Designing Imidazolium Poly(amide-amide) and Poly(amide-imide) Ionenes and Their Interactions with Mono- and Tris(imidazolium) Ionic Liquids
Abstract
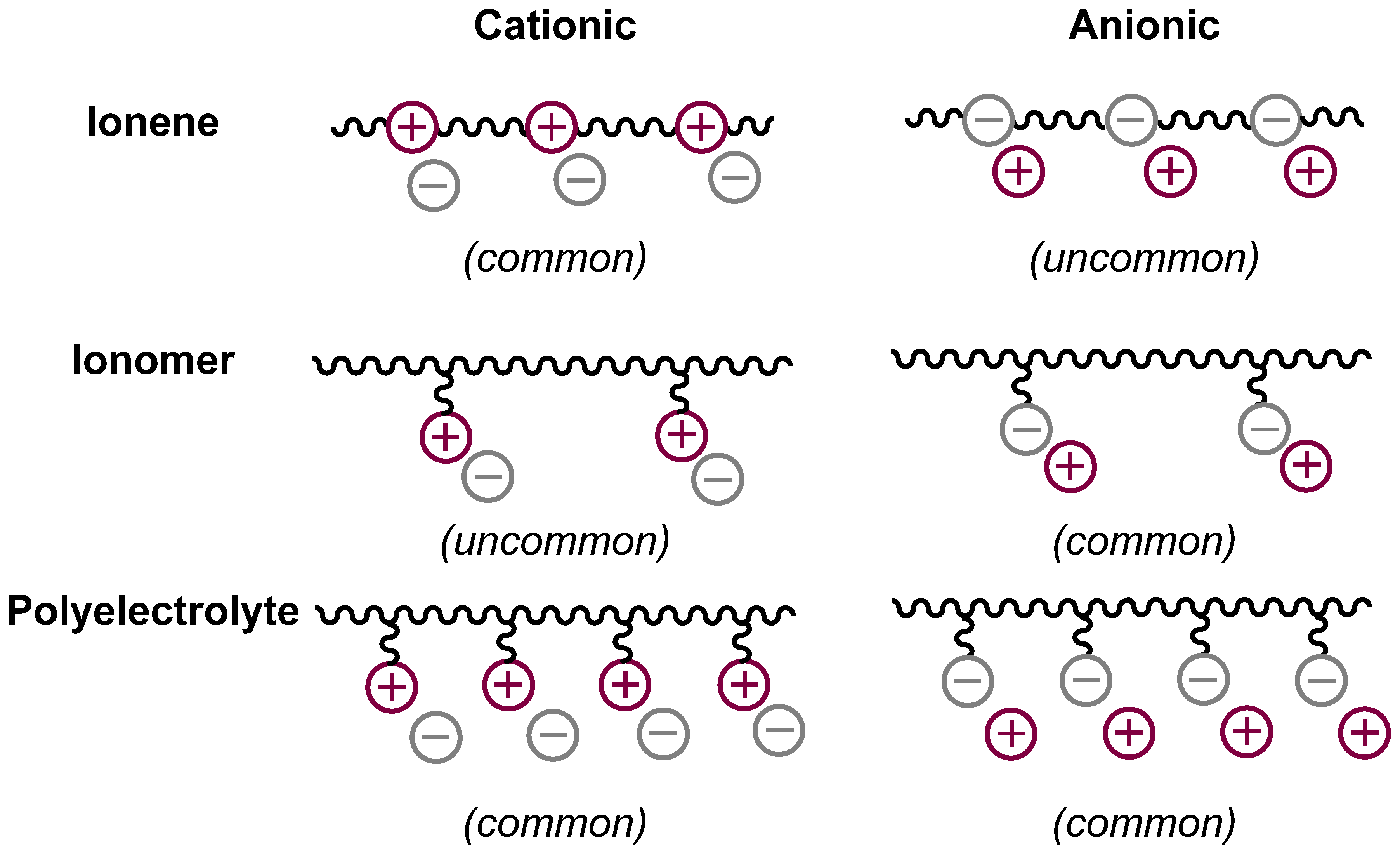
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Monomers and Small Molecules
2.3.1. Synthesis of Bis(imidazoles)
2.3.2. Synthesis of N,N’-(1,4-phenylene)bis(4-(chloromethyl)benzamide) “Diamide Dichloride Linkage”
2.3.3. Synthesis of N1,N3,N5-tris(4-(1H-imidazol-1-yl)phenyl)benzene-1,3,5-tricarboxamide and 1,1’,1’’-(((benzene-1,3,5-tricarbonyl)tris(azanediyl))tris(benzene-4,1-diyl))tris(3-methyl-1H-imidazol-3-ium) “[BTA(MeIm+)3][Tf2N‒]3”
2.4. Synthesis of Poly(amide), Poly(imide) and Poly(imide-amide) Ionenes
2.4.1. Synthesis of [TC API Amide][Tf2N]
2.4.2. Synthesis of [TC I4A Amide][Tf2N]
2.4.3. Synthesis of [6FDA API Amide][Tf2N]
2.4.4. Synthesis of [6FDA I4A Amide][Tf2N]
2.4.5. Synthesis of [PMDA API Amide][Tf2N]
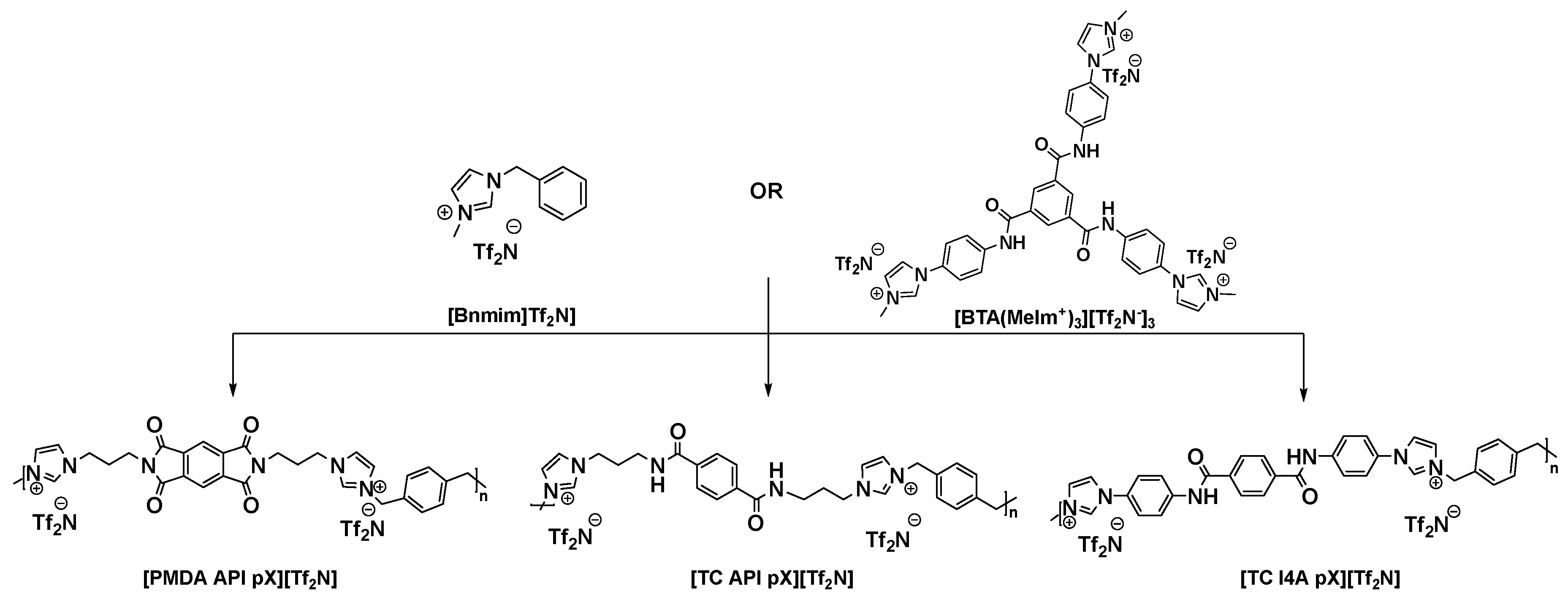
2.5. Preparation of Ionene: [Bnmim][Tf2N] and Ionene: [BTA(MeIm+)3][Tf2N‒]3 Composites
3. Results and Discussion
3.1. Molecular Weight Confirmation
3.2. Thermal Characterization
3.2.1. Thermal Behavior of Mixed PAA and PAI Ionenes
3.2.2. Thermal Behavior of Xylyl PA and PI Ionenes with [BTA(MeIm+)3][Tf2N−]3
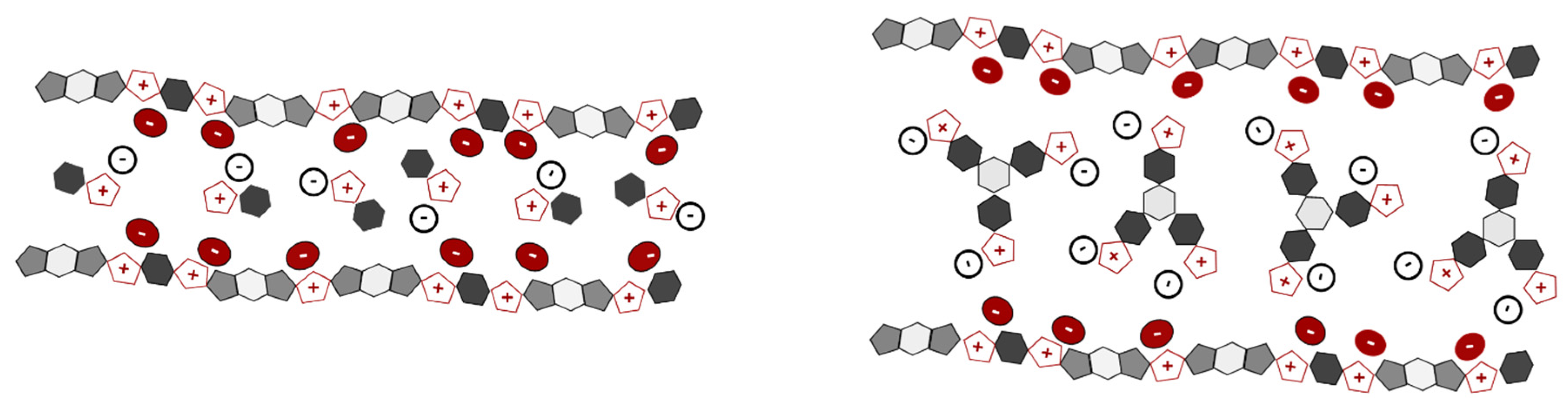
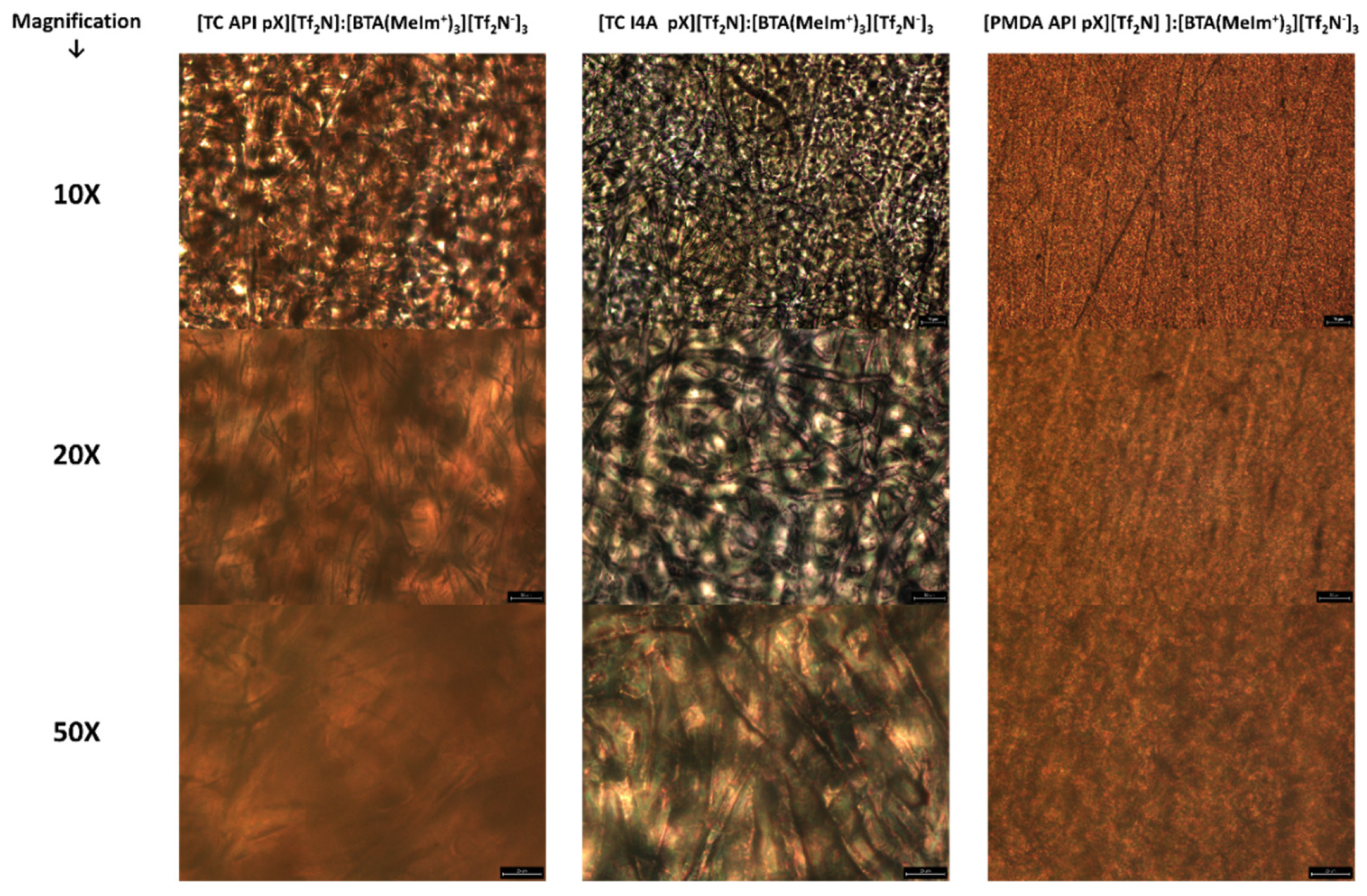
3.3. Structural Characterization
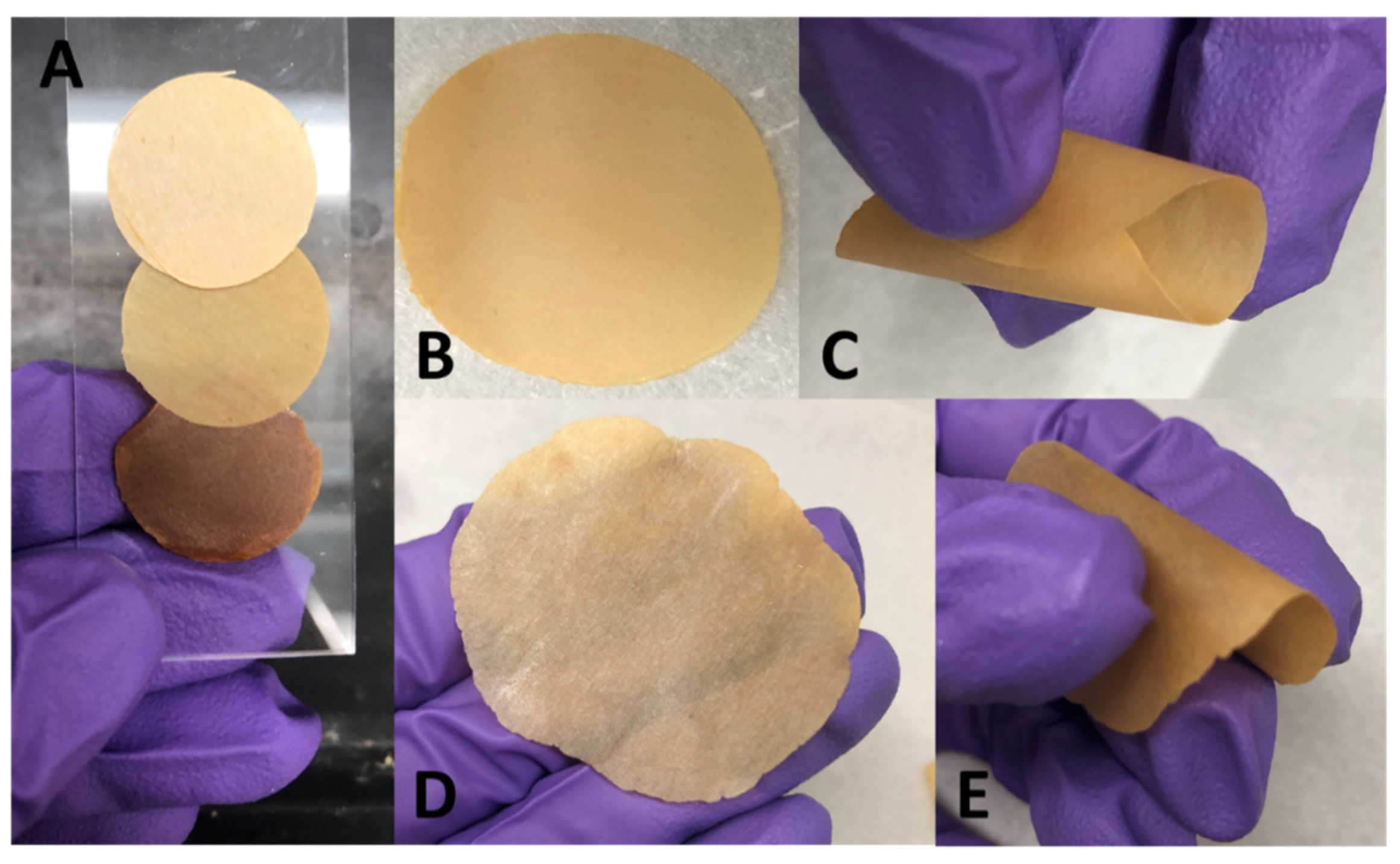
3.4. Material Behavior and Potential Applicability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly(ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Nulwala, H.; Mirjafari, A.; Zhou, X. Ionic liquids and poly(ionic liquid)s for 3D printing–A focused mini-review. Eur. Polym. J. 2018, 108, 390–398. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E. Recent Advances in the Design of Ionenes: Toward Convergence with High-Performance Polymers. Macromol. Chem. Phys. 2019. [Google Scholar] [CrossRef]
- Kammakakam, I.; Rao, A.H.N.; Yoon, H.W.; Nam, S.; Park, H.B.; Kim, T.-H. An imidazolium-based ionene blended with crosslinked PEO as a novel polymer membrane for selective CO2 separation. Macromol. Res. 2014, 22, 907–916. [Google Scholar] [CrossRef]
- O’Harra, K.E.; Kammakakam, I.; Devriese, E.M.; Noll, D.M.; Bara, J.E.; Jackson, E.M. Synthesis and Performance of 6FDA-Based Polyimide-Ionenes and Composites with Ionic Liquids as Gas Separation Membranes. Membranes 2019, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- O’Harra, K.E.; Kammakakam, I.; Noll, D.M.; Turflinger, E.M.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes. Membranes 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Mittenthal, M.S.; Flowers, B.S.; Bara, J.E.; Whitley, J.W.; Spear, S.K.; Roveda, J.D.; Wallace, D.A.; Shannon, M.S.; Holler, R.; Martens, R.; et al. Ionic Polyimides: Hybrid Polymer Architectures and Composites with Ionic Liquids for Advanced Gas Separation Membranes. Ind. Eng. Chem. Res. 2017, 56, 5055–5069. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Self-healing imidazolium-based ionene-polyamide membranes: An experimental study on physical and gas transport properties. Polym. Int. 2019, 68, 1123–1129. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Bara, J.E.; Jackson, E.M. Design and Synthesis of Imidazolium-Mediated Tröger’s Base-Containing Ionene Polymers for Advanced CO2 Separation Membranes. ACS Omega 2019, 4, 3439–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. N-Spirocyclic Quaternary Ammonium Ionenes for Anion-Exchange Membranes. J. Am. Chem. Soc. 2017, 139, 2888–2891. [Google Scholar] [CrossRef]
- Venkataraman, S.; Tan, J.P.K.; Chong, S.T.; Chu, C.Y.H.; Wilianto, E.A.; Cheng, C.X.; Yang, Y.Y. Identification of Structural Attributes Contributing to the Potency and Selectivity of Antimicrobial Polyionenes: Amides Are Better Than Esters. Biomacromolecules 2019, 20, 2737–2742. [Google Scholar] [CrossRef]
- Tan, J.P.K.; Tan, J.; Park, N.; Xu, K.; Chan, E.D.; Yang, C.; Piunova, V.A.; Ji, Z.; Lim, A.; Shao, J.; et al. Upcycling Poly(ethylene terephthalate) Refuse to Advanced Therapeutics for the Treatment of Nosocomial and Mycobacterial Infections. Macromolecules 2019, 52, 7878–7885. [Google Scholar] [CrossRef]
- Liu, S.; Ono, R.J.; Wu, H.; Teo, J.Y.; Liang, Z.C.; Xu, K.; Zhang, M.; Zhong, G.; Tan, J.P.K.; Ng, M.; et al. Highly potent antimicrobial polyionenes with rapid killing kinetics, skin biocompatibility and in vivo bactericidal activity. Biomaterials 2017, 127, 36–48. [Google Scholar] [CrossRef]
- Krumm, C.; Trump, S.; Benski, L.; Wilken, J.; Oberhaus, F.; Koller, M.; Tiller, J.C. Fast-Acting Antibacterial, Self-Deactivating Polyionene Esters. ACS Appl. Mater. Interfaces 2020, 12, 21201–21209. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Chu, H.; Lim, S.; Yim, T.; Kim, Y.-J.; Kim, T.-H. Preparation and characterization of imidazolium-PEO-based Ionene/PVDF(HFP)/LiTFSI as a novel Gel polymer electrolyte. Macromol. Res. 2014, 23, 38–44. [Google Scholar] [CrossRef]
- Strużyńska-Piron, I.; Jung, M.; Maljusch, A.; Conradi, O.; Kim, S.; Jang, J.H.; Kim, H.-J.; Kwon, Y.; Nam, S.W.; Henkensmeier, D. Imidazole based ionenes, their blends with PBI-OO and applicability as membrane in a vanadium Redox flow battery. Eur. Polym. J. 2017, 96, 383–392. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, L.; Qin, D.; Du, B.; Wang, J.; Chen, L. Single ion solid-state composite electrolytes with high electrochemical stability based on a poly(perfluoroalkylsulfonyl)-imide ionene polymer. J. Mater. Chem. A 2014, 2, 15952–15957. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Boton, L.B.; Kim, H. Triazole-based ionene exhibiting tunable structure and ionic conductivity obtained via cycloaddition reaction: A new polyelectrolyte for electrochromic devices. Sol. Energy Mater. Sol. Cells 2018, 188, 210–218. [Google Scholar] [CrossRef]
- Samanta, S.K.; Scherf, U. Cationic Main-Chain Polyelectrolytes with Pyridinium-Based p-Phenylenevinylene Units and Their Aggregation-Induced Gelation. Macromol. Chem. Phys. 2017, 218, 1600374. [Google Scholar] [CrossRef]
- Yoshida, M. Ionic gelators: Oligomeric and polymeric electrolytes as novel gel forming materials. Chem. Rec. 2010, 10, 230–242. [Google Scholar] [CrossRef]
- Bachl, J.; Bertran, O.; Mayr, J.; Alemán, C.; Díaz Díaz, D. Aromatic ionene topology and counterion-tuned gelation of acidic aqueous solutions. Soft Matter 2017, 13, 3031–3041. [Google Scholar] [CrossRef]
- Gómez-Valdemoro, A.; San-José, N.; García, F.C.; De La Peña, J.L.; Serna, F.; García, J.M. Novel aromatic polyamides with main chain and pendant 1,2,4-triazole moieties and their application to the extraction/elimination of mercury cations from aqueous media. Polym. Chem. 2010, 1, 1291–1301. [Google Scholar] [CrossRef]
- Demarteau, J.; O’Harra, K.E.; Bara, J.E.; Sardon, H. Valorization of plastic wastes for the synthesis of imidazolium based self-supported elastomeric ionenes. ChemSusChem 2020, 13. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E.; Durbin, M.M.; Dennis, G.P.; Jackson, E.M.; Thomas, B.; Odutola, J.A. Synthesis and Characterization of Ionene-Polyamide Materials as Candidates for New Gas Separation Membranes. MRS Adv. 2018, 3, 3091–3102. [Google Scholar] [CrossRef]
- Mikhailenko, V.L.; Kizhnyaev, V.N.; Verkhoturova, S.I.; Apartsin, K.A.; Gusarova, N.K.; Grigor’ev, E.G.; Trofimov, B.A. Synthesis and properties of a new family of phosphorus- and nitrogen-containing ionenes. Dokl. Chem. 2016, 465, 286–290. [Google Scholar] [CrossRef]
- Xuehui, S.; Yu-kun, Y.; Fengcai, L. Novel polyimide ionene: Synthesis and characterization of polyimides containing aromatic bipyridinium salt. Polymer 1997, 38, 4737–4741. [Google Scholar] [CrossRef]
- Williams, S.R.; Salas-de la Cruz, D.; Winey, K.I.; Long, T.E. Ionene segmented block copolymers containing imidazolium cations: Structure–property relationships as a function of hard segment content. Polymer 2010, 51, 1252–1257. [Google Scholar] [CrossRef]
- Hemp, S.T.; Zhang, M.; Tamami, M.; Long, T.E. Phosphonium ionenes from well-defined step-growth polymerization: Thermal and melt rheological properties. Polym. Chem. 2013, 4, 3582–3590. [Google Scholar] [CrossRef]
- Feng, D.; Wilkes, G.L.; Lee, B.; McGrath, J.E. Structure-property behaviour of segmented poly (tetramethylene oxide)-based bipyridinium ionene elastomers. Polymer 1992, 33, 526–535. [Google Scholar] [CrossRef]
- Kizhnyaev, V.N.; Krakhotkina, E.A.; Petrova, T.L.; Kazantseva, M.V.; Pokatilov, F.A.; Verkhozina, O.N. Synthesis and properties of azole-containing ionenes. Polym. Sci. Ser. B 2011, 53, 144–150. [Google Scholar] [CrossRef]
- Nagaya, J.; Minakata, A.; Tanioka, A. Conductance and Counterion Activity of Ionene Solutions. Langmuir 1999, 15, 4129–4134. [Google Scholar] [CrossRef]
- O’Harra, K.E.; Kammakakam, I.; Bara, J.E.; Jackson, E.M. Understanding the effects of backbone chemistry and anion type on the structure and thermal behaviors of imidazolium polyimide-ionenes. Polym. Int. 2019, 69, 1547–1556. [Google Scholar] [CrossRef]
- Malikova, N.; Čebašek, S.; Glenisson, V.; Bhowmik, D.; Carrot, G.; Vlachy, V. Aqueous solutions of ionenes: Interactions and counterion specific effects as seen by neutron scattering. Phys. Chem. Chem. Phys. 2012, 14, 12898–12904. [Google Scholar] [CrossRef]
- Williams, S.R.; Long, T.E. Recent advances in the synthesis and structure–property relationships of ammonium ionenes. Prog. Polym. Sci. 2009, 34, 762–782. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, T.K.; Bara, J.E.; Lafrate, A.L.; Gin, D.L.; Noble, R.D. Main-chain imidazolium polymer membranes for CO2 separations: An initial study of a new ionic liquid-inspired platform. J. Membr. Sci. 2010, 359, 37–43. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and Performance of Polymerizable Room-Temperature Ionic Liquids as Gas Separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Wu, F.; Huang, C.-L.; Zeng, J.-B.; Li, S.-L.; Wang, Y.-Z. Synthesis and characterization of segmented poly(butylene succinate) urethane ionenes containing secondary amine cation. Polymer 2014, 55, 4358–4368. [Google Scholar] [CrossRef]
- Venkateshwaran, L.N.; Leir, C.E.; Wilkes, G.L. Selective plasticization of the ionic domains in a segmented thermoplastic ionene cationomer. J. Appl. Polym. Sci. 1991, 43, 951–966. [Google Scholar] [CrossRef]
- Schreiner, C.; Bridge, A.T.; Hunley, M.T.; Long, T.E.; Green, M.D. Segmented imidazolium ionenes: Solution rheology, thermomechanical properties, and electrospinning. Polymer 2017, 114, 257–265. [Google Scholar] [CrossRef]
- Lee, K.M.; Wycisk, R.; Litt, M.; Pintauro, P.N. Alkaline fuel cell membranes from xylylene block ionenes. J. Membr. Sci. 2011, 383, 254–261. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709–1726. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Li, H.-P.; Cui, G.-K.; Li, Z.-Y.; Wang, J.-J. Recent Progress in Self-Assembly of Ionic Liquid Surfactants and Its Regulation and Control in Aqueous Solutions. Acta Phys. Chim. Sin. 2016, 32, 249–260. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, J. Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions. Chem. Commun. 2016, 52, 6744–6764. [Google Scholar] [CrossRef]
- Hao, J.; Zemb, T. Self-assembled structures and chemical reactions in room-temperature ionic liquids. Curr. Opin. Colloid Interface Sci. 2007, 12, 129–137. [Google Scholar] [CrossRef]
- Kimizuka, N.; Nakashima, T. Molecular Self-assembly in Ionic Liquids. Electrochem. Asp. Ion. Liq. 2011, 1, 169–182. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Schenning, A.P.H.J.; Meijer, E.W. nsight into the Mechanisms of Cooperative Self-Assembly: The “Sergeants-and-Soldiers” Principle of Chiral and Achiral C3-Symmetrical Discotic Triamides. J. Am. Chem. Soc. 2008, 130, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Cantekin, S.; de Greef, T.F.; Palmans, A.R. Benzene-1,3,5-tricarboxamide: A versatile ordering moiety for supramolecular chemistry. Chem. Soc. Rev. 2012, 41, 6125–6137. [Google Scholar] [CrossRef]
- Misawa, Y.; Koumura, N.; Matsumoto, H.; Tamaoki, N.; Yoshida, M. Hydrogels Based on Surfactant-Free Ionene Polymers with N,N′-(p-Phenylene)dibenzamide Linkages. Macromolecules 2008, 41, 8841–8846. [Google Scholar] [CrossRef]
- Tiffner, M.; Häring, M.; Díaz, D.D.; Waser, M. Cationic Polymers Bearing Quaternary Ammonium Groups-Catalyzed CO2 Fixation with Epoxides. Top. Catal. 2018, 61, 1545–1550. [Google Scholar] [CrossRef]
- Saborío, M.G.; Bertran, O.; Lanzalaco, S.; Häring, M.; Franco, L.; Puiggalí, J.; Díaz, D.D.; Estrany, F.; Alemán, C. Isomeric cationic ionenes as n-dopant agents of poly(3,4-ethylenedioxythiophene) for in situ gelation. Soft Matter 2018, 14, 6374–6385. [Google Scholar] [CrossRef] [PubMed]
- Bachl, J.; Zanuy, D.; López-Pérez, D.E.; Revilla-López, G.; Cativiela, C.; Alemán, C.; Díaz, D.D. Synergistic Computational-Experimental Approach to Improve Ionene Polymer-Based Functional Hydrogels. Adv. Funct. Mater. 2014, 24, 4893–4904. [Google Scholar] [CrossRef]
- Häring, M.; Grijalvo, S.; Haldar, D.; Saldías, C.; Díaz, D.D. Polymer topology-controlled self-healing properties of polyelectrolyte hydrogels based on DABCO-containing aromatic ionenes. Eur. Polym. J. 2019, 115, 221–224. [Google Scholar] [CrossRef]
- Kammakakam, I.; Kim, H.W.; Nam, S.; Park, H.B.; Kim, T.-H. Alkyl imidazolium-functionalized cardo-based poly(ether ketone)s as novel polymer membranes for O2/N2 and CO2/N2 separations. Polymer 2013, 54, 3534–3541. [Google Scholar] [CrossRef]
- Tamami, M.; Williams, S.R.; Park, J.K.; Moore, R.B.; Long, T.E. Poly(propylene glycol)-based ammonium ionenes as segmented ion-containing block copolymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4159–4167. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, Z.; Zheng, Y.; Gao, C. Scalable Synthesis of Positively Charged Sequence-Defined Functional Polymers. J. Am. Chem. Soc. 2019, 141, 4541–4546. [Google Scholar] [CrossRef]
- Suckow, M.; Roy, M.; Sahre, K.; Häußler, L.; Singha, N.K.; Voit, B.; Böhme, F. Synthesis of polymeric ionic liquids with unidirectional chain topology by AB step growth polymerization. Polymer 2017, 111, 123–129. [Google Scholar] [CrossRef]
- Halasa, A.F.; Wathen, G.D.; Hsu, W.L.; Matrana, B.A.; Massie, J.M. Relationship between interchain spacing of amorphous polymers and blend miscibility as determined by wide-angle X-ray scattering. J. Appl. Polym. Sci. 1991, 43, 183–190. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Zhang, P.; Sun, B.; Li, J. Effect of molecular structures on polyimide properties: Comparison between estimations and experiments. J. Appl. Polym. Sci. 2007, 103, 998–1003. [Google Scholar] [CrossRef]
- Shimazu, A.; Miyazaki, T.; Ikeda, K. Interpretation of d-spacing determined by wide angle X-ray scattering in 6FDA-based polyimide by molecular modeling. J. Membr. Sci. 2000, 166, 113–118. [Google Scholar] [CrossRef]
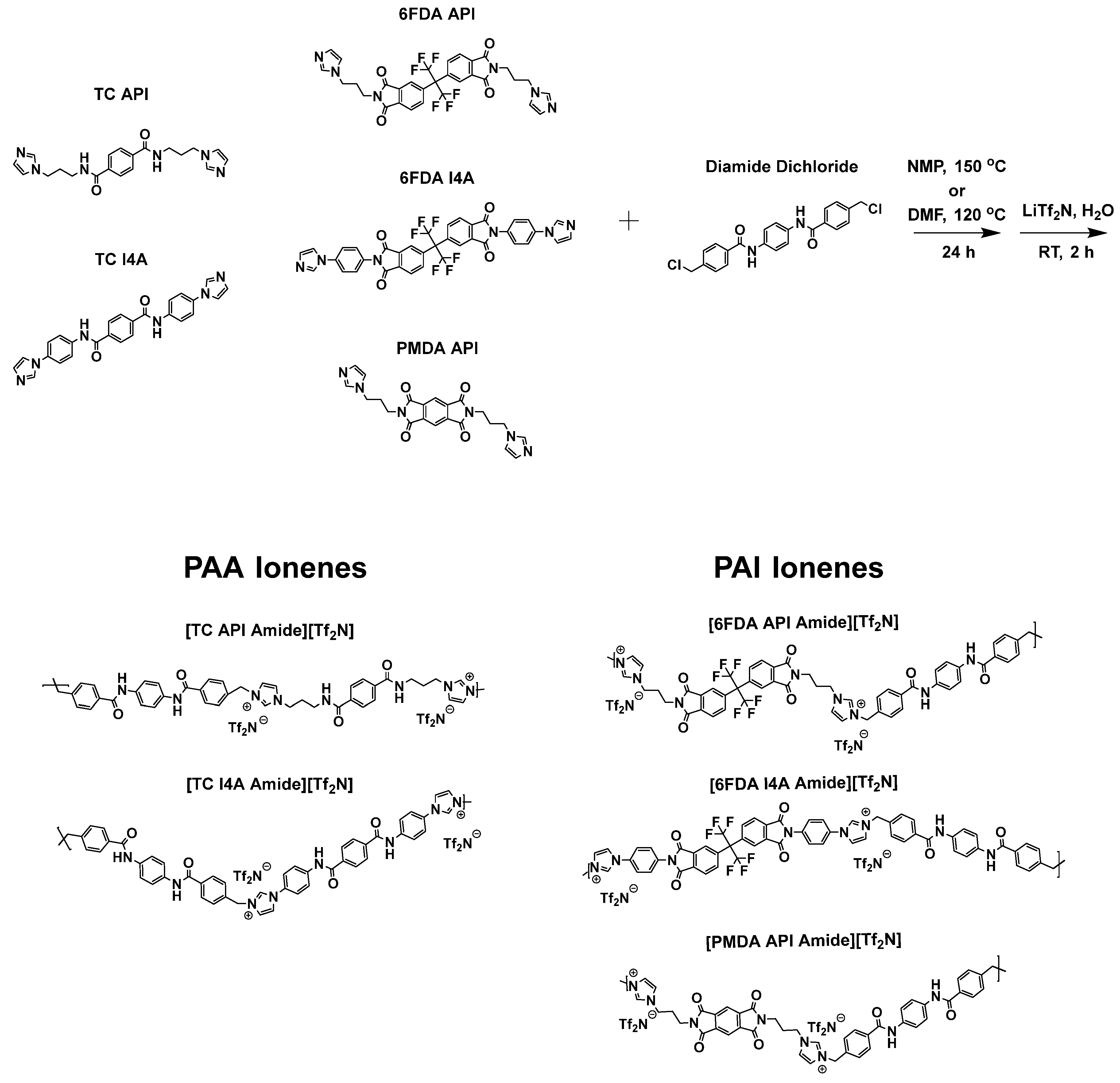

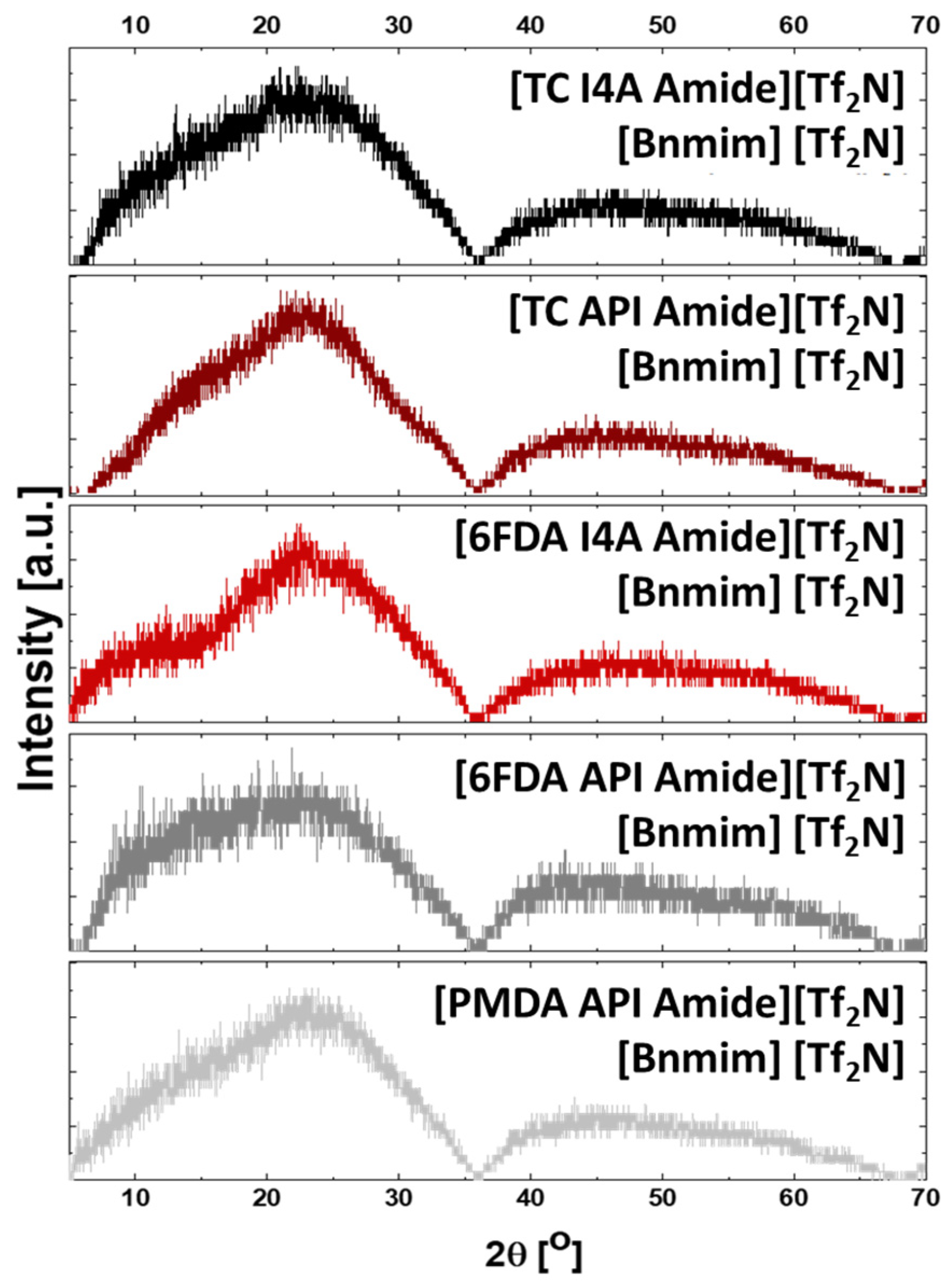


| Ionene | Tg (°C) | Td, onset (°C) | MWRU (g/mol) | d-Spacing (Å) | Density (g/cm3) | ||
|---|---|---|---|---|---|---|---|
| Neat Ionene | Ionene:IL Composite | ||||||
| [6FDA API Amide][Tf2N] | 139 | 363 | 35 | 1561.23 | 53.8 | 4.49 | 1.57 |
| [6FDA I4A Amide][Tf2N] | 160 | 392 | 34 | 1629.26 | 55.7 | 5.19 | 1.55 |
| [TC API Amide][Tf2N] | 105 | 333 | 67 | 1283.12 | 86.1 | 4.52 | 1.49 |
| [TC I4A Amide][Tf2N] | 177 | 418 | 44 | 1351.16 | 60.1 | 4.52 | 1.40 |
| [PMDA API Amide][Tf2N] | 145 | 305 | 35 | 1335.11 | 46.7 | 4.56 | 1.52 |
| Ionene: IL | Tg (°C) | Tm (°C) | d-Spacings (Å) | Density (g/cm3) | MWRU (g/mol) | MWAdd (g/mol) | Ionene:IL Mass (g) |
|---|---|---|---|---|---|---|---|
| [TC API pX][Tf2N]:Neat | 82 | 130 | 4.70 | 1.49 | 1044.88 | - | - |
| [TC I4A pX][Tf2N]:Neat | 136 | - | 4.26 | 1.52 | 1112.91 | - | - |
| [PMDA API pX][Tf2N]:Neat | 91 | 190 | 5.08 | 1.66 | 1096.86 | - | - |
| [TC API pX][Tf2N]:[Bnmim][Tf2N] | - | - | 4.69 | - | 1044.88 | 453.37 | 0.5:0.43 |
| [TC I4A pX][Tf2N]:[Bnmim][Tf2N] | - | - | 3.8/4.5/5.9/6.7 | - | 1112.91 | 453.37 | 0.5:0.41 |
| [PMDA API pX][Tf2N]:[Bnmim][Tf2N] | 70 | 144 | 5.10/5.56 | - | 1096.86 | 453.37 | 0.5:0.41 |
| [TC API pX][Tf2N]:[BTA(MeIm+)3][Tf2N−]3 | 38 | 160 | 4.45 | 1.59 | 1044.88 | 1519.18 | 0.5:0.48 |
| [TC I4A pX][Tf2N]:[BTA(MeIm+)3][Tf2N−]3 | 52 | - | 3.60/4.49 | 1.49 | 1112.91 | 1519.18 | 0.5:0.46 |
| [PMDA API pX][Tf2N]:[BTA(MeIm+)3][Tf2N−]3 | 70 | 155 | 4.58 | 1.56 | 1096.86 | 1519.18 | 0.5:0.46 |
| Neat [BTA(MeIm+)3][Tf2N−]3 | - | 66.9 | - | - | - | 1519.18 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Harra, K.E.; Noll, D.M.; Kammakakam, I.; DeVriese, E.M.; Solis, G.; Jackson, E.M.; Bara, J.E. Designing Imidazolium Poly(amide-amide) and Poly(amide-imide) Ionenes and Their Interactions with Mono- and Tris(imidazolium) Ionic Liquids. Polymers 2020, 12, 1254. https://doi.org/10.3390/polym12061254
O’Harra KE, Noll DM, Kammakakam I, DeVriese EM, Solis G, Jackson EM, Bara JE. Designing Imidazolium Poly(amide-amide) and Poly(amide-imide) Ionenes and Their Interactions with Mono- and Tris(imidazolium) Ionic Liquids. Polymers. 2020; 12(6):1254. https://doi.org/10.3390/polym12061254
Chicago/Turabian StyleO’Harra, Kathryn E., Danielle M. Noll, Irshad Kammakakam, Emily M. DeVriese, Gala Solis, Enrique M. Jackson, and Jason E. Bara. 2020. "Designing Imidazolium Poly(amide-amide) and Poly(amide-imide) Ionenes and Their Interactions with Mono- and Tris(imidazolium) Ionic Liquids" Polymers 12, no. 6: 1254. https://doi.org/10.3390/polym12061254