Why Is the Sensory Response of Organic Probes within a Polymer Film Different in Solution and in the Solid-State? Evidence and Application to the Detection of Amino Acids in Human Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental
3.1. Measurement Techniques
3.2. Preparation of the Sensory Monomer
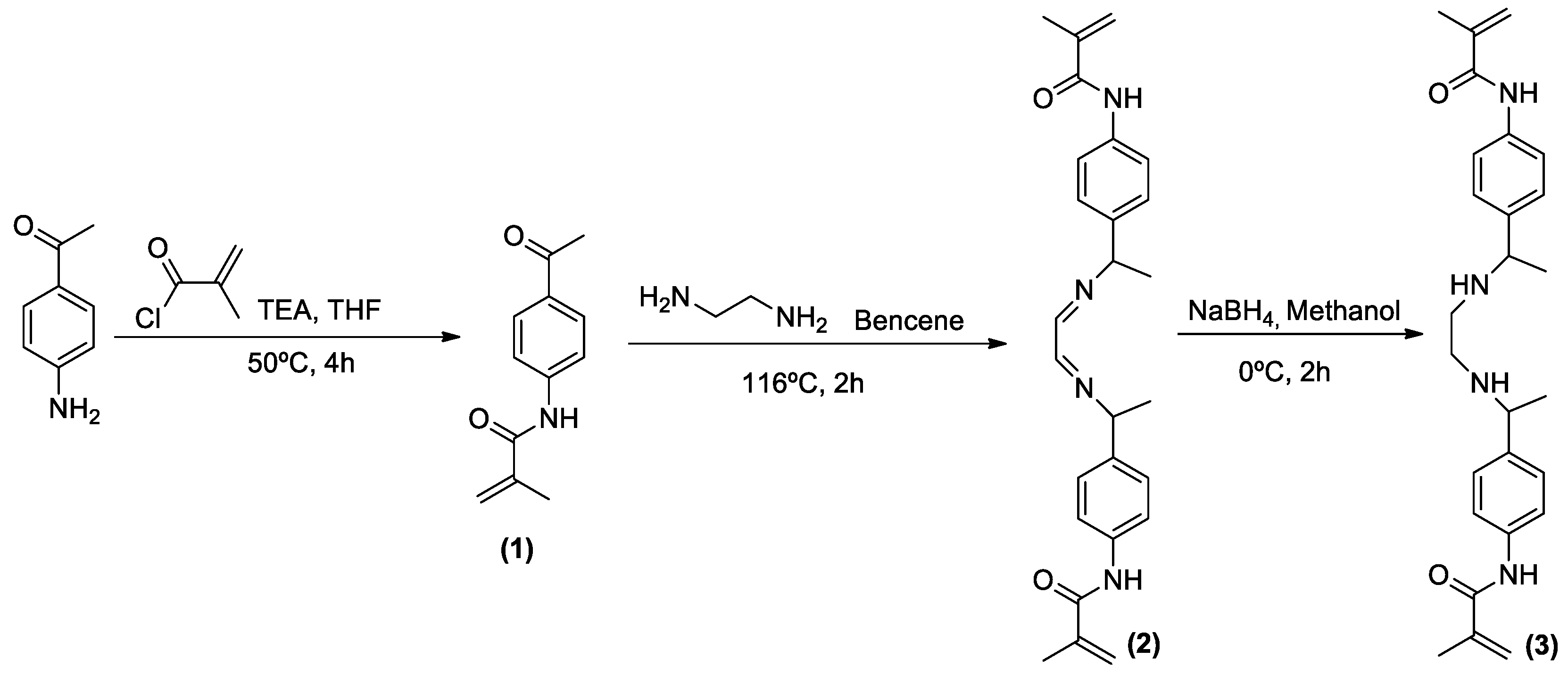
3.2.1. Synthesis of N-(4-acetylphenyl)methacrylamide (1)
3.2.2. Synthesis of N,N′-(((ethane-1,2-diylidenebis(azanylylidene))bis(ethane-1,1-diyl))bis(4,1-phenylene))-bis(2-methacrylamide) (2)
3.2.3. Synthesis of N,N′-(((ethane-1,2-diylbis(azanediyl))bis(ethane-1,1-diyl))bis(4,1-phenylene))bis(2-methacrylamide) (3)
3.3. Preparation of the Sensory Film
3.4. Ethical Statement
4. Results and Discussion
4.1. Mechanical and Thermal Properties of the Films
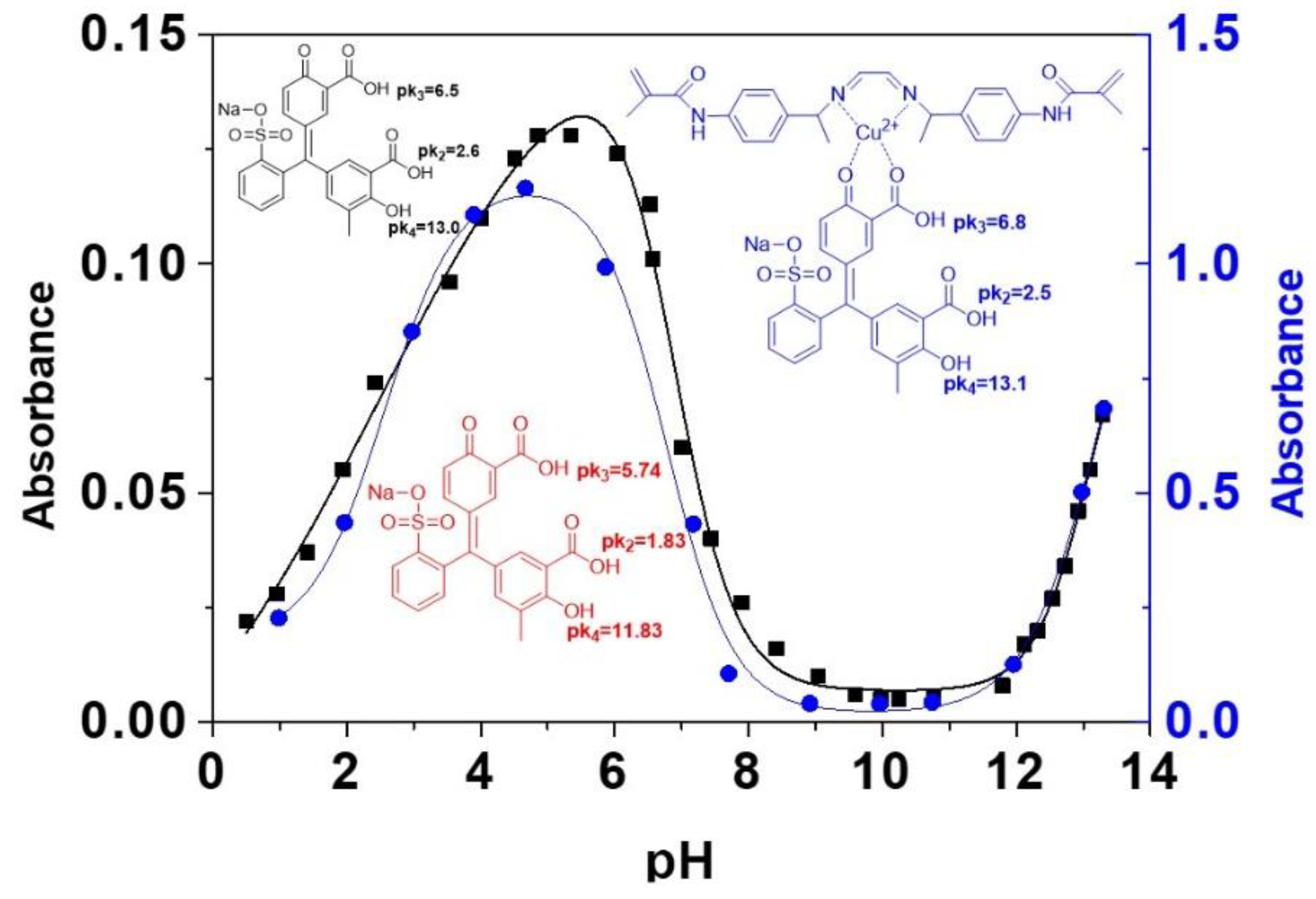
4.2. The behaviour of the Sensory Film at Different pH
4.3. Interference Study
4.4. Why Films Are Not Only a Support but Deeply Influence the Performance of the Sensory Probe?
4.5. Analysis of the Interaction of the Dye with Solvents both in Solution and in the Amorphous and Solid-State (within the Solvent Swelled Film)
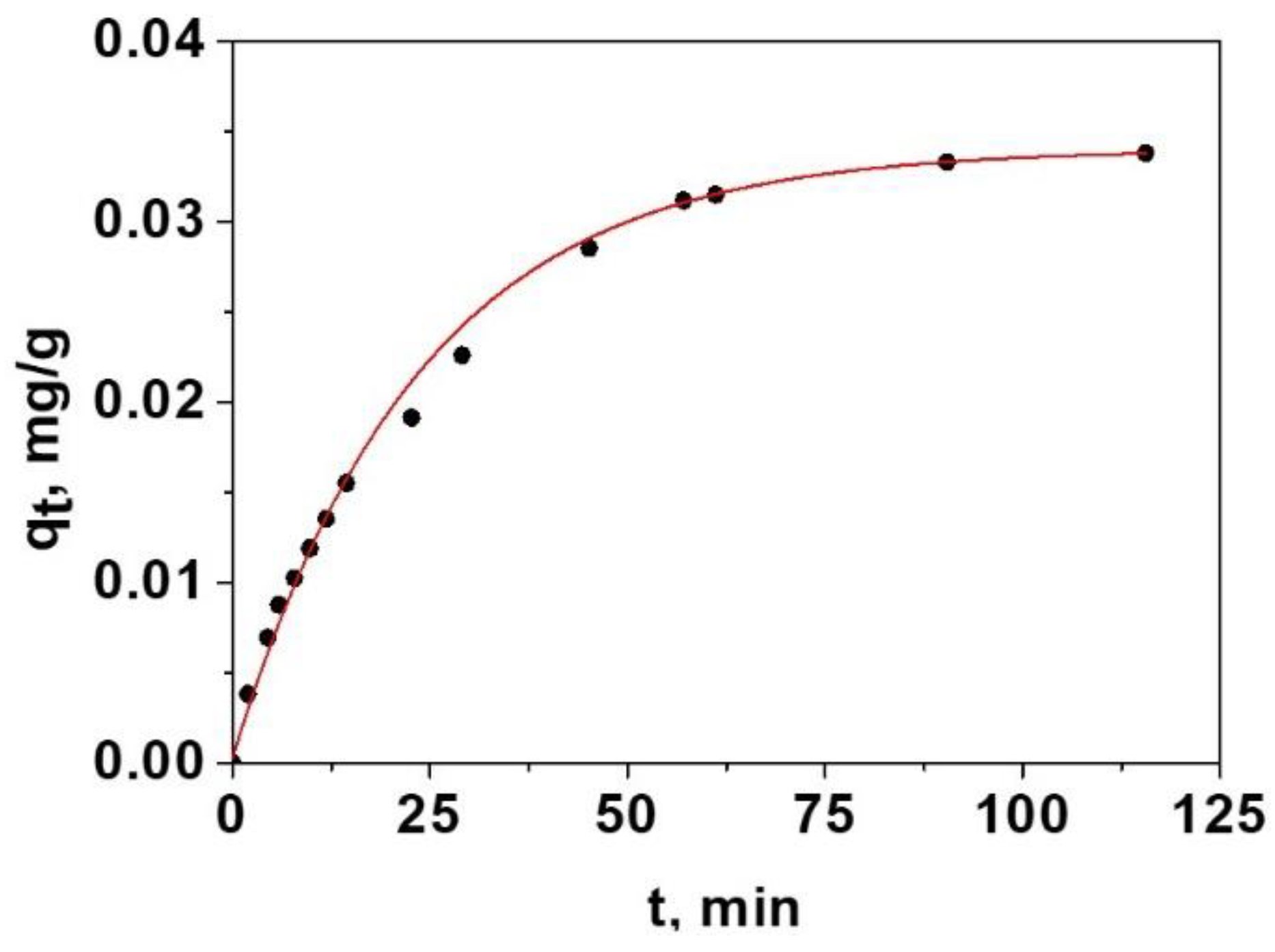
4.6. Diffusion of Species in Solution into the Swelled Film
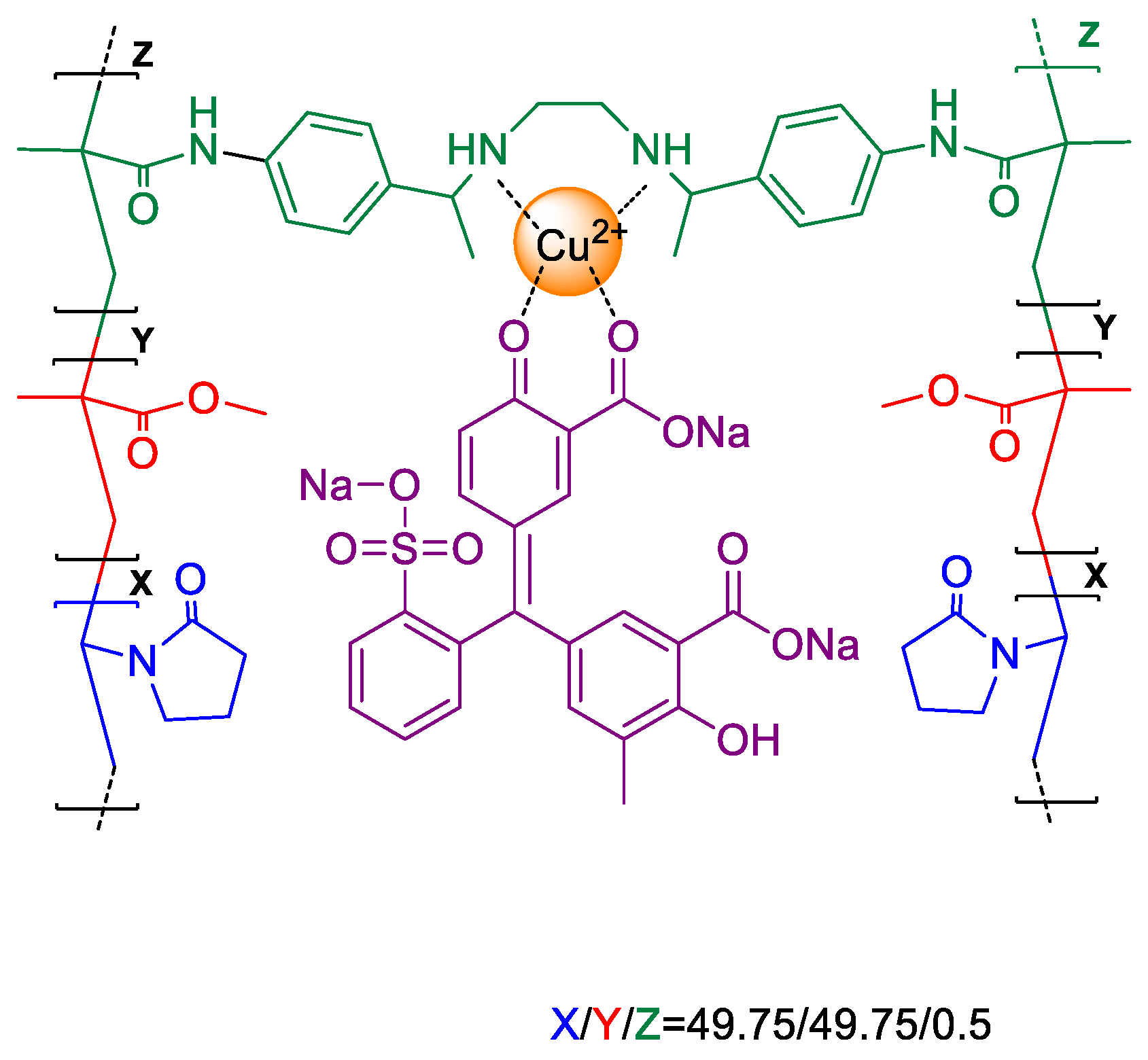
4.7. Analysis of the Complex Formation between the Polymer, Cu(II) and Dye
4.8. Colourimetric Sensing of Amino Acids
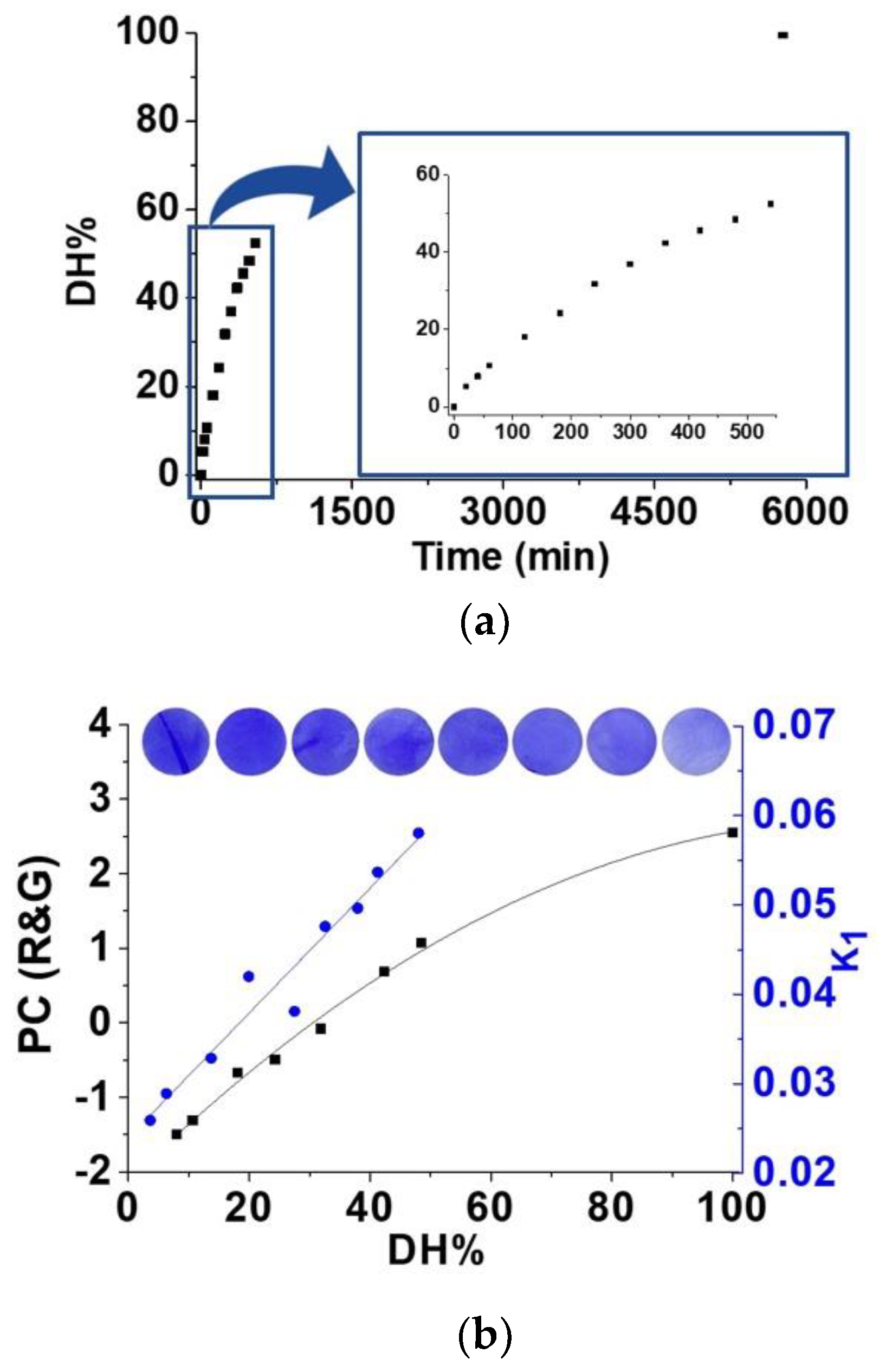
4.9. Proof of Concept. Sensing Amino Acids from a Beefsteak and A Human Chronic Wound
4.10. Reusability of the Sensory Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nathan, A.J.; Scobell, A. How China Sees America; Wiley: New York, NY, USA, 2012; ISBN 0-471-98369-1. [Google Scholar]
- Bustamante, S.E.; Vallejos, S.; Pascual-Portal, B.S.; Muñoz, A.; Mendia, A.; Rivas, B.L.; García, F.C.; García, J.M. Polymer films containing chemically anchored diazonium salts with long-term stability as colorimetric sensors. J. Hazard. Mater. 2019, 365, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Reglero, J.A.; García, F.C.; García, J.M. Direct visual detection and quantification of mercury in fresh fish meat using facilely prepared polymeric sensory labels. J. Mater. Chem. A 2017, 5, 13710–13716. [Google Scholar] [CrossRef]
- Bustamante Fonseca, S.E.; Rivas, B.L.; García Pérez, J.M.; Vallejos Calzada, S.; García, F. Synthesis of a polymeric sensor containing an occluded pyrylium salt and its application in the colorimetric detection of trimethylamine vapors. J. Appl. Polym. Sci. 2018, 135, 46185. [Google Scholar] [CrossRef]
- Vallejos, S.; Hernando, E.; Trigo, M.; García, F.C.; García-Valverde, M.; Iturbe, D.; Cabero, M.J.; Quesada, R.; García, J.M. Polymeric chemosensor for the detection and quantification of chloride in human sweat. Application to the diagnosis of cystic fibrosis. J. Mater. Chem. B 2018, 6, 3735–3741. [Google Scholar] [CrossRef] [Green Version]
- González-Ceballos, L.; Melero, B.; Trigo-López, M.; Vallejos, S.; Muñoz, A.; García, F.C.; Fernandez-Muiño, M.A.; Sancho, M.T.; García, J.M. Functional aromatic polyamides for the preparation of coated fibres as smart labels for the visual detection of biogenic amine vapours and fish spoilage. Sens. Actuators B Chem. 2020, 304, 127249. [Google Scholar] [CrossRef]
- Vallejos, S.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Solid sensory polymer substrates for the quantification of iron in blood, wine and water by a scalable RGB technique. J. Mater. Chem. A 2013, 1, 15435–15441. [Google Scholar] [CrossRef]
- Vallejos, S.; Estévez, P.; Ibeas, S.; García, F.C.; Serna, F.; García, J.M. An organic/inorganic hybrid membrane as a solid “Turn-On” fluorescent chemosensor for coenzyme a (CoA), cysteine (Cys), and glutathione (GSH) in aqueous media. Sensors 2012, 12, 2969–2982. [Google Scholar] [CrossRef] [Green Version]
- Vallejos, S.; Moreno, D.; Ibeas, S.; Muñoz, A.; García, F.C.; García, J.M. Polymeric chemosensor for the colorimetric determination of the total polyphenol index (TPI) in wines. Food Control 2019, 106, 106684. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Nherera, L.; Digby, L.; Di Vincenzo, P.; Clark, J.; Gilpin, C. Quantifying the economic value of diagnostics in wound care in the UK Background: The burden of chronic wounds. In Proceedings of the European Wound Management Association; Woundcheck: Copenhagen, Danmark, 2013; p. C1418. [Google Scholar]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Gómez Fernández, P. Revisión del tratamiento de las úlceras venosas: Terapia compresiva. RqR Enfermería Comunitaria 2015, 3, 43–54. [Google Scholar]
- Becker, K.; Boykin, J.; Crossland, M.; Davis, P.; Doughty, D.; Driver, V.; von Eiff, C.; Harding, K.; Lindholm, C.; Lubbers, M.; et al. Diagnostics and Wound. A Consensus Document. World Union of Wound Healing Societies (WUWHS); Principles of Best Practice: A World Union of Wound Healing Societies’ Initiative: London, UK, 2008. [Google Scholar]
- International Consensus The role of proteases in wound diagnostics. In An Expert Working Group Review; Wounds Int.: London, UK, 2011.
- Westby, M.J.; Norman, G.; Dumville, J.C.; Stubbs, N.; Cullum, N. Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst. Rev. 2016, 2016, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, K.; Song, S.; Cheng, Y.; Guo, L.; Pei, Y.; Lv, X.; Aastrup, T.; Pei, Z. Fabrication of carbohydrate chips based on polydopamine for real-time determination of carbohydrate-lectin interactions by QCM biosensor. Polymers (Basel) 2018, 10, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers (Basel) 2017, 9, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Shangguan, Y.; Zheng, Q. Ferrocene-modified polyelectrolyte film-coated electrode and its application in glucose detection. Polymers (Basel) 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Bagal-Kestwal, D.R.; Chiang, B.H. Exploration of chitinous scaffold-based interfaces for glucose sensing assemblies. Polymers (Basel) 2019, 11, 1958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Khan, I.M.; Ji, H.; Wang, Z.; Tian, H.; Cao, W.; Mi, W. A Label-Free Fluorescent Aptasensor for Detection of Staphylococcal Enterotoxin A Based on Aptamer-Functionalized Silver Nanoclusters. Polymers (Basel) 2020, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.L.; Murphy, S.M.; Hamilton, C.J.; Tighe, B.J. Polymer membranes in clinical sensor applications. III. Hydrogels as reactive matrix membranes in fibre optic sensors. Biomaterials 1992, 13, 991–999. [Google Scholar] [CrossRef]
- Retama, J.R.; Lopez-Ruiz, B.; Lopez-Cabarcos, E. Microstructural modifications induced by the entrapped glucose oxidase in cross-linked polyacrylamide microgels used as glucose sensors. Biomaterials 2003, 24, 2965–2973. [Google Scholar] [CrossRef]
- Klueh, U.; Dorsky, D.I.; Kreutzer, D.L. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularisation. Biomaterials 2005, 26, 1155–1163. [Google Scholar] [CrossRef]
- Gerritsen, M.; Kros, A.; Sprakel, V.; Lutterman, J.A.; Nolte, R.J.M.; Jansen, J.A. Biocompatibility evaluation of sol-gel coatings for subcutaneously implantable glucose sensors. Biomaterials 2000, 21, 71–78. [Google Scholar] [CrossRef]
- Tian, Y.; Su, F.; Weber, W.; Nandakumar, V.; Shumway, B.R.; Jin, Y.; Zhou, X.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. A series of naphthalimide derivatives as intra and extracellular pH sensors. Biomaterials 2010, 31, 7411–7422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz Folmer-Andersen, J.; Kitamura, M.; Anslyn, E.V. Pattern-based discrimination of enantiomeric and structurally similar amino acids: An optical mimic of the mammalian taste response. J. Am. Chem. Soc. 2006, 128, 5652–5653. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Anslyn, E.V. Indicator-displacement assays. Coord. Chem. Rev. 2006, 250, 3118–3127. [Google Scholar] [CrossRef]
- Gupta, V.K.; Goyal, R.N.; Sharma, R.A. Anion recognition using newly synthesised hydrogen bonding disubstituted phenylhydrazone-based receptors: Poly(vinyl chloride)-based sensor for acetate. Talanta 2008, 76, 859–864. [Google Scholar] [CrossRef]
- Marcus, Y. The Properties of Solvents, 4th ed.; Wiley: Chichester, UK, 1998; ISBN 0-471-98369-1. [Google Scholar]
- Kamlet, M.J.; Taft, R.W. The Solvatochromic Comparison Method. I. The β-Scale Of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The Solvatochromic Comparison Method. 2. The α-Scale of Solvent Hydrogen-Bond Donor (HBD) Acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The Solvatochromic Comparison Method. 6. The π* Scale of Solvent Polarities1. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear Solvation Energy Relationships. 23. A Comprehensive Collection of the Solvatochromic Parameters, π, α, and β, and Some Methods for Simplifying the Generalised Solvatochromic Equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Mir, A.A.; Amooey, A.A.; Ghasemi, S. Adsorption of direct yellow 12 from aqueous solutions by an iron oxide-gelatin nanoadsorbent; kinetic, isotherm and mechanism analysis. J. Clean. Prod. 2018, 170, 570–580. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Liu, X.; Yang, L.; Pan, F.; Lv, J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Doǧan, M.; Abak, H.; Alkan, M. Adsorption of methylene blue onto hazelnut shell: Kinetics, mechanism and activation parameters. J. Hazard. Mater. 2009, 164, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interface Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. Diffusion in a Plane Sheet; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Chatterjee, A.; Schiewer, S. Multi-resistance kinetic models for biosorption of Cd by raw and immobilised citrus peels in batch and packed-bed columns. Chem. Eng. J. 2014, 244, 105–116. [Google Scholar] [CrossRef]
- Illanes, C.O.; Ochoa, N.A.; Marchese, J. Kinetic sorption of Cr(VI) into solvent impregnated porous microspheres. Chem. Eng. J. 2008, 136, 92–98. [Google Scholar] [CrossRef]
- Dávila-Guzman, N.E.; Cerino-Córdova, F.J.; Diaz-Flores, P.E.; Rangel-Mendez, J.R.; Sánchez-González, M.N.; Soto-Regalado, E. Equilibrium and kinetic studies of ferulic acid adsorption by Amberlite XAD-16. Chem. Eng. J. 2012, 183, 112–116. [Google Scholar] [CrossRef]
- Sun, X.; Chen, J.H.; Su, Z.; Huang, Y.; Dong, X. Highly effective removal of Cu(II) by a novel 3-aminopropyltriethoxysilane functionalised polyethyleneimine/sodium alginate porous membrane adsorbent. Chem. Eng. J. 2016, 290, 1–11. [Google Scholar] [CrossRef]
- Kiernan, J.A. Chromoxane cyanine R. II. Staining of animal tissues by the dye and its iron complexes. J. Microsc. 1984, 134, 25–39. [Google Scholar] [CrossRef]
- Levina, A.; Muzart, J. Enantioselective allylic oxidation in the presence of the Cu(I) Cu(II)-proline catalytic system. Tetrahedron Asymmetry 1995, 6, 147–156. [Google Scholar] [CrossRef]
- Conato, C.; Contino, A.; Maccarrone, G.; Magrì, A.; Remelli, M.; Tabbì, G. Copper(II) complexes with L-lysine and L-ornithine: Is the side-chain involved in the coordination? A thermodynamic and spectroscopic study. Thermochim. Acta 2000, 362, 13–23. [Google Scholar] [CrossRef]
- Ilavarasi, R.; Rao, M.N.S.; Udupa, M.R. Synthesis and characterisation of copper(II) complexes of adenine and aminoacids. Proc. Indian Acad. Sci. Chem. Sci. 1997, 109, 79–87. [Google Scholar] [CrossRef]
- Valora, G.; Bonomo, R.P.; Tabbì, G. An EPR and voltammetric study of simple and mixed copper(II) complexes with L- or D-glutamate and L-arginate in aqueous solution. Inorg. Chim. Acta 2016, 453, 62–68. [Google Scholar] [CrossRef]
- Scarpa, M.; Vianello, F.; Signor, L.; Zennaro, L.; Rigo, A. Ascorbate Oxidation Catalysed by Bis(histidine)copper(II). Inorg. Chem. 1996, 35, 5201–5206. [Google Scholar] [CrossRef]
- Solans, X.; Ruíz-Ramírez, L.; Martínez, A.; Gasque, L.; Moreno-Esparza, R. Mixed chelate complexes. III. Structures of (L-alaninato)(aqua)(2,2’-bipyridine)copper(II) nitrate monohydrate and aqua(2,2’-bipyridine)(L-tyrosinato)copper(II) chloride trihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 1785–1788. [Google Scholar] [CrossRef] [Green Version]
- Su, C.C.; Tai, T.Y.; Wu, S.P.; Wang, S.L.; Liao, F.L. Spectroscopic and electronic properties of mixed ligand aminoacidatocopper(II) complexes: Molecular structure of [Cu(4,7-dimethyl-1,10-phenanthroline)(L-phenylalaninato)](ClO4). Polyhedron 1999, 18, 2361–2368. [Google Scholar] [CrossRef]
- Gala, L.; Lawson, M.; Jomova, K.; Zelenicky, L.; Congradyova, A.; Mazur, M.; Valko, M. EPR spectroscopy of a clinically active (1:2) copper(ii)-histidine complex used in the treatment of menkes disease: A fourier transform analysis of a fluid cw-epr spectrum. Molecules 2014, 19, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.M.M.; Paniago, E.B.; Carvalho, S. Copper(II) mixed ligands complexes of hydroxamic acids with glycine, histamine and histidine. J. Braz. Chem. Soc. 1997, 8, 537–548. [Google Scholar] [CrossRef]
- Marti, E.M.; Quash, A.; Methivier, C.; Dubot, P.; Pradier, C.M. Interaction of S-histidine, an amino acid, with copper and gold surfaces, a comparison based on RAIRS analyses. Colloids Surf. A Physicochem. Eng. Asp. 2004, 249, 85–89. [Google Scholar] [CrossRef]
- Mukherjee, G.; Ghosh, T. Metal ion interaction with penicillins: Part VIII. Equilibrium study of mixed ligand complex formation of Co (II), Ni (II), Cu (II) and Zn (II) with ampicillin and some amino acids. Proc. Indian Acad. Sci. Chem. Sci. 1996, 108, 371–378. [Google Scholar] [CrossRef]
- Estrader, M.; Diaz, C.; Ribas, J.; Solans, X.; Font-Bardía, M. Synthesis, characterisation and magnetic properties of six new copper(II) complexes with aminoacids as bridging ligand, exhibiting ferromagnetic coupling. Inorg. Chim. Acta 2008, 361, 3963–3969. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of proteins from the oral tissues-I. A comparison of human oral epithelium, epidermis and nail proteins. Arch. Oral Biol. 1963, 8, 449–458. [Google Scholar] [CrossRef]
- Keeley, F.W.; Partridge, S.M. Amino acid composition and calcification of human aortic elastin. Atherosclerosis 1974, 19, 287–296. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Martens, P.; Thomas, N.R. The amino-acid composition of human hard tissue collagens in osteogenesis imperfecta and dentinogenesis imperfecta. Calcif. Tissue Res. 1973, 12, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Szeinberg, A.; Szeinberg, B.; Cohen, B.E. Screening method for detection of specific aminoacidemias. Clin. Chim. Acta 1969, 23, 93–95. [Google Scholar] [CrossRef]
- Katsikis, S.; Marin-Montesinos, I.; Ludwig, C.; Günther, U.L. Detecting acetylated aminoacids in blood serum using hyperpolarised 13C-1H-2D-NMR. J. Magn. Reson. 2019, 305, 175–179. [Google Scholar] [CrossRef]
- Aubaid, A.H.; Risan, A.Z.; Naem, A.K. Detection of sugars and aminoacids in antigens of Trichophyton mentagrophytes var. erinacei. Mycoses 1999, 42, 249–253. [Google Scholar] [CrossRef]
- Veledo, M.T.; de Frutos, M.; Diez-Masa, J.C. On-capillary derivatisation and analysis of amino acids in human plasma by capillary electrophoresis with laser-induced fluorescence detection: Application to diagnosis of aminoacidopathies. Electrophoresis 2006, 27, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, P.; Mopper, K. High Performance Liquid Chromatographic Determination of Subpicomole Amounts of Amino Acids by Precolumn Fluorescence Derivatization with o-Phthaldialdehyde. Anal. Chem. 1979, 51, 1667–1674. [Google Scholar] [CrossRef]
- Petritis, K.; Elfakir, C.; Dreux, M. HPLC-CLND for the Analysis of Underivatized Amino Acids. LC GC Eur. 2001, 14, 389–395. [Google Scholar]
- Pasieka, A.E.; Thomas, M.E. The detection of β-alanine in biological fluids. Clin. Biochem. 1968, 2, 423–429. [Google Scholar] [CrossRef]
- Saifer, A. Rapid screening methods for the detection of inherited and acquired aminoacidopathies. Adv. Clin. Chem. 1971, 14, 145–218. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, K.; Vanheule, R.; van Belle, M. A new simple screening method for detecting pathological amino-acidemias with collection of blood on paper. Clin. Chim. Acta 1967, 15, 362–364. [Google Scholar] [CrossRef]


| Polymers | Mechanical Properties of Water Swelled Films, Young Modulus (MPa) | Thermal Properties, in N2 | ||
|---|---|---|---|---|
| Thermal Resistance | Thermal Transition | |||
| T5 (°C) | T10 (°C) | Tg (°C) | ||
| F(3) | 116 | 355 | 372 | 137 |
| F(3)-Cu | 108 | 355 | 374 | 144 |
| F(3)-Cu-D | 98 | 347 | 366 | 140 |
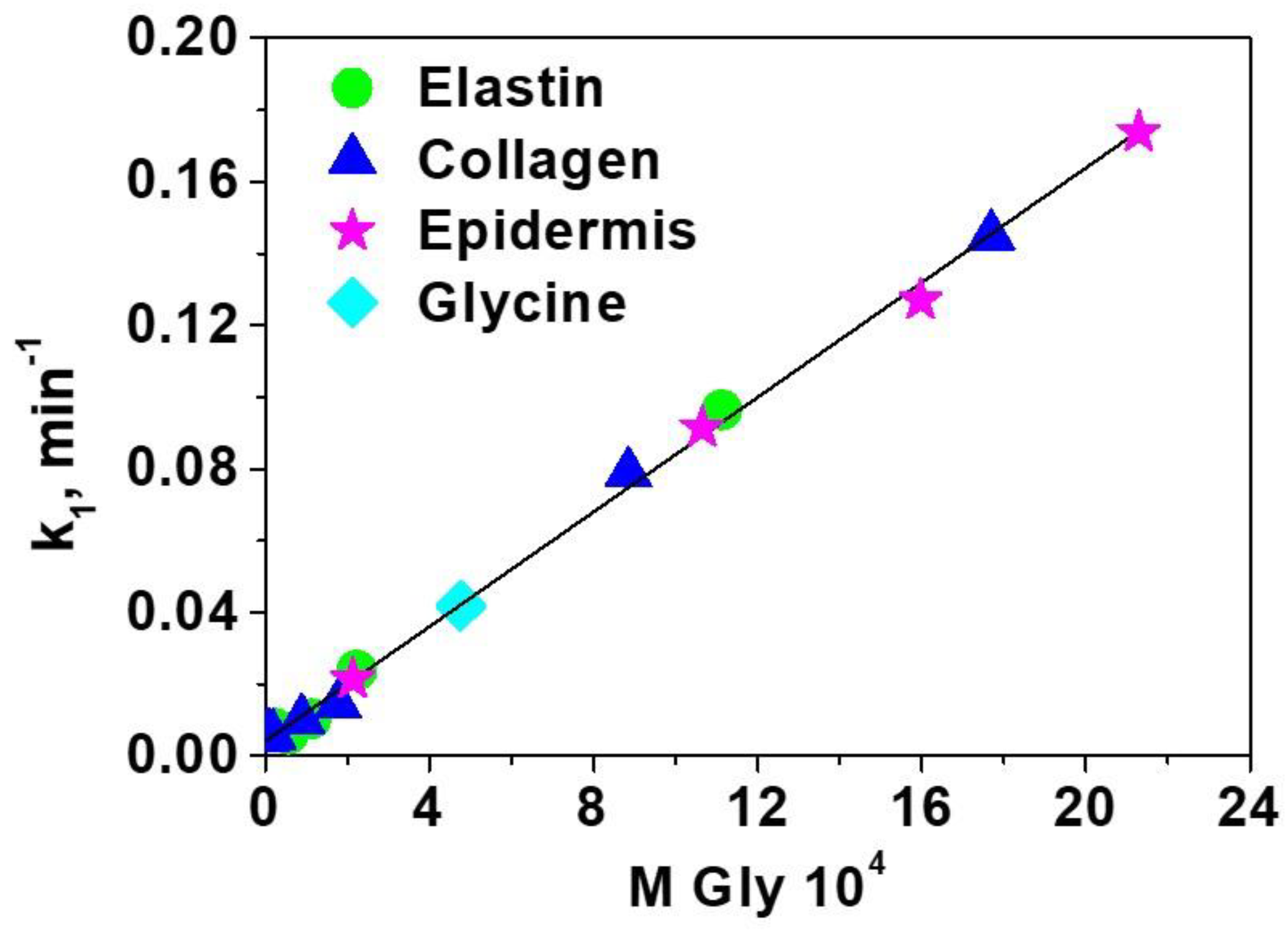
| Amino Acid | kf 104, cm/min | Ds 104, cm2/min | Bi | R |
|---|---|---|---|---|
| Arginine | 0.31 ± 0.07 | 0.009 ± 0.001 | 0.17 ± 0.06 | 0.9975 |
| Aspartic Acid | 0.47 ± 0.02 | 0.021 ± 0.006 | 0.11 ± 0.04 | 0.9977 |
| Phenylalanine | 0.57 ± 0.09 | 0.024 ± 0.004 | 0.12 ± 0.04 | 0.9989 |
| Glutamic Acid | 0.89 ± 0.02 | 0.038 ± 0.007 | 0.12 ± 0.02 | 0.9989 |
| Hydroxyproline | 1.18 ± 0.04 | 0.041 ± 0.003 | 0.14 ± 0.02 | 0.9977 |
| Proline | 1.41 ± 0.05 | 0.049 ± 0.003 | 0.14 ± 0.01 | 0.9968 |
| Alanine | 1.75 ± 0.05 | 0.067 ± 0.003 | 0.13 ± 0.01 | 0.9981 |
| Valine | 2.00 ± 0.1 | 0.082 ± 0.004 | 0.12 ± 0.01 | 0.9975 |
| Glycine | 2.20 ± 0.1 | 0.092 ± 0.003 | 0.12 ± 0.01 | 0.9973 |
| Method | Detection Method | Low Cost | Response Time | Naked Eye Detection | Limit of Detection | Reference |
|---|---|---|---|---|---|---|
| Reference method | UV-vis | no | 15 min | no | - | [62] |
| Screening method | Chromatography | yes | 5 min | yes | qualitative method | [63] |
| hyperpolarised 13C-1H-2D-NMR | NMR | no | 4 h | no | - | [64] |
| Trichophyton mentagrophytes var. erinacei | UV-vis | no | 2 w | no | - | [65] |
| CE-LIF | Electrophoresis and Fluorimetry | no | 4 h | no | 25–50 nM | [66] |
| HPLC | HPLC | no | 3 h | no | 50–60 fmol | [67] |
| HPLC-CLND | HPLC (CLND) | no | 3 h | no | 0.0025–0.0075 mM | [68] |
| High Voltage Electrophoresis | Electrophoresis | no | 22 h | no | - | [69] |
| Chromatography | Chromatography | no | 3 h | no | 0.5–10 µg | [70] |
| Screening method | Chromatography | no | 24 h | no | 1.5–7.5 w% | [71] |
| RGB | Digital pictures (RGB parameters defining the digital colours) | Yes | 5–60 min | yes | 1.58 × 10−4 M | This work |
| Kinetics | UV-vis | Yes | 5–300 min | yes | 1.89 × 10−6 M | This work |
| Initial rate | UV-vis | yes | 1–5 min | yes | 1.2 × 1 0−4 M | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guembe-García, M.; Peredo-Guzmán, P.D.; Santaolalla-García, V.; Moradillo-Renuncio, N.; Ibeas, S.; Mendía, A.; García, F.C.; García, J.M.; Vallejos, S. Why Is the Sensory Response of Organic Probes within a Polymer Film Different in Solution and in the Solid-State? Evidence and Application to the Detection of Amino Acids in Human Chronic Wounds. Polymers 2020, 12, 1249. https://doi.org/10.3390/polym12061249
Guembe-García M, Peredo-Guzmán PD, Santaolalla-García V, Moradillo-Renuncio N, Ibeas S, Mendía A, García FC, García JM, Vallejos S. Why Is the Sensory Response of Organic Probes within a Polymer Film Different in Solution and in the Solid-State? Evidence and Application to the Detection of Amino Acids in Human Chronic Wounds. Polymers. 2020; 12(6):1249. https://doi.org/10.3390/polym12061249
Chicago/Turabian StyleGuembe-García, Marta, Patricia D. Peredo-Guzmán, Victoria Santaolalla-García, Natalia Moradillo-Renuncio, Saturnino Ibeas, Aranzazu Mendía, Félix Clemente García, José Miguel García, and Saúl Vallejos. 2020. "Why Is the Sensory Response of Organic Probes within a Polymer Film Different in Solution and in the Solid-State? Evidence and Application to the Detection of Amino Acids in Human Chronic Wounds" Polymers 12, no. 6: 1249. https://doi.org/10.3390/polym12061249
APA StyleGuembe-García, M., Peredo-Guzmán, P. D., Santaolalla-García, V., Moradillo-Renuncio, N., Ibeas, S., Mendía, A., García, F. C., García, J. M., & Vallejos, S. (2020). Why Is the Sensory Response of Organic Probes within a Polymer Film Different in Solution and in the Solid-State? Evidence and Application to the Detection of Amino Acids in Human Chronic Wounds. Polymers, 12(6), 1249. https://doi.org/10.3390/polym12061249







