Degradable Poly(ethylene oxide)-Like Plasma Polymer Films Used for the Controlled Release of Nisin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Film Deposition
2.3. Characterization of Thin Films
2.4. Characterization of Permeation of Nisin
3. Results
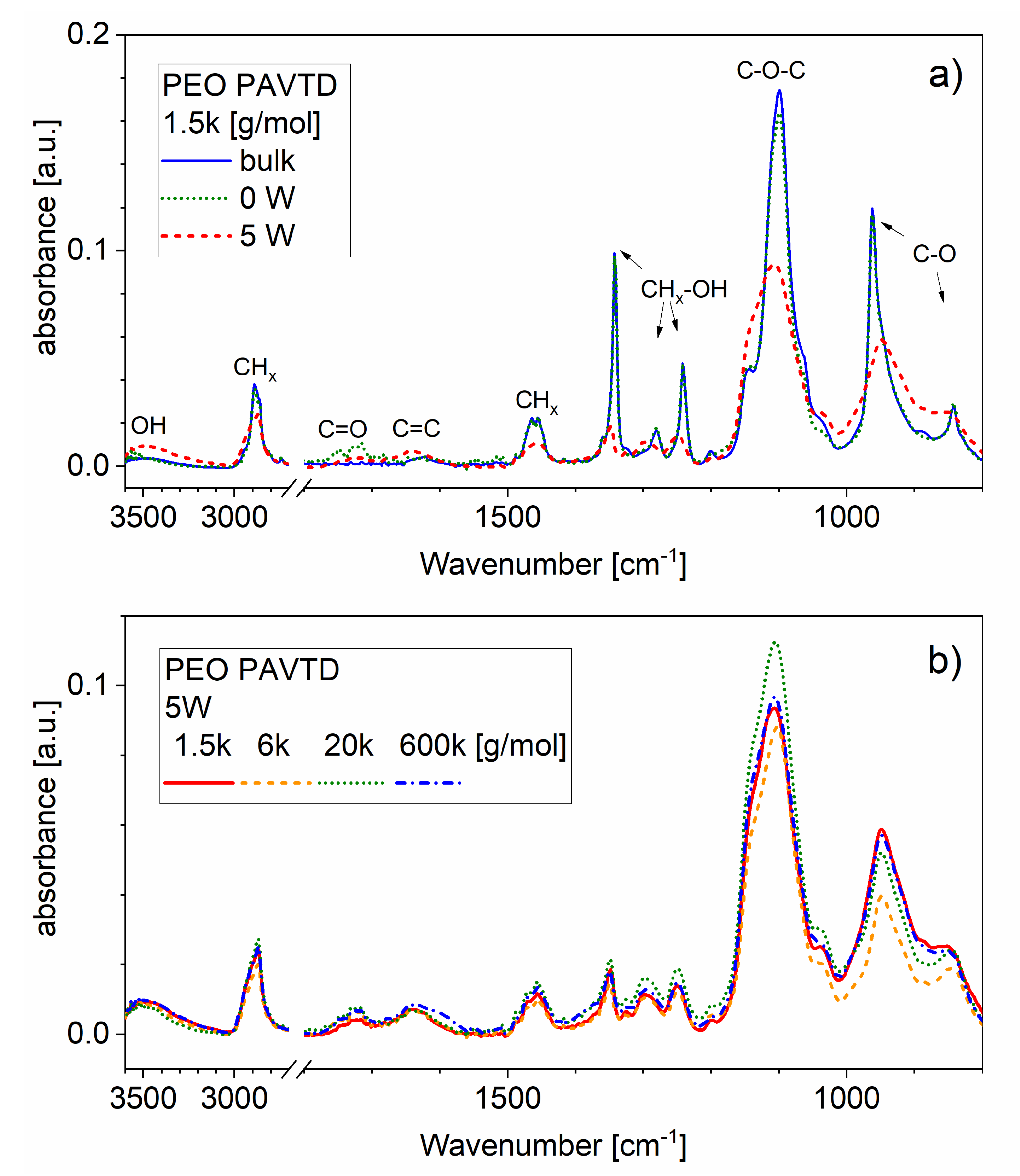
3.1. Influence of Molar Weight of Precursor Material on Properties of Thin Films
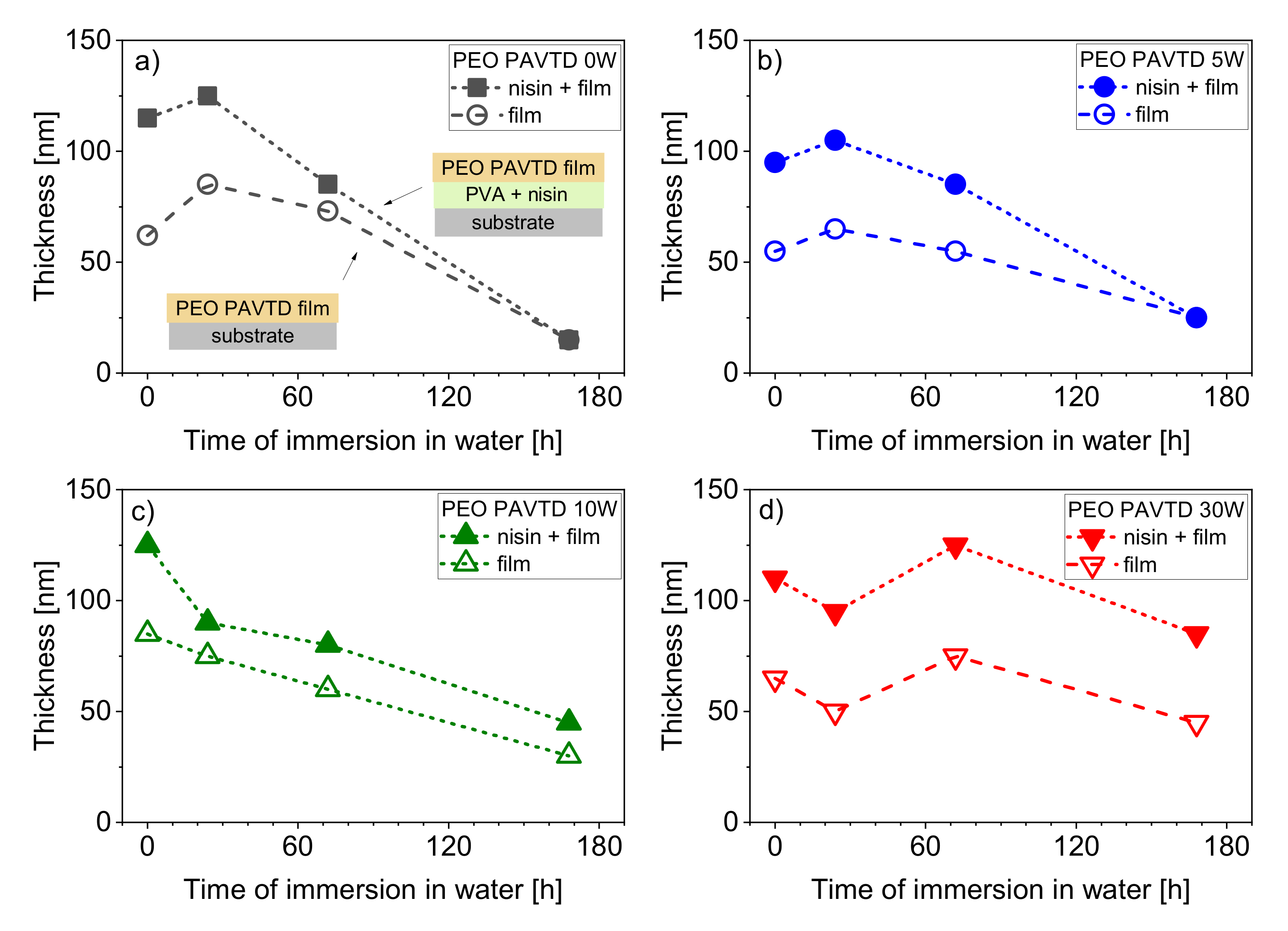
3.2. Control of Film Degradability and Nisin Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, W.; Cheng, W.; Wang, G.; Liu, Y. Developments in antimicrobial polymers. J. Polym. Sci. Pol. Chem. 2017, 55, 632–639. [Google Scholar] [CrossRef]
- Holcapkova, P.; Hurajova, A.; Bazant, P.; Pummerova, M.; Sedlarik, V. Thermal stability of bacteriocin nisin in polylactide-based films. Polym. Degrad. Stabil. 2018, 158, 31–39. [Google Scholar] [CrossRef]
- Kolarova Raskova, Z.; Stahel, P.; Sedlarikova, J.; Musilova, L.; Stupavska, M.; Lehocky, M. The effect of plasma pretreatment and cross-linking degree on the physical and antimicrobial properties of nisin-coated PVA films. Materials 2018, 11, 1451. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Tan, K.L.; Kang, E.T. Surface modification of poly(tetrafluoroethylene) films via grafting of poly(ethylene glycol) for reduction in protein adsorption. J. Biomater. Sci. Polym. Ed. 2000, 11, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Wei, O.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Knoll, W.; Förch, R. Pulsed plasma polymerized di(ethylene glycol) monovinyl ether coating for nonfouling surfaces. Chem. Mater. 2006, 18, 4840–4844. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Zheng, J.; Ratner, B.D.; Jiang, S. Protein adsorption on oligo(ethylene glycol)-terminated alkanethiolate self-assembled monolayers: The molecular basis for nonfouling behaviour. J. Phys. Chem. B 2005, 109, 2934–2941. [Google Scholar] [CrossRef]
- Zanini, S.; Grimoldi, E.; Riccardi, C. Development of controlled releasing surfaces by plasma deposited multilayers. Mater. Chem. Phys. 2013, 138, 850–855. [Google Scholar] [CrossRef]
- Stloukal, P.; Novak, I.; Micusik, M.; Prochazka, M.; Kucharczyk, P.; Chodak, I.; Lehocky, M.; Sedlarik, V. Effect of plasma treatment on the release kinetics of a chemotherapy drug from biodegradable polyester films and polyester urethane films. Int. J. Polym. Mater. 2017, 67, 161–173. [Google Scholar] [CrossRef]
- Vasilev, K.; Ramiasa-MacGregor, M. Nanoengineered plasma polymer films for biomedical applications. Adv. Mater. Lett. 2018, 9, 42–52. [Google Scholar] [CrossRef]
- Sardella, E.; Palumbo, F.; Camporeale, G.; Favia, P. Non-equilibrium plasma processing for the preparation of antibacterial surfaces. Materials 2016, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of plasma-enhanced chemical vapour deposition as a method for thin-film fabrication with biological applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Förch, R.; Chifen, A.N.; Bousquet, A.; Khor, H.L.; Jungblut, M.; Chu, L.-Q.; Zhang, Z.; Osey-Mensah, I.; Sinner, E.-K.; Knoll, W. Recent and expected roles of plasma-polymerized films for biomedical applications. Chem. Vap. Depos. 2007, 13, 280–294. [Google Scholar] [CrossRef]
- Gordeev, I.; Choukourov, A.; Šimek, M.; Prukner, V.; Biederman, H. PEO-like Plasma Polymers Prepared by Atmospheric Pressure Surface Dielectric Barrier Discharge. Plasma Process. Polym. 2012, 9, 782–791. [Google Scholar] [CrossRef]
- Stallard, C.H.P.; Solar, P.; Biederman, H.; Dowling, D.P. Deposition of non-fouling PEO-like coatings using a low temperature atmospheric pressure plasma jet. Plasma Process. Polym. 2016, 13, 241–252. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Li, X.; Tu, Q.; Sun, H.; Huang, N. Interaction of platelets, fibrinogen and endothelial cells with plasma deposited PEO-like films. Appl. Surf. Sci. 2012, 258, 3378–3385. [Google Scholar] [CrossRef]
- Cutter, C.N.; Willett, J.L.; Siragusa, G.R. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Choukourov, A.; Hanus, J.; Kousal, J.; Grinevich, A.; Pihosh, Y.; Slavinska, D.; Biederman, H. Thin polymer films from polyimide vacuum thermal degradation with and without a glow discharge. Vacuum 2006, 80, 923–929. [Google Scholar] [CrossRef]
- Choukourov, A.; Gordeev, I.; Ponti, J.; Uboldi, C.; Melnichuk, I.; Vaidulych, M.; Kousal, J.; Nikitin, D.; Hanykova, L.; Krakovsky, I.; et al. Microphase-separated PE/PEO thin films prepared by plasma-assisted vapour phase deposition. ACS Appl. Mater. Interfaces 2016, 8, 8201–8212. [Google Scholar] [CrossRef]
- Kousal, J.; Krtous, Z.; Kolarova Raskova, Z.; Sedlarikova, J.; Schäfer, J.; Kucerova, L.; Shelemin, A.; Solar, P.; Hurajova, A.; Biederman, H.; et al. Degradable plasma polymer films with tailored hydrolysis behaviour. Vacuum 2019. accepted. [Google Scholar] [CrossRef]
- Yakut, S.; Ulutas, H.K.; Melnichuk, I.; Choukourov, A.; Biederman, H.; Deger, D. Dielectric properties of plasma polymerized poly(ethylene oxide) thin films. Thin Solid Films 2016, 616, 279–286. [Google Scholar] [CrossRef]
- Choukourov, A.; Polonskyi, O.; Hanus, J.; Kousal, J.; Grinevich, A.; Slavinska, D.; Biederman, H. PEO-like coatings prepared by plasma-based techniques. Plasma Process. Polym. 2009, 6, S21–S24. [Google Scholar] [CrossRef]
- Choukourov, A.; Gordeev, I.; Polonskyi, O.; Artemenko, A.; Hanykova, L.; Krakovsky, I.; Kylian, O.; Slavinska, D.; Biederman, H. Poly(ethylene oxide)-like Plasma Polymers Produced by Plasma-Assisted Vacuum Evaporation. Plasma Process. Polym. 2010, 7, 445–458. [Google Scholar] [CrossRef]
- Choukourov, A.; Gordeev, I.; Arzhakov, D.; Artemenko, A.; Kousal, J.; Kylian, O.; Slavinska, D.; Biederman, H. Does cross-link density of PEO-like plasma polymer influence their resistance to adsorption of fibrinogen? Plasma Process. Polym. 2012, 9, 48–58. [Google Scholar] [CrossRef]
- Kim, H.; Fassihi, R. Application of binary polymer in drug release rate modulation. 2. Influence of formulation variables and hydrodynamic conditions on release kinetics. J. Pharm. Sci. 1997, 86, 323–328. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier Ltd.: Cambridge, UK, 2015. [Google Scholar]
- Lopez-Garcia, J.; Cupessala, F.; Humpolicek, P.; Lehocky, M. Physical and morphological changes of poly(tetrafluoroethylene) after using non-thermal plasma-treatments. Materials 2018, 11, 2013. [Google Scholar] [CrossRef] [Green Version]
- Masruroh, T.N.; Zahirah, N.T.; Sakti, S.P.; Santjojo, D.J.D.; Masruroh, T.N. The effect of molecular weight on the surface wettability of polystyrene treated with nitrogen plasma. In Proceedings of the IOP Conference Series: Materials Science and Engineering, The 1st Materials Research Society Indonesia Conference and Congress, Yogyakarta, Indonesia, 8–12 October 2017; Volume 432. [Google Scholar] [CrossRef]
- Kolska, Z.; Kasalkova Slepickova, N.; Siegel, J.; Svorcik, V. Electrokinetic Potential for Characterization of Nanosctructured Solid Flat Surfaces. J. Nano Res. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H. Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag-In vitro assay. Radiat. Phys. Chem. 2007, 76, 1011–1016. [Google Scholar] [CrossRef]
- Pleskunov, P.; Nikitin, D.; Tafiichuk, R.; Khalakhan, I.; Kolská, Z.; Choukourov, A. Nanophase-separated poly(acrylic acid)/poly(ethylene oxide) plasma polymers for the spatially localized attachment of biomolecules. Plasma Process. Polym. 2019. [Google Scholar] [CrossRef]
- Xiang, C.H.; Taylor, A.G.; Frey, M.W. Controlled release of non-ionic compounds from poly(lactic acid)/cellulose nanocrystal nanocomposite fibers. J. Appl. Polym. Sci. 2013, 127, 79–86. [Google Scholar] [CrossRef]
- Hrabalikova, M.; Holcapkova, P.; Suly, P.; Sedlarik, V. Immobilization of bacteriocin nisin into a poly(vinyl alcohol) polymer matrix crosslinked with nontoxic dicarboxylic acid. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef] [Green Version]



| Molar Weight (Mw, g/mol) | Polydispersity (PDI) | ||||
|---|---|---|---|---|---|
| Bulk Polymer | PAVTD Film | ||||
| 0 W | 5 W | bulk | 0 W | 5 W | |
| 1.5k | 5500 | 6500 | 1.26 | 1.5 | 2.6 |
| 6k | 6000 | 15000 | 1.56 | 1.7 | 3.2 |
| 20k | 12000 | 60000 | 1.31 | 2.4 | 4.6 |
| 600k | 2300 | 60000 | 1.07 | 1.8 | 3.8 |
| PEO PAVTD | AFM RMS Roughness [nm] | |
|---|---|---|
| Precursor | 0 W | 5 W |
| 1.5k | 2.9 ± 0.2 | 1.5 ± 0.1 |
| 6k | 6.7 ± 3.8 | 6.1 ± 1.9 |
| 20k | 8.7 ± 1.3 | 8.7 ± 1.6 |
| 600k | 9.8 ± 0.4 | 3.6 ± 1.0 |
| PEO PAVTD | K [h−1/2] | b | R2 |
|---|---|---|---|
| 0 W | 0.27 ± 0.07 | 0.17 ± 0.13 | 0.977 |
| 5 W | 0.33 ± 0.07 | −0.20 ± 0.13 | 0.984 |
| 10 W | 0.22 ± 0.03 | −0.11 ± 0.10 | 0.918 |
| 30 W | 0.09 ± 0.02 | −0.03 ± 0.07 | 0.902 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kousal, J.; Sedlaříková, J.; Kolářová-Rašková, Z.; Krtouš, Z.; Kučerová, L.; Hurajová, A.; Vaidulych, M.; Hanuš, J.; Lehocký, M. Degradable Poly(ethylene oxide)-Like Plasma Polymer Films Used for the Controlled Release of Nisin. Polymers 2020, 12, 1263. https://doi.org/10.3390/polym12061263
Kousal J, Sedlaříková J, Kolářová-Rašková Z, Krtouš Z, Kučerová L, Hurajová A, Vaidulych M, Hanuš J, Lehocký M. Degradable Poly(ethylene oxide)-Like Plasma Polymer Films Used for the Controlled Release of Nisin. Polymers. 2020; 12(6):1263. https://doi.org/10.3390/polym12061263
Chicago/Turabian StyleKousal, Jaroslav, Jana Sedlaříková, Zuzana Kolářová-Rašková, Zdeněk Krtouš, Liliana Kučerová, Anna Hurajová, Mykhailo Vaidulych, Jan Hanuš, and Marián Lehocký. 2020. "Degradable Poly(ethylene oxide)-Like Plasma Polymer Films Used for the Controlled Release of Nisin" Polymers 12, no. 6: 1263. https://doi.org/10.3390/polym12061263





