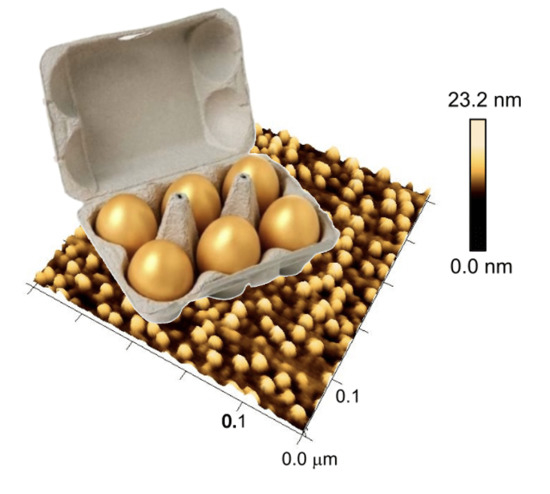
Controlled Arrangement of Gold Nanoparticles on Planar Surfaces via Constrained Dewetting of Surface-Grafted RAFT Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomic Force Microscopy and s-SNOM
2.2. Spectroscopic Ellipsometry
2.3. Size-Exclusion Chromatography
2.4. Chemicals
2.5. Glass Coverslips with Gold Nanolayer
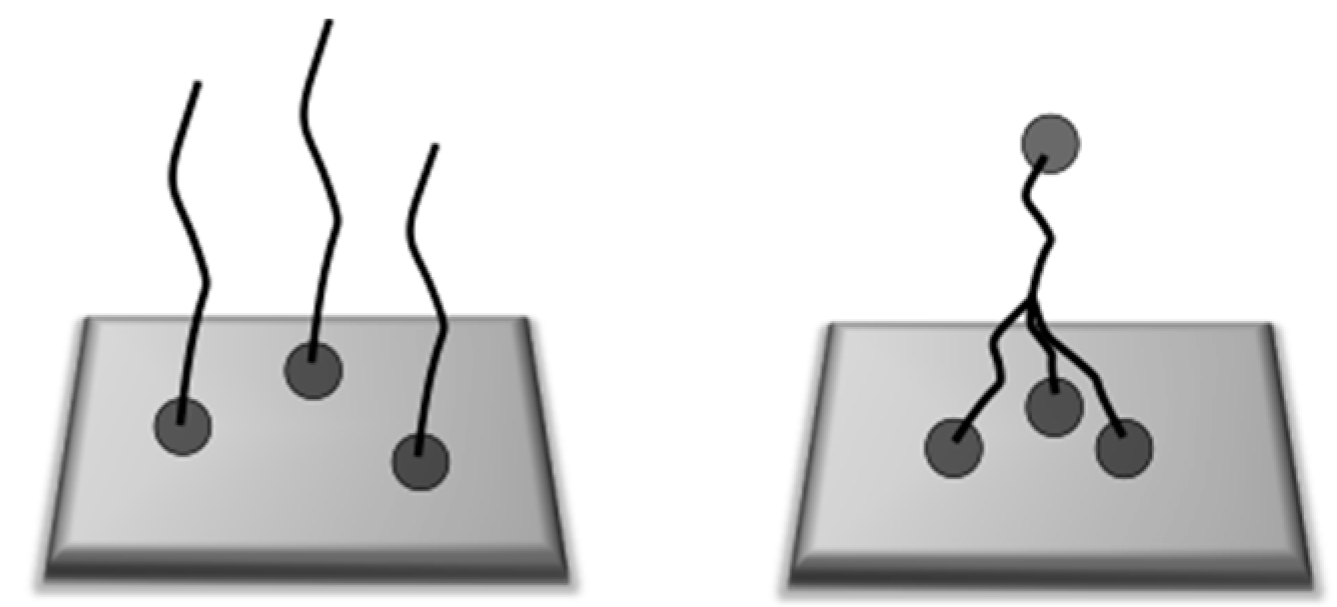
2.6. Homopolymerization with Styrene
2.7. Synthesis of Gold Nanoparticles
2.8. Immobilization of RAFT-Terminated Polystyrene
2.9. Nanostructure Formation
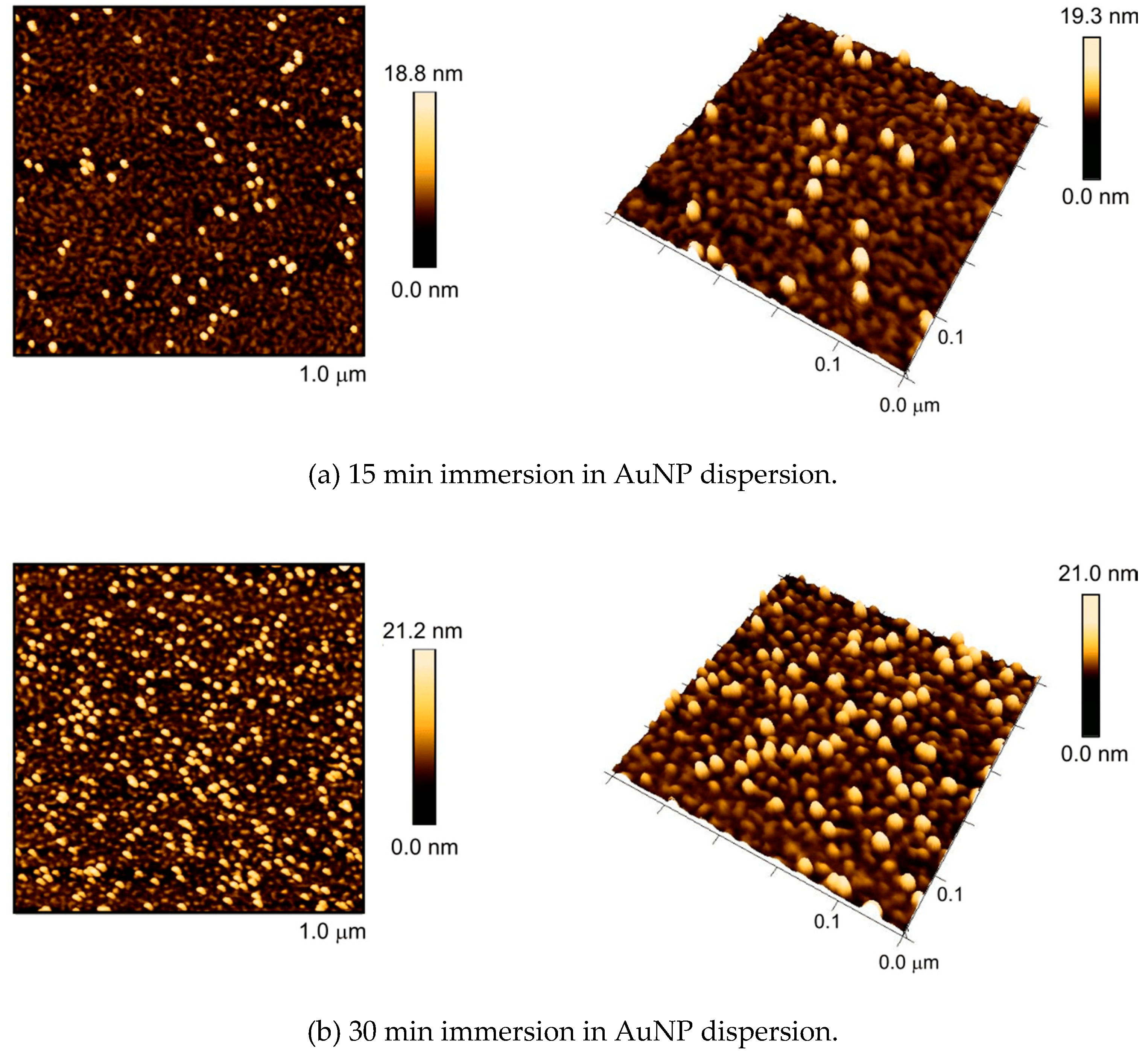
2.10. Selective Self-Assembly of Gold Nanoparticles
3. Results and Discussion
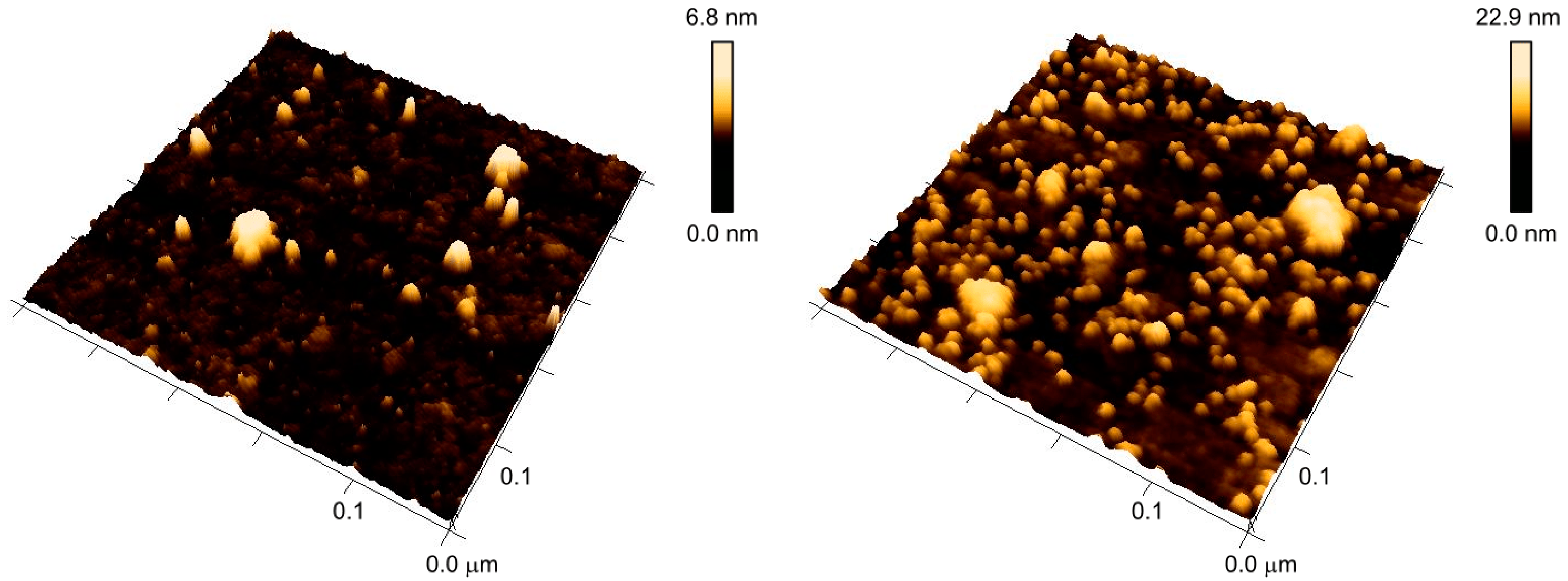
3.1. Reversibility of the Surface Morphologies
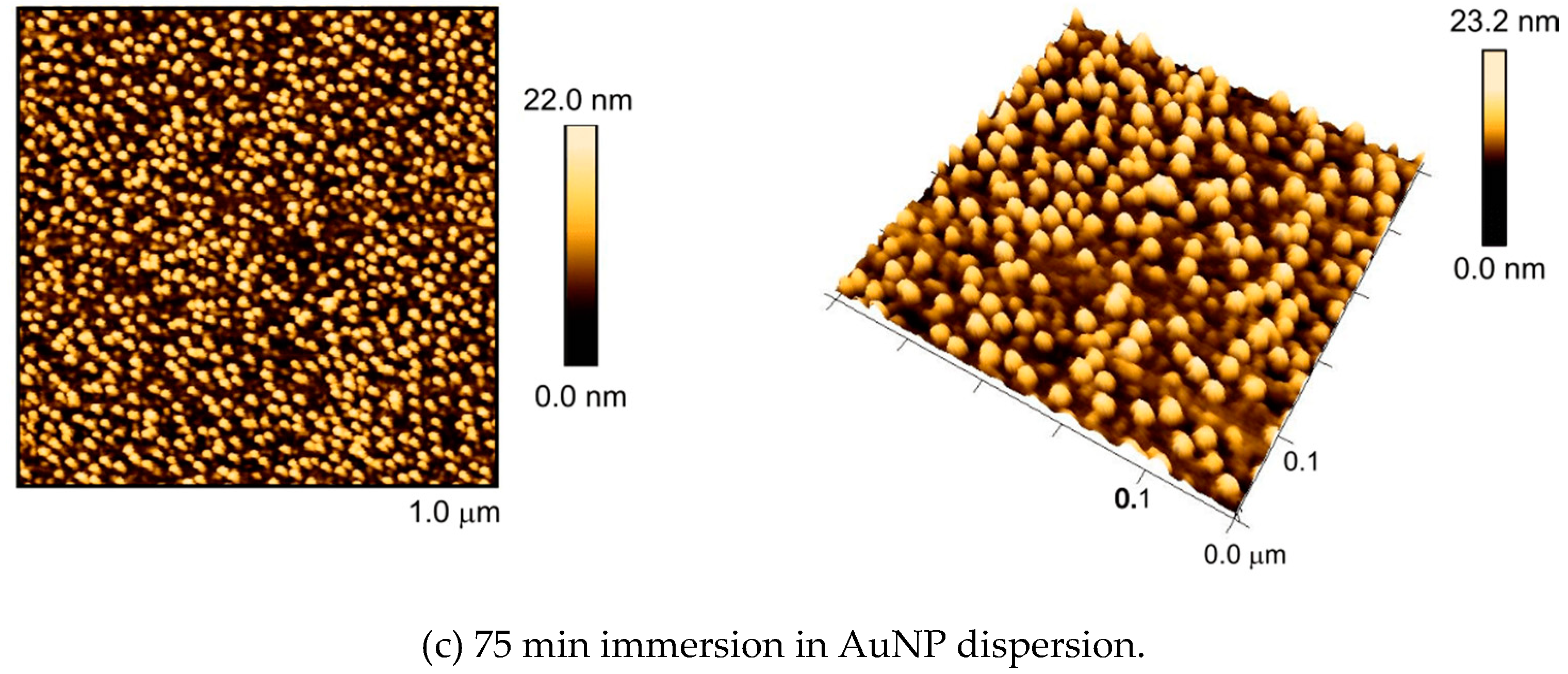
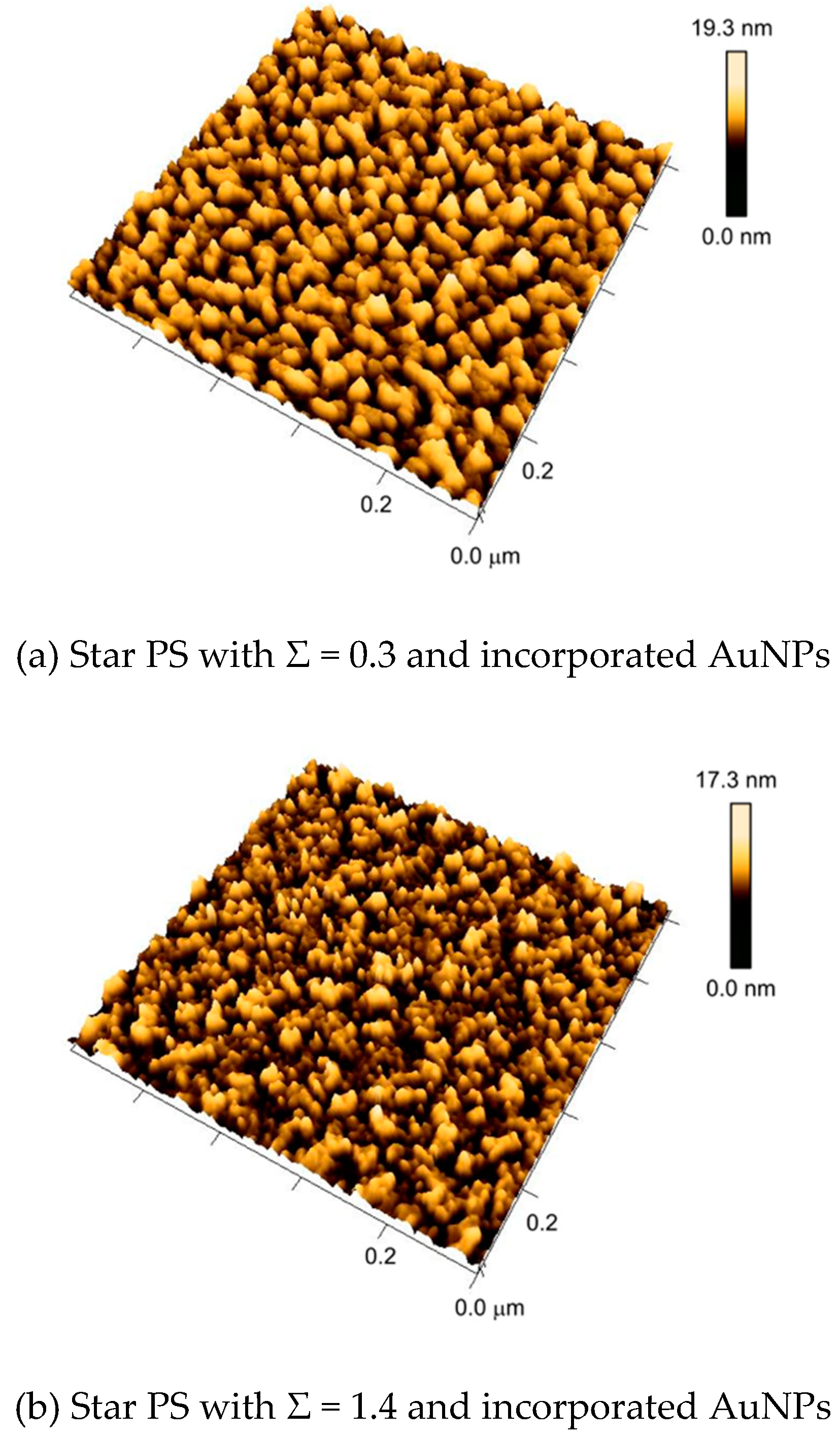
3.2. Self-Assembly of AuNPs upon Surfaces with Nanostructures from Constrained Dewetting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmelmer, U.; Paul, A.; Küller, A.; Steenackers, M.; Ulman, A.; Grunze, M.; Gölzhäuser, A.; Jordan, R. Nanostructured polymer brushes. Small 2007, 3, 459. [Google Scholar] [CrossRef] [PubMed]
- Minko, S.; Usov, D.; Goreshnik, E.; Stamm, M. Environment-Adopting Surfaces with Reversibly Switchable Morphology. Macromol. Rapid Commun. 2001, 22, 206. [Google Scholar] [CrossRef]
- Orski, S.V.; Fries, K.H.; Sontag, S.K.; Locklin, J. Fabrication of nanostructures using polymer brushes. J. Mater. Chem. 2011, 21, 14135. [Google Scholar] [CrossRef]
- Manfrinato, V.R.; Stein, A.; Zhang, L.; Nam, C.-Y.; Yager, K.G.; Stach, E.A.; Black, C.T. Aberration-Corrected Electron Beam Lithography at the One Nanometer Length Scale. Nano Lett. 2017, 17, 4562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, S.; Mirkin, C.A. Surface and site-specific ring-opening metathesis polymerization initiated by dip-pen nanolithography. Angew. Chem. Int. Ed. 2003, 42, 4785. [Google Scholar] [CrossRef] [PubMed]
- Kaholek, M.; Lee, W.K.; Ahn, S.J.; Ma, H.; Caster, K.C.; LaMattina, B.; Zauscher, S. Stimulus-Responsive Poly(N-isopropylacrylamide) Brushes and Nanopatterns Prepared by Surface-Initiated Polymerization. Chem. Mater. 2004, 16, 3688. [Google Scholar] [CrossRef]
- Slim, C.; Tran, Y.; Chehimi, M.M.; Garraud, N.; Roger, J.P.; Combellas, C.; Kanoufi, F. Microelectrochemical patterning of surfaces with polymer brushes. Chem. Mater. 2008, 20, 6677. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Katz, E.; Minko, S. Chemical Gating with Nanostructured Responsive Polymer Brushes: Mixed Brush versus Homopolymer Brush. ACS Nano 2008, 2, 41. [Google Scholar] [CrossRef]
- Minko, S.; Usov, D.; Froeck, C.; Stamm, M.; Müller, M.; Scholl, A. Lateral versus perpendicular segregation in mixed polymer brushes. Phys. Rev. Lett. 2002, 88, 4. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J.; Zhou, W.; Cheng, S.Z. Nanopattern Formation from Tethered PS-b-PMMA Brushes upon Treatment with Selective Solvents. J. Am. Chem. Soc. 2000, 122, 2407. [Google Scholar] [CrossRef]
- Siqueira, D.F.; Köhler, K.; Stamm, M. Structures at the surface of dry thin films of grafted copolymers. Langmuir 1995, 11, 3092. [Google Scholar] [CrossRef]
- Lee, T.; Hendy, S.; Neto, C. Tunable nanopatterns via the constrained dewetting of polymer brushes. Macromolecules 2013, 46, 6326. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.; Ahn, C.H.; Jo, W.H.; Bright, J.N.; Williams, D.R. Constrained dewetting of polymers grafted onto a nonadsorbing surface in poor solvents: From pancake micelles to the holey layer. Macromolecules 2005, 38, 2974. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Davis, T.P.; Heuts, J.P.A.; Stenzel, M.H.; Vana, P.; Whittaker, M. RAFTing Down Under: Tales of Missing Radicals, Fancy Architectures, and Mysterious Holes. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 365. [Google Scholar] [CrossRef]
- Ebeling, E.; Vana, P. RAFT-Polymers with Single and Multiple Trithiocarbonate Groups as Uniform Gold-Nanoparticle Coatings. Macromolecules 2013, 46, 4862. [Google Scholar] [CrossRef]
- Rossner, C.; Ebeling, B.; Vana, P. Spherical Gold-Nanoparticle Assemblies with Tunable Interparticle Distances mediated by Multifunctional RAFT Polymers. ACS Macro Lett. 2013, 2, 1073. [Google Scholar] [CrossRef]
- Rossner, C.; Roddatis, V.; Lopatin, S.; Vana, P. Functionalization of Planet-Satellite Nanostructures Revealed by Nanoscopic Localization of Distinct Macromolecular Species. Macromol. Rapid Commun. 2016, 37, 1742. [Google Scholar] [CrossRef]
- Tebbe, M.; Galati, E.; Walker, G.C.; Kumacheva, E. Homopolymer Nanolithography. Small 2017, 13, 1702043. [Google Scholar] [CrossRef]
- Boschmann, D.; Vana, P. Z-RAFT Star Polymerizations of Acrylates: Star Coupling via Intermolecular Chain Transfer to Polymer. Macromolecules 2007, 40, 2683. [Google Scholar] [CrossRef]
- Rossner, C.; Vana, P. Planet-satellite nanostructures made to order by RAFT star polymers. Angew. Chem. Int. Ed. 2014, 53, 12639. [Google Scholar] [CrossRef]
- Peng, W.; Rossner, C.; Roddatis, V.; Vana, P. Gold-Planet–Silver-Satellite Nanostructures Using RAFT Star Polymer. ACS Macro Lett. 2016, 5, 1227. [Google Scholar] [CrossRef]
- Brittain, W.J.; Minko, S. A structural definition of polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3505. [Google Scholar] [CrossRef]




| Reaction Time/h | Mn (Homopolymer)/kg/mol | Ð (Homopolymer) | Mn (Star Polymer) */kg/mol | Ð (Star Polymer) |
|---|---|---|---|---|
| 6 | 21 | 1.10 | 22 | 1.13 |
| 12 | 40 | 1.08 | 60 | 1.12 |
| 24 | 64 | 1.07 | 84 | 1.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrich, K.; Peng, W.; Vana, P. Controlled Arrangement of Gold Nanoparticles on Planar Surfaces via Constrained Dewetting of Surface-Grafted RAFT Polymer. Polymers 2020, 12, 1214. https://doi.org/10.3390/polym12061214
Hendrich K, Peng W, Vana P. Controlled Arrangement of Gold Nanoparticles on Planar Surfaces via Constrained Dewetting of Surface-Grafted RAFT Polymer. Polymers. 2020; 12(6):1214. https://doi.org/10.3390/polym12061214
Chicago/Turabian StyleHendrich, Katharina, Wentao Peng, and Philipp Vana. 2020. "Controlled Arrangement of Gold Nanoparticles on Planar Surfaces via Constrained Dewetting of Surface-Grafted RAFT Polymer" Polymers 12, no. 6: 1214. https://doi.org/10.3390/polym12061214