Highly Effective Sensitizers Based on Merocyanine Dyes for Visible Light Initiated Radical Polymerization
Abstract
:1. Introduction
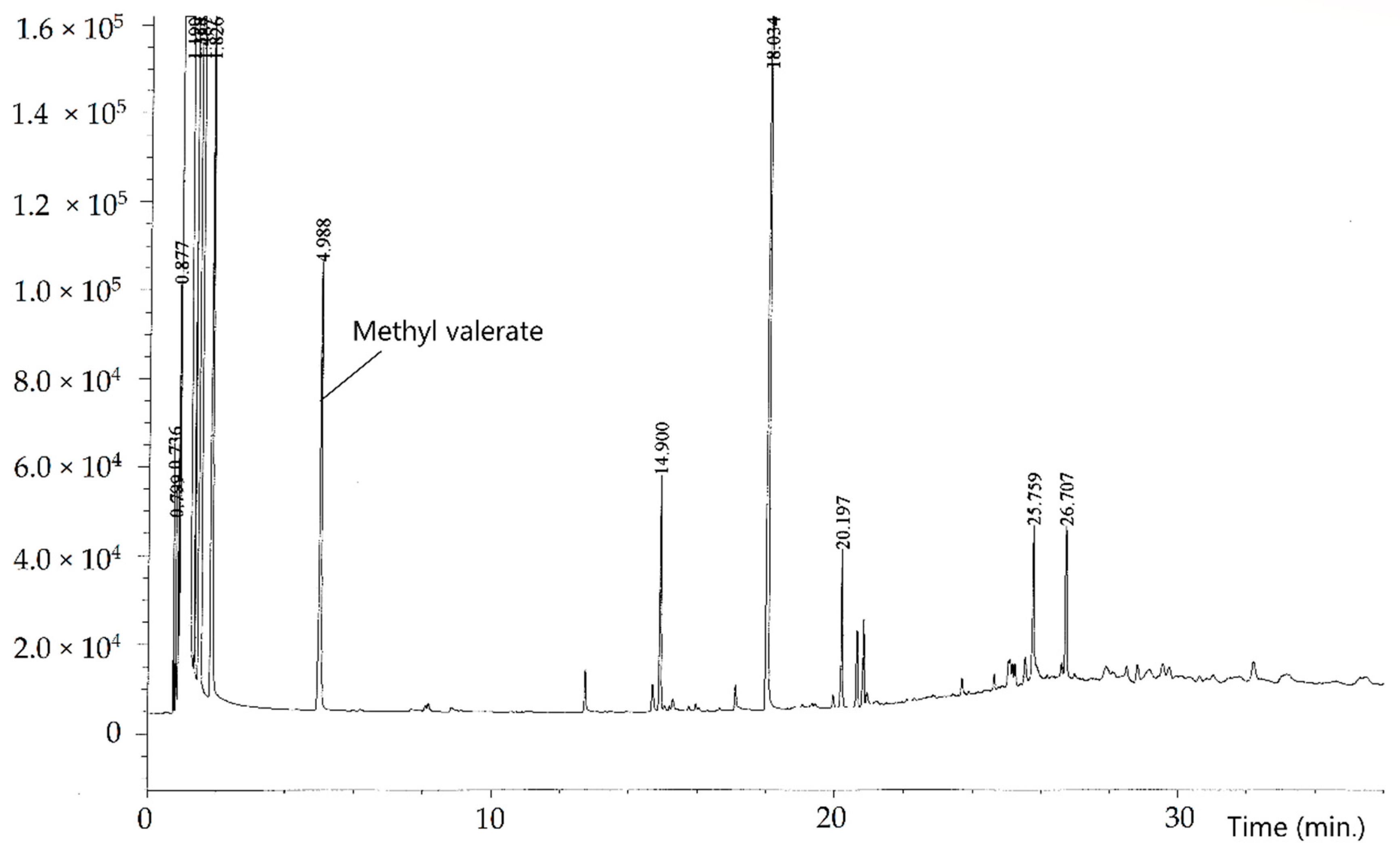
2. Materials and Methods
3. Results
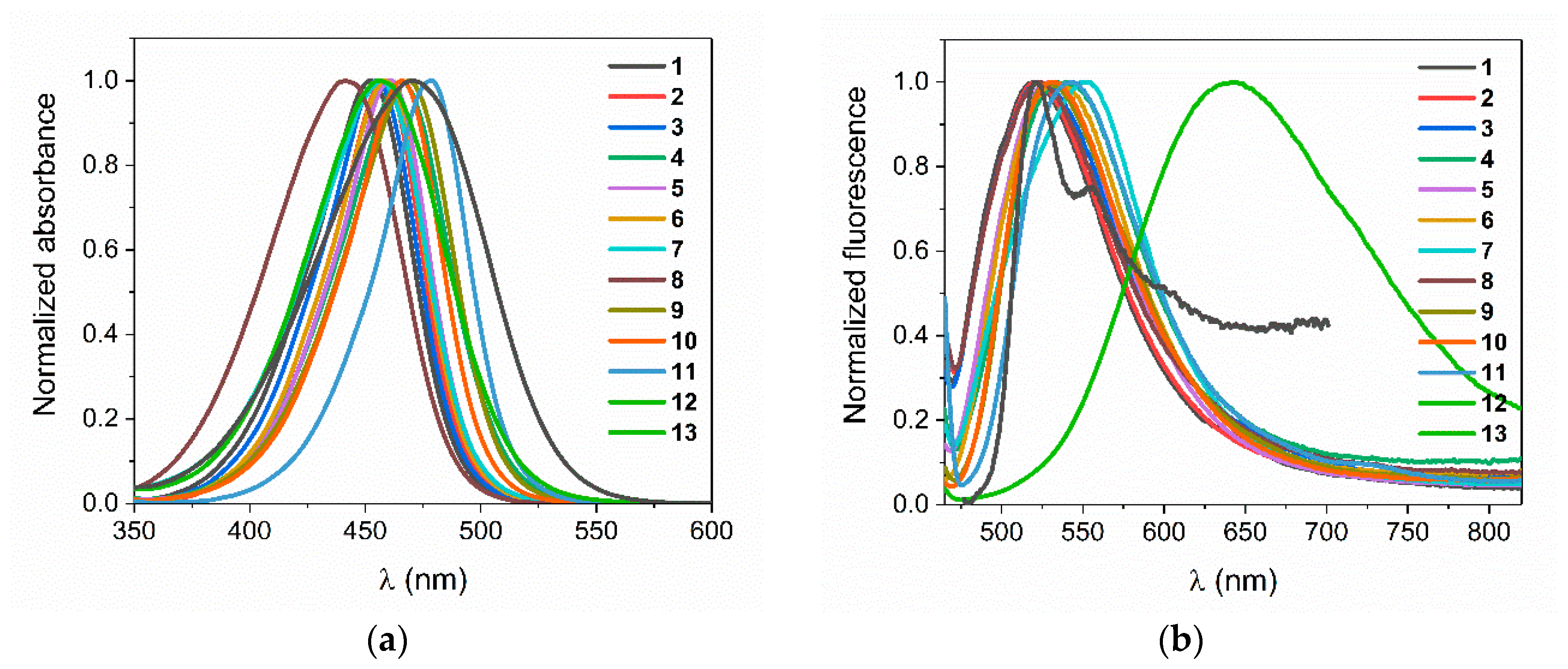
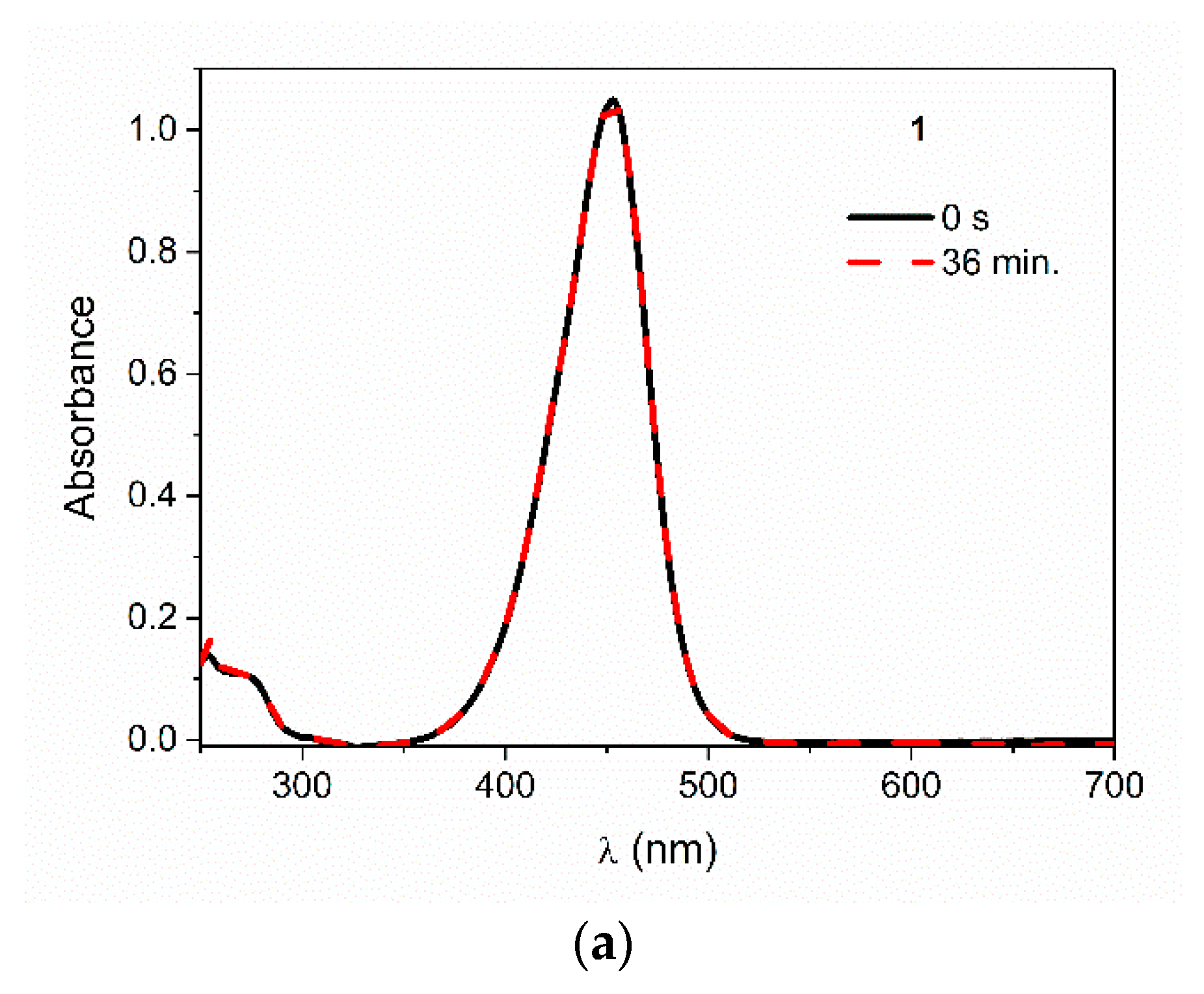
3.1. Photostability
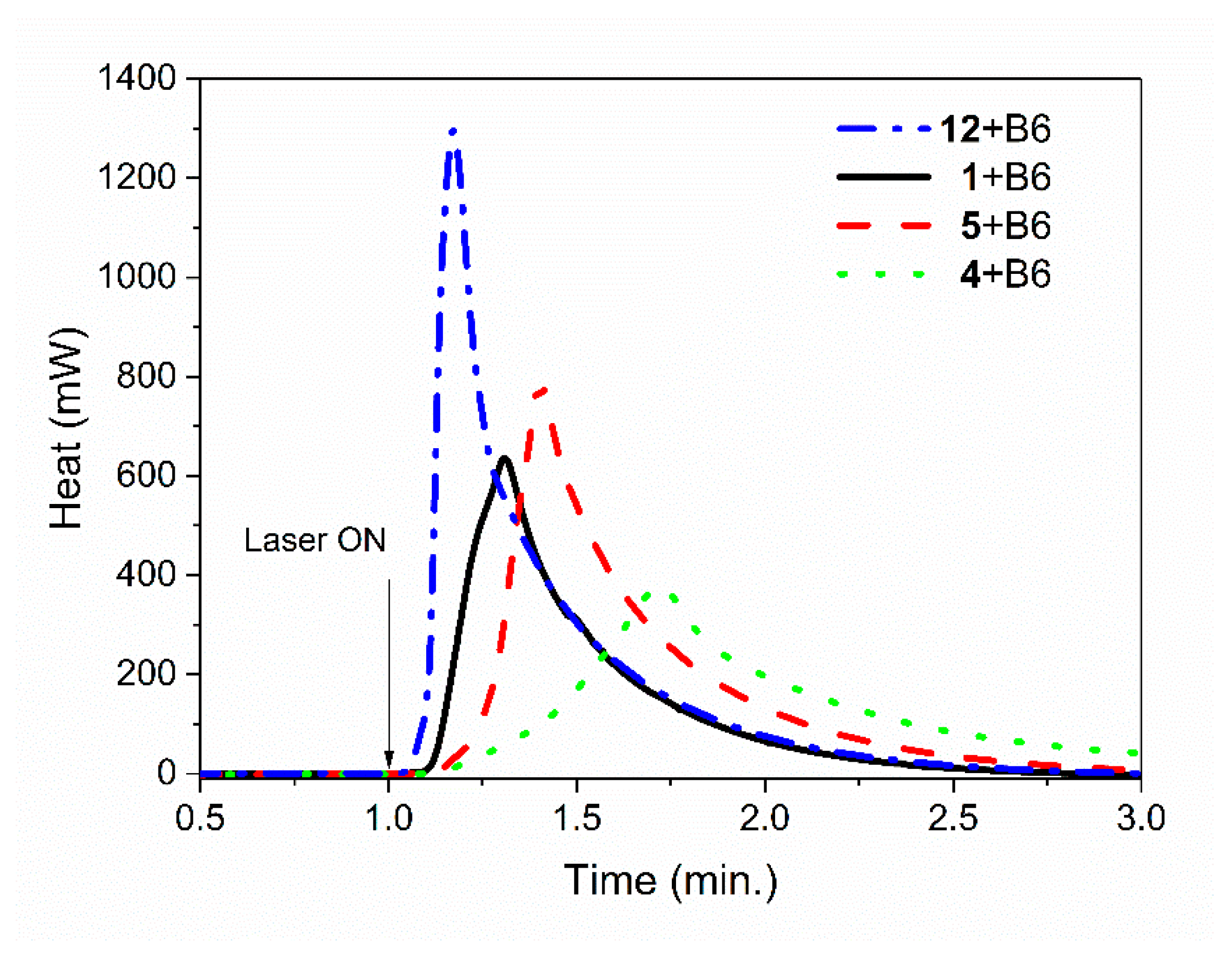
3.2. Photoinitiating Polymerization Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; A John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Pączkowski, J.; Neckers, D.C. Photoinduced electron transfer initiating systems for free radical polymerization. In Electron Transfer in Chemistry; Balzani, V., Ed.; WILEY-VCH Verlag GmbH: New York-Weinheim, NY, USA, 2001; Volume 5, pp. 516–585. [Google Scholar]
- Pączkowski, J.; Kabatc, J.; Jędrzejewska, B. Polymethine dyes as fluorescent probes and visible-light photoinitiators for free radical polymerization. In Heterocyclic Polymethine Dyes; Gupta, R.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 14, pp. 183–220. [Google Scholar]
- Pączkowski, J. Electron-transfer photoinitiators of free radical polymerization. The effect of the co-iniciator structure on photoinitiation ability. In Photochemistry and UV Curing: New Trends; Fouassier, J.-P., Ed.; Research Signpost: Kerala, India, 2006; p. 101. [Google Scholar]
- Oster, G. Dye-Sensitized photopolymerization. Nature 1954, 173, 300–301. [Google Scholar] [CrossRef]
- Topa, M.; Petko, F.; Galek, M.; Machowski, K.; Pilch, M.; Szymaszek, P.; Ortyl, J. Applicability of 1,6-Diphenylquinolin-2-one derivatives as fluorescent sensors for monitoring the progress of photopolymerisation processes and as photosensitisers for bimolecular photoinitiating systems. Polymers 2019, 11, 1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, X.; Yin, J. Study of methoxyphenylquinoxalines (MOPQs) as photoinitiators in the negative photo-resist. Prog. Org. Coat. 2010, 67, 225–232. [Google Scholar] [CrossRef]
- Podsiadły, R.; Sokołowska, J. Synthesis of novel oxidizable polymerization sensitizers based on the dithiinoquinoxaline skeleton. Dyes Pigment. 2012, 92, 1300–1307. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on pyrene-based photoinitiators of polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Han, W.; You, J.; Li, H.; Zhao, D.; Nie, J.; Wang, T. Curcuminoid-Based difluoroboron dyes as high-performance photosensitizers in long-wavelength (yellow and red) cationic photopolymerization. Macromol. Rapid Commun. 2019, 40, 1900291. [Google Scholar] [CrossRef]
- Han, W.; Shi, Y.; Xue, T.; Wang, T. Synthesis and electrochemical, linear and third-order nonlinear optical properties of ferrocene-based D-π-A dyes as novel photoredox catalysts in photopolymerization under visible LED irradiations. Dyes Pigment. 2019, 166, 140–148. [Google Scholar] [CrossRef]
- Yoon, J.; Jung, Y.J.; Yoon, J.B.; Damodar, K.; Kim, H.-J.; Shin, M.; Seo, M.; Cho, D.W.; Lee, J.T.; Lee, J.K. The heavy-atom effect on xanthene dyes for photopolymerization by visible light. Polym. Chem. 2019, 10, 5737–5742. [Google Scholar] [CrossRef]
- Xue, T.; Zhao, D.; Hao, T.; Li, X.; Wang, T.; Nie, J. Synthesis, one/two-photon optical and electrochemical properties and the photopolymerization-sensitizing effect of anthracene-based dyes: Influence of the donor groups. New J. Chem. 2019, 43, 6737–6745. [Google Scholar] [CrossRef]
- Oldring, P.K.T. Chemistry and technology of UV and EB formulation for coatings, inks and paints. In Speciality Finishes; Wiley & Sita Techn. Ltd.: London, UK, 1997; Volume 5. [Google Scholar]
- Sokołowska, J.; Podsiadły, R.; Stoczkiewicz, J. Styryl dyes as new photoinitiators for free radical polymerization. Dyes Pigment. 2008, 77, 510–514. [Google Scholar] [CrossRef]
- Encinas, M.V.; Rufs, A.M.; Bertolotti, S.G.; Previtali, C.M. Xanthene dyes/amine as photoinitiators of radical polymerization: A comparative and photochemical study in aqueous medium. Polymer 2009, 50, 2762–2767. [Google Scholar] [CrossRef]
- Wan, X.; Zhao, Y.; Xue, J.; Wu, F.; Fang, X. Water-soluble benzylidene cyclopentanone dye for two-photon photopolymerization. J. Photochem. Photobiol. A Chem. 2009, 202, 74–79. [Google Scholar] [CrossRef]
- Jędrzejewska, B. Factors affecting the TMPTA radical polymerization photoinitiated by phenyltrialkylborates paired with tri-cationic hemicyanine dye. Kinetic studies. Colloid Polym. Sci. 2013, 291, 2225–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jędrzejewska, B.; Pietrzak, M.; Rafiński, Z. Phenyltrialkylborates as co-initiators with cyanine dyes in visible light polymerization of acrylates. Polymer 2011, 52, 2110–2119. [Google Scholar] [CrossRef]
- Gould, I.R.; Shukla, D.; Giesen, D.; Farid, S. Energetics of electron-transfer reactions of photoinitiated polymerization: Dye-Sensitized fragmentation of N-Alkoxypyridinium salts. Helv. Chim. Acta 2001, 84, 2796–2812. [Google Scholar] [CrossRef]
- Podsiadły, R.; Sokołowska, J.; Kolińska, J. Heterocyclic dyes as visible photoinitiators for free-radical and cationic polymerization. In Recent Research Developments in Photochemistry and Photobiology; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2011; p. 17. [Google Scholar]
- Tarzi, O.I.; Allonas, X.; Ley, C.; Fouassier, J.-P. Pyrromethene derivatives in three-component photoinitiating systems for free radical photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2594–2603. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic electronics: An el dorado in the quest of new photocatalysts for polymerization reactions. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis-Scope, Reactivity, and Efficiency; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Würthner, F.; Yao, S.; Debaerdemaeker, T.; Wortmann, R. Dimerization of merocyanine dyes. Structural and energetic characterization of dipolar dye aggregates and implications for nonlinear optical materials. J. Am. Chem. Soc. 2002, 124, 9431–9447. [Google Scholar] [CrossRef]
- Ding, S.; Yao, B.; Schobben, L.; Hong, Y. Barbituric acid based fluorogens: Synthesis, aggregation-induced emission, and protein fibril detection. Molecules 2020, 25, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, C.; Halbhuber, A.; Keil, D.; Strehmel, B. NIR-Sensitized photoinitiated radical polymerization and proton generation with cyanines and LED arrays. Prog. Org. Coat. 2016, 100, 32–46. [Google Scholar] [CrossRef]
- Shirinian, V.Z.; Shimkin, A.A. Merocyanines: Synthesis and application. In Topics in Heterocyclic Chemistry; Gupta, R.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 14, pp. 75–105. [Google Scholar]
- Kulinich, A.V.; Ishchenko, A.A. Merocyanine dyes: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2009, 78, 141–164. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S. Merocyanine dyes containing imide functional groups: Synthesis and studies on hydrogen bonding to melamine receptors. J. Org. Chem. 2003, 68, 8943–8949. [Google Scholar] [CrossRef] [PubMed]
- Mercaldi, G.F.; D’Antonio, E.L.; Aguessi, A.; Rodriguez, A.; Cordeiro, A.T. Discovery of antichagasic inhibitors by high-throughput screening with Trypanosoma cruzi glucokinase. Bioorg. Med. Chem. Lett. 2019, 29, 1948–1953. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S.; Schilling, J.; Wortmann, R.; Redi-Abshiro, M.; Mecher, E.; Gallego-Gomez, F.; Meerholz, K. ATOP dyes. Optimization of a multifunctional merocyanine chromophore for high refractive index modulation in photorefractive materials. J. Am. Chem. Soc. 2001, 123, 2810–2824. [Google Scholar] [CrossRef]
- Karatsu, T.; Yanai, M.; Yagai, S.; Mizukami, J.; Urano, T.; Kitamura, A. Evaluation of sensitizing ability of barbiturate-functionalized non-ionic cyanine dyes; application for photoinduced radical generation system initiated by near IR light. J. Photochem. Photobiol. A Chem. 2005, 170, 123–129. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push–pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Kawamura, K.; Amemiya, T.; Nakai, Y.; Takashima, M. Novel and efficient dye-linked photoinitiator generating a radical via an intramolecular electron-transfer process. J. Photopolym. Sci. Technol. 2009, 22, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Kadoma, Y.; Fujisawa, S. Radical-Scavenging activity of thiols, thiobarbituric acid derivatives And phenolic antioxidants determined using the induction period method for radical polymerization of Methyl methacrylate. Polymers 2012, 4, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- Su, H.-L.; Hsu, J.-M.; Pan, J.-P.; Wang, T.-H.; Yu, F.-E.; Chern, C.-S. Kinetic and structural studies of the polymerization of N,N′-bismaleimide-4,4′-diphenylmethane with barbituric acid. Polym. Eng. Sci. 2011, 51, 1188–1197. [Google Scholar] [CrossRef]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H. Polymer Handbook, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, Canada; Singapore, 1989. [Google Scholar]
- Avci, D.; Nobles, J.; Mathias, L.J. Synthesis and photopolymerization kinetics of new flexible diacrylate and dimethacrylate crosslinkers based on C18 diacid. Polymer 2003, 44, 963–968. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Jakubiak, J.; Rabek, J.F. Modeling of the kinetics of linear and crosslinking photopolymerization. Part III. Polimery 2001, 46, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Anseth, K.S.; Decker, C.; Bowman, C.N. Real-Time infrared characterization of reaction diffusion during multifunctional monomer polymerizations. Macromolecules 1995, 28, 4040–4043. [Google Scholar] [CrossRef]
- Jakubiak, J.; Sionkowska, A.; Lindén, L.Å.; Rabek, J.F. Isothermal photo differential scanning calorimetry. Crosslinking polymerization of multifunctional monomers in presence of visible light photoinitiators. J. Therm. Anal. Calorim. 2001, 65, 435. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.; Yin, J. Polymeric amine bearing side-chain thioxanthone as a novel photoinitiator for photopolymerization. Polymer 2004, 45, 133–140. [Google Scholar] [CrossRef]
- Kabatc, J.; Jędrzejewska, B.; Pączkowski, J. New heterobicationic hemicyanine dyes: Synthesis, spectroscopic properties, and photoinitiating ability. J. Polym. Sci. Part. A Polym. Chem. 2006, 44, 6345–6359. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Marcus, R.A. On the theory of electron-transfer reactions. VI. unified treatment for homogeneous and electrode reactions. J. Chem. Phys. 1965, 43, 679–701. [Google Scholar] [CrossRef] [Green Version]
- Pa̧czkowski, J.; Pietrzak, M.; Kucybała, Z. Generalization of the kinetic scheme for photoinduced polymerization via an intermolecular electron transfer process. 2. application of the marcus theory. Macromolecules 1996, 29, 5057–5064. [Google Scholar] [CrossRef]
- Eberson, L. Electron. Transfer in Organic Chemistry; Springer: New York, NY, USA, 1987. [Google Scholar]






| Abbr. | Substituent, R | ||||
|---|---|---|---|---|---|
| 1 | 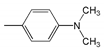 | 452.5 | 65,000 | 521 | 2906 |
| 2 | 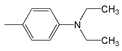 | 458 | 86,800 | 525 | 2786 |
| 3 | 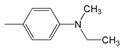 | 455 | 77,500 | 527 | 3003 |
| 4 | 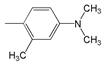 | 465.5 | 58,500 | 539 | 2929 |
| 5 | 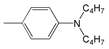 | 460.5 | 83,200 | 532 | 2919 |
| 6 | 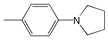 | 458.5 | 76,200 | 529 | 2907 |
| 7 | 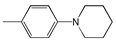 | 457 | 61,800 | 545 | 3533 |
| 8 |  | 442.5 | 50,600 | 521 | 3405 |
| 9 | 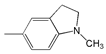 | 469 | 61,200 | 534 | 2630 |
| 10 | 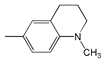 | 465 | 78,700 | 532 | 2708 |
| 11 | 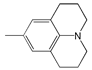 | 477 | 93,300 | 541 | 2480 |
| 12 | 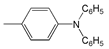 | 456 | 40,600 | 642 | 6354 |
| 13 |  | 471 | 20,600 | 542 | 2781 |
| Abbr. | Ered (V) | E00 (eV) | ΔGet (eV) | Rp max av (μmol/s) a) | Rp max av (μmol/s) b) | Φpa) | Φpb) | Cfav (%) a) | Cfav (%) b) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1.212 | 2.641 | −0.665 | 8.04 | 6.99 | 98.60 | 85.73 | 58 | 51 |
| 2 | −1.268 | 2.613 | −0.581 | 7.50 | 7.08 | 91.97 | 86.83 | 68 | 55 |
| 3 | −1.276 | 2.624 | −0.584 | 6.84 | 6.22 | 83.91 | 74.67 | 57 | 50 |
| 4 | −1.25 | 2.508 | −0.494 | 4.01 | 1.10 | 49.15 | 13.47 | 54 | 53 |
| 5 | −1.308 | 2.597 | −0.525 | 9.10 | 7.04 | 111.56 | 86.34 | 63 | 53 |
| 6 | −1.298 | 2.591 | −0.529 | 9.38 | 7.05 | 115.05 | 86.39 | 65 | 55 |
| 7 | −1.226 | 2.528 | −0.538 | 6.88 | 6.37 | 84.29 | 78.14 | 63 | 54 |
| 8 | −1.268 | 2.652 | −0.620 | 9.17 | 6.88 | 112.40 | 84.30 | 60 | 53 |
| 9 | −1.168 | 2.528 | −0.596 | 7.21 | 4.28 | 81.62 | 52.45 | 54 | 42 |
| 10 | −1.264 | 2.565 | −0.537 | 7.64 | 7.46 | 93.65 | 91.51 | 61 | 57 |
| 11 | −1.104 | 2.508 | −0.640 | 6.40 | 4.78 | 78.48 | 58.63 | 57 | 54 |
| 12 | −1.224 | 2.387 | −0.399 | 16.91 | 15.98 | 207.37 | 195.91 | 73 | 54 |
| 14 | −1.29 | 2.487 | −0.433 | 0.70 | 0.34 | 8.60 | 4.22 | 36 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejewska, B.; Wejnerowska, G. Highly Effective Sensitizers Based on Merocyanine Dyes for Visible Light Initiated Radical Polymerization. Polymers 2020, 12, 1242. https://doi.org/10.3390/polym12061242
Jędrzejewska B, Wejnerowska G. Highly Effective Sensitizers Based on Merocyanine Dyes for Visible Light Initiated Radical Polymerization. Polymers. 2020; 12(6):1242. https://doi.org/10.3390/polym12061242
Chicago/Turabian StyleJędrzejewska, Beata, and Grażyna Wejnerowska. 2020. "Highly Effective Sensitizers Based on Merocyanine Dyes for Visible Light Initiated Radical Polymerization" Polymers 12, no. 6: 1242. https://doi.org/10.3390/polym12061242






