liang, Y.; Deng, Y.; Wang, C. Simple fabrication of multi-functional melamine sponges. Mater. Lett. 2017, 190, 119–122. [Google Scholar] [CrossRef]
- Charara, M.; Luo, W.; Saha, M.C.; Liu, Y. Investigation of Lightweight and Flexible Carbon Nanofiber/Poly Dimethylsiloxane Nanocomposite Sponge for Piezoresistive Sensor Application. Adv. Eng. Mater. 2019, 21, 1801068. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.D.; Pereira, E.; Freitas, R. Toxic Effects of Metal Nanoparticles in Marine Invertebrates. In Nanostructured Materials for Treating Aquatic Pollution; Gonçalves, G.A.B., Marques, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–224. ISBN 978-3-030-33745-2. [Google Scholar]
- Zeng, J.; Xu, P.; Chen, G.; Zeng, G.; Chen, A.; Hu, L.; Huang, Z.; He, K.; Guo, Z.; Liu, W.; et al. Effects of silver nanoparticles with different dosing regimens and exposure media on artificial ecosystem. J. Environ. Sci. (China) 2019, 75, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Muhammad, P.; Ahmad, W.; Hussain, Z.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Surface Plasmonic-Assisted Photocatalysis and Optoelectronic Devices with Noble Metal Nanocrystals: Design, Synthesis, and Applications. Adv. Funct. Mater. 2020, 30, 1906744. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Fragouli, D.; Athanassiou, A. Facile oil removal from water-in-oil stable emulsions using PU foams. Materials 2018, 11, 2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Zaini, N.A.; Ismail, H.; Rusli, A. A Short Review on Sepiolite-Filled Polymer Nanocomposites. Polym. Plast. Technol. Eng. 2017, 56, 1665–1679. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, C.; Ji, Y.; Tian, Y.; Wei, H.; Li, C.; Li, Z.; Zhu, T.; Sun, Q.; Man, B.; et al. 3D Ultrasensitive Polymers-Plasmonic Hybrid Flexible Platform for In-Situ Detection. Polymers 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Du, B.; Huang, Y.; Zheng, J. Ultrasmall noble metal nanoparticles: Breakthroughs and biomedical implications. Nano Today 2018, 21, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Kulkarni, G.U. Removal of organic compounds from water by using a gold nanoparticle-poly(dimethylsiloxane) nanocomposite foam. ChemSusChem 2011, 4, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, L.; Yan, K.; Zhang, L.; Yu, Q.J. Polydopamine nanocluster decorated electrospun nanofibrous membrane for separation of oil/water emulsions. J. Memb. Sci. 2018, 547, 156–162. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef] [Green Version]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Pérez-Jiménez, L.E.; Solis-Cortazar, J.C.; Rojas-Blanco, L.; Perez-Hernandez, G.; Martinez, O.S.; Palomera, R.C.; Paraguay-Delgado, F.; Zamudio-Torres, I.; Morales, E.R. Enhancement of optoelectronic properties of TiO2 films containing Pt nanoparticles. Results Phys. 2019, 12, 1680–1685. [Google Scholar] [CrossRef]
- Song, Q.; Li, M.; Wang, L.; Ma, X.; Liu, F.; Liu, X. Mechanism and optimization of electrochemical system for simultaneous removal of nitrate and ammonia. J. Hazard. Mater. 2019, 363, 119–126. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Song, Y.; Liu, Q.; Zhang, Y.; Zhuang, Y.; Chen, S. Synthesis of ZnFe2O4/ZnO heterostructures decorated three-dimensional graphene foam as peroxidase mimetics for colorimetric assay of hydroquinone. Sens. Actuators B Chem. 2019, 283, 130–137. [Google Scholar] [CrossRef]
- Mergu, N.; Kim, H.; Heo, G.; Son, Y.A. Fabrication and topochemically controlled diacetylene-based polymer and its colorimetric application toward HCl detection. Dye. Pigment. 2020, 174, 108061. [Google Scholar] [CrossRef]
- Mergu, N.; Kim, H.; Ryu, J.; Son, Y.A. A simple and fast responsive colorimetric moisture sensor based on symmetrical conjugated polymer. Sens. Actuators B Chem. 2020, 311, 127906. [Google Scholar] [CrossRef]
- Ko, Y.; Jeong, H.Y.; Kwon, G.; Kim, D.; Lee, C.; You, J. pH-responsive polyaniline/polyethylene glycol composite arrays for colorimetric sensor application. Sens. Actuators B Chem. 2020, 305, 127447. [Google Scholar] [CrossRef]
- Pardo-Alonso, S.; Solórzano, E.; Rodriguez-Perez, M.A. Time-resolved X-ray imaging of nanofiller-polyurethane reactive foam systems. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 119–125. [Google Scholar] [CrossRef]
- Prade, F.; Schaff, F.; Senck, S.; Meyer, P.; Mohr, J.; Kastner, J.; Pfeiffer, F. Nondestructive characterization of fiber orientation in short fiber reinforced polymer composites with X-ray vector radiography. NDT E Int. 2017, 86, 65–72. [Google Scholar] [CrossRef]
- Roels, S.; Carmeliet, J. Analysis of moisture flow in porous materials using microfocus X-ray radiography. Int. J. Heat Mass Transf. 2006, 49, 4762–4772. [Google Scholar] [CrossRef]
- Solórzano, E.; Pinto, J.; Pardo, S.; Garcia-Moreno, F.; Rodriguez-Perez, M.A. Application of a microfocus X-ray imaging apparatus to the study of cellular polymers. Polym. Test. 2013, 32, 321–329. [Google Scholar] [CrossRef]
- Melnichenko, Y.B.; Wignall, G.D.; Cole, D.R.; Frielinghaus, H. Adsorption of supercritical CO2 in aerogels as studied by small-angle neutron scattering and neutron transmission techniques. J. Chem. Phys. 2006, 124, 204711. [Google Scholar] [CrossRef] [Green Version]
- Bracewell, B.L.; Veigele, W.J. Tables of X-ray Mass Attenuation Coefficients for 87 Elements at Selected Wavelengths. In Developments in Applied Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1971; pp. 357–400. [Google Scholar]







| Samples | Reaction Time (days) | Precursor Solution (mg/mL) | L* | a* | b* |
|---|---|---|---|---|---|
| Me_Pure | -- | 74.78 ± 0.72 | −1.33 ± 0.31 | −3.93 ± 0.44 | |
| Me_1d-0.85 | 1 | 0.85 | 51.82 ± 2.06 | 7.93 ± 0.53 | 14.32 ± 2.43 |
| Me_2d-0.85 | 2 | 0.85 | 47.10 ± 2.30 | 8.14 ± 1.12 | 13.39 ± 3.54 |
| Me_3d-0.85 | 3 | 0.85 | 37.97 ± 0.85 | 8.65 ± 1.35 | 14.14 ± 0.62 |
| Me_4d-0.85 | 4 | 0.85 | 35.71 ± 0.23 | 9.13 ± 0.52 | 13.26 ± 0.38 |
| Me_5d-0.85 | 5 | 0.85 | 32.97 ± 0.86 | 9.54 ± 0.46 | 12.05 ± 1.77 |
| Me_6 d-0.85 | 6 | 0.85 | 29.95 ± 1.67 | 10.90 ± 1.64 | 8.56 ± 2.47 |
| Me_7d-0.85 | 7 | 0.85 | 28.94 ± 0.28 | 8.11 ± 0.79 | 9.61 ± 0.68 |
| Me_1d-1.70 | 1 | 1.70 | 48.28 ± 0.65 | 9.10 ± 0.93 | 17.00 ± 1.18 |
| Me_2d-1.70 | 2 | 1.70 | 41.87 ± 0.41 | 9.80 ±0.38 | 17.84 ± 0.48 |
| Me_3d-1.70 | 3 | 1.70 | 38.42 ± 0.65 | 9.86 ± 0.85 | 16.83 ± 0.47 |
| Me_4d-1.70 | 4 | 1.70 | 34.94 ± 0.11 | 10.14 ± 0.36 | 15.30 ± 0.20 |
| Me_5d-1.70 | 5 | 1.70 | 30.99 ± 0.16 | 9.56 ± 0.23 | 11.66 ± 0.06 |
| Me_6d-1.70 | 6 | 1.70 | 28.33 ± 0.95 | 8.12 ± 1.08 | 9.59 ± 0.40 |
| Me_7d-1.70 | 7 | 1.70 | 27.74 ± 0.29 | 8.33 ± 0.97 | 8.31 ± 1.70 |
| Samples | TGA-ICP | ΔL-ICP | ΔE-ICP | X-ray-ICP |
|---|---|---|---|---|
| ME/Ag | 2.05 | 0.31 | 0.85 | 1.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-Solares, S.; Cimavilla-Roman, P.; Rodriguez-Perez, M.A.; Pinto, J. Non-Invasive Approaches for the Evaluation of the Functionalization of Melamine Foams with In-Situ Synthesized Silver Nanoparticles. Polymers 2020, 12, 996. https://doi.org/10.3390/polym12050996
Barroso-Solares S, Cimavilla-Roman P, Rodriguez-Perez MA, Pinto J. Non-Invasive Approaches for the Evaluation of the Functionalization of Melamine Foams with In-Situ Synthesized Silver Nanoparticles. Polymers. 2020; 12(5):996. https://doi.org/10.3390/polym12050996
Chicago/Turabian StyleBarroso-Solares, Suset, Paula Cimavilla-Roman, Miguel Angel Rodriguez-Perez, and Javier Pinto. 2020. "Non-Invasive Approaches for the Evaluation of the Functionalization of Melamine Foams with In-Situ Synthesized Silver Nanoparticles" Polymers 12, no. 5: 996. https://doi.org/10.3390/polym12050996





