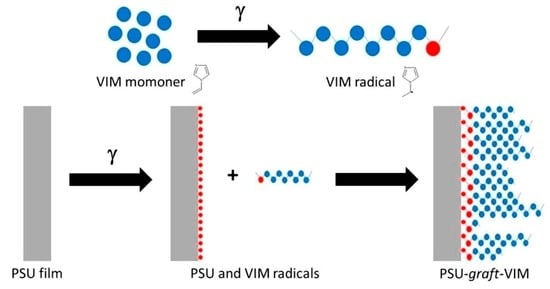
Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grafted Membranes Preparation
2.3. Characterization of PSU Grafted Membranes
3. Results and Discussion
3.1. Grafting Yield of VIM onto PSU Membranes
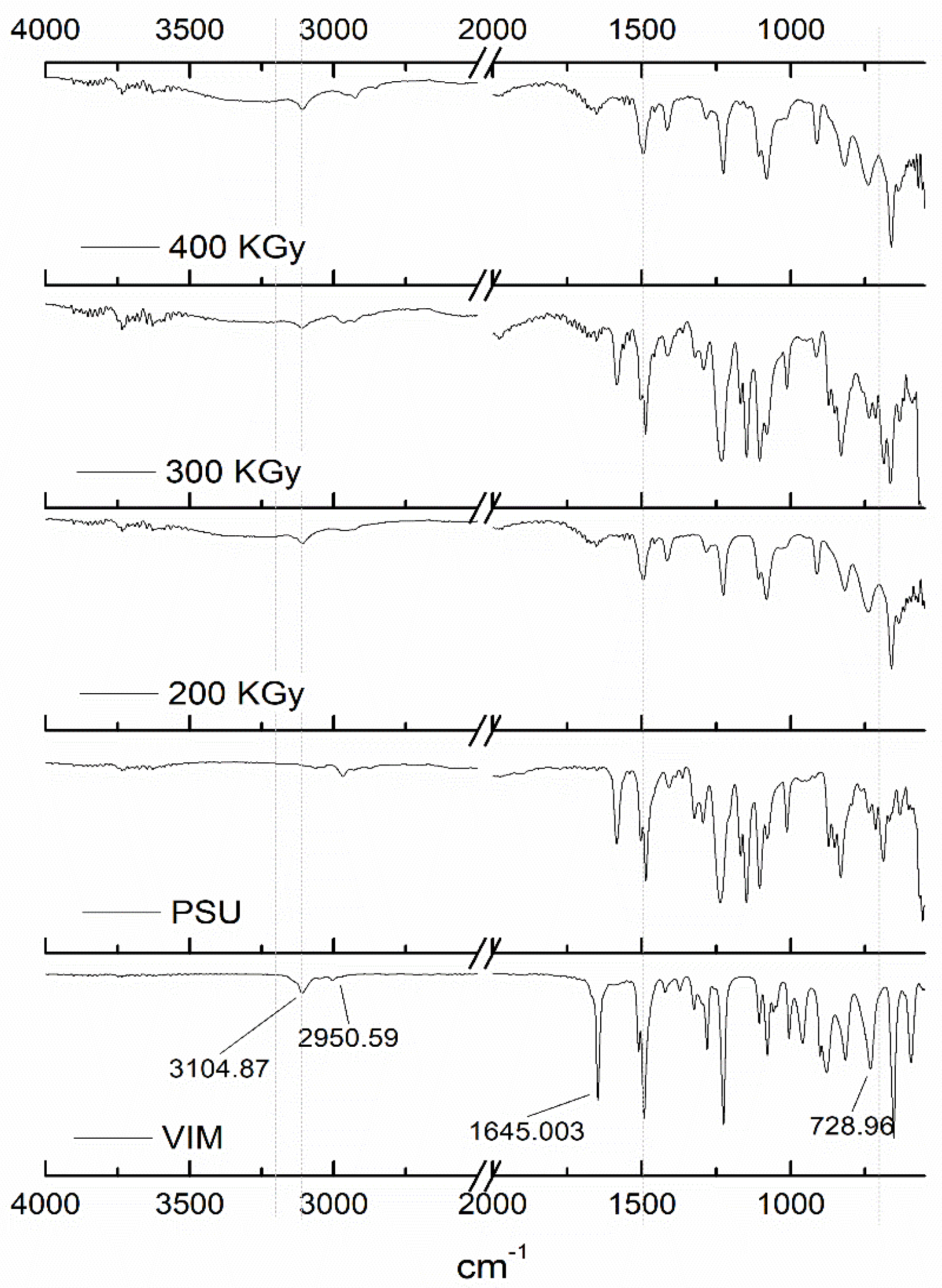
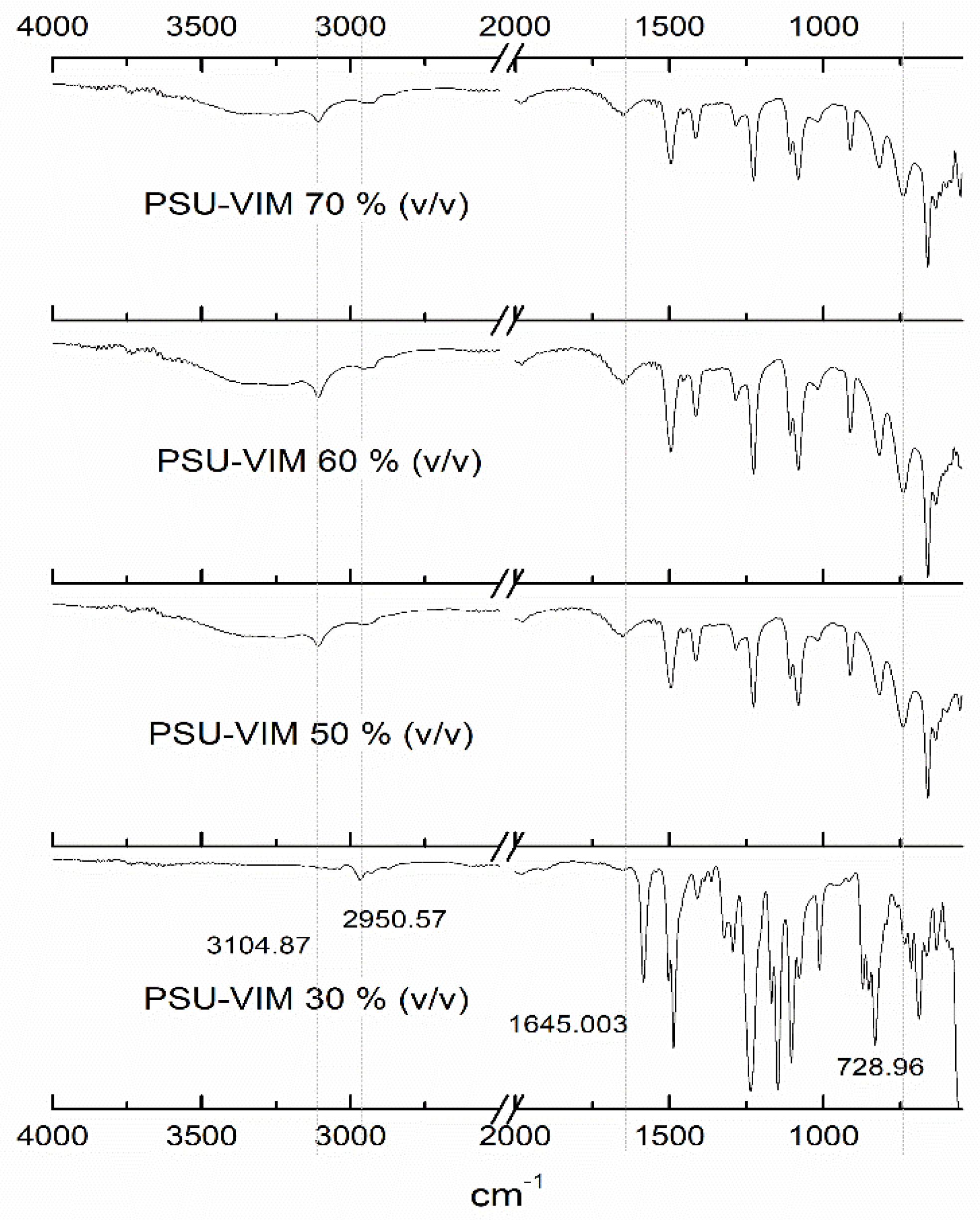
3.2. Chemical Structure of the Grafted PSU Membranes
3.3. Surface Morphology of the Grafted PSU Membranes
3.4. Hydrophilicity Properties of the Grafted PSU Membranes
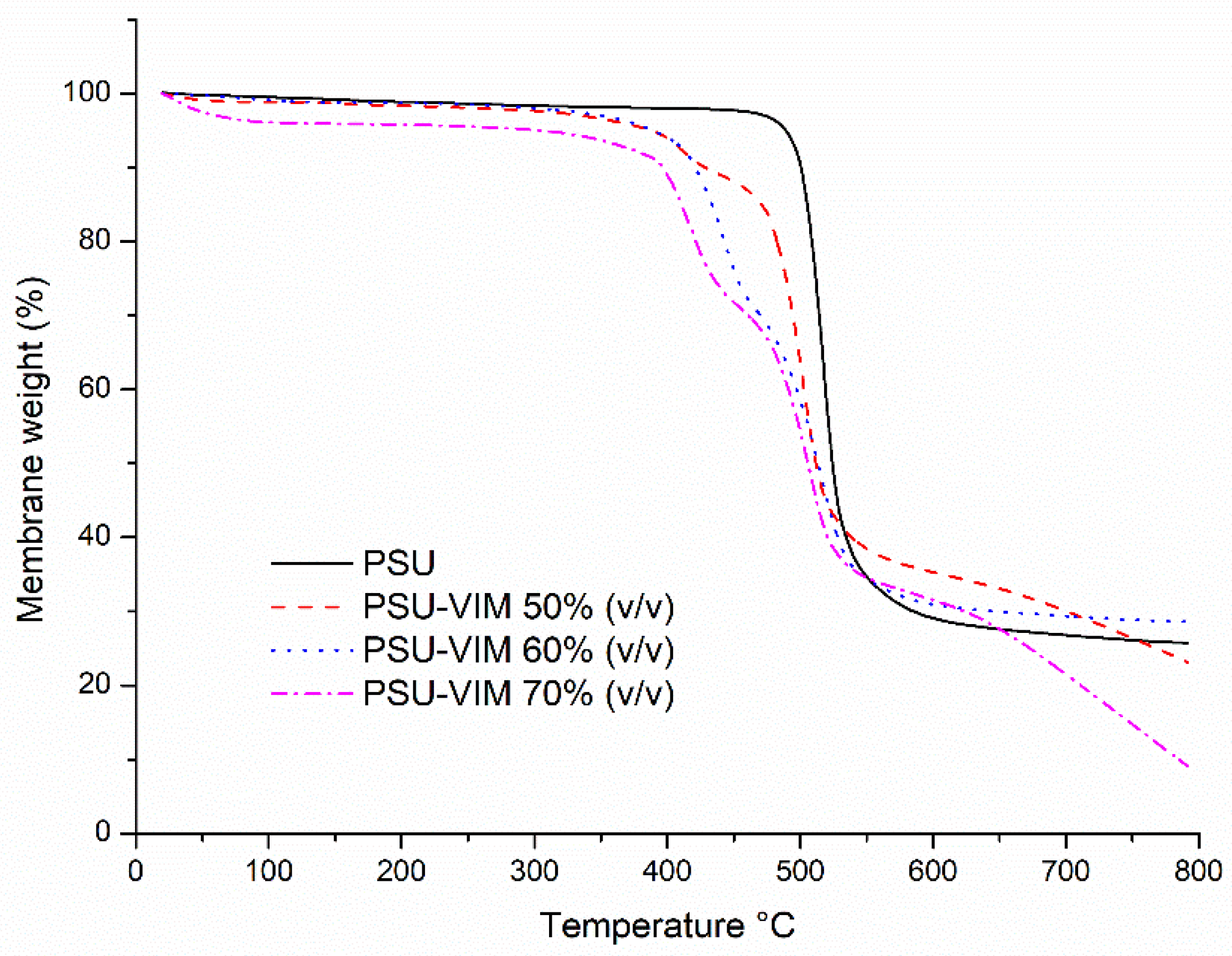
3.5. Thermal Behavior of the Grafted PSU Membranes
3.6. Desalination Test for the Grafted PSU Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Restrepo-Flórez, J.-M.; Maldovan, M. Breaking Separation Limits in Membrane Technology. J. Membr. Sci. 2018, 566, 301–306. [Google Scholar] [CrossRef]
- Hu, F.; Fang, C.; Wang, Z.; Liu, C.; Zhu, B.; Zhu, L. Poly (N-Vinyl Imidazole) Gel Composite Porous Membranes for Rapid Separation of Dyes through Permeating Adsorption. Sep. Purif. Technol. 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Tiron, L.G.; Vlad, M.; Baltă, Ş. Research on Hydrophilic Nature of Polyvinylpyrrolidone on Polysulfone Membrane Filtration. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012059. [Google Scholar] [CrossRef]
- Guo, J.; Khan, S.; Cho, S.-H.; Kim, J. ZnS Nanoparticles as New Additive for Polyethersulfone Membrane in Humic Acid Filtration. J. Ind. Eng. Chem. 2019, 79, 71–78. [Google Scholar] [CrossRef]
- Soleymani Lashkenrai, A.; Najafi, M.; Peyravi, M.; Jahanshahi, M.; Mosavian, M.T.H.; Amiri, A.; Shahavi, M.H. Direct Filtration Procedure to Attain Antibacterial TFC Membrane: A Facile Developing Route of Membrane Surface Properties and Fouling Resistance. Chem. Eng. Res. Des. 2019, 149, 158–168. [Google Scholar] [CrossRef]
- Kazemi, A.S.; Patterson, B.; LaRue, R.J.; Papangelakis, P.; Yoo, S.M.; Ghosh, R.; Latulippe, D.R. Microscale Parallel-Structured, Cross-Flow Filtration System for Evaluation and Optimization of the Filtration Performance of Hollow-Fiber Membranes. Sep. Purif. Technol. 2019, 215, 299–307. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for Next-Generation Desalination and Water Purification Membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Lu, Y.; Meng, H.; Li, C.; Chen, B.; Lei, Z. Adsorptivity of a Hyper Cross-Linked Ionic Polymer Poly(Vinyl Imidazole)-1,4-Bis(Chloromethyl)Benzene for Thiophenic Sulfurs in Model Oil. Energy Fuels 2016, 30, 5035–5041. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Hassan, M.S.; Zohdy, M.H. Adsorption Kinetics of Toxic Heavy Metal Ions from Aqueous Solutions onto Grafted Jute Fibers with Acrylic Acid by Gamma Irradiation. J. Nat. Fibers 2018, 15, 506–516. [Google Scholar] [CrossRef]
- Xu, F.; Wei, M.; Zhang, X.; Song, Y.; Zhou, W.; Wang, Y. How Pore Hydrophilicity Influences Water Permeability? Research 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Zaidi, S.M.J.; Mauritz, K.A.; Hassan, M.K. Membrane Surface Modification and Functionalization. In Functional Polymers; Polymers and Polymeric Composites: A Reference, Series; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 391–416. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification Methods for Poly(Arylsulfone) Membranes: A Mini-Review Focusing on Surface Modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for Next-Generation Molecularly Selective Synthetic Membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Akamatsu, K.; Noto, W.; Fukuzawa, H.; Hara, A.; Nakao, S. Grafting of Carboxybetaine Polymers to Polyethylene Membranes via Plasma Graft Polymerization to Improve Low-Fouling Properties and to Tune the Molecular Weight Cut-Off. Sep. Purif. Technol. 2018, 204, 298–303. [Google Scholar] [CrossRef]
- Yu, H.; Shao, P.; Fang, L.; Pei, J.; Ding, L.; Pavlostathis, S.G.; Luo, X. Palladium Ion-Imprinted Polymers with PHEMA Polymer Brushes: Role of Grafting Polymerization Degree in Anti-Interference. Chem. Eng. J. 2019, 359, 176–185. [Google Scholar] [CrossRef]
- Zych, A.; Verdelli, A.; Soliman, M.; Pinalli, R.; Pedrini, A.; Vachon, J.; Dalcanale, E. Strain-Reporting Pyrene-Grafted Polyethylene. Eur. Polym. J. 2019, 111, 69–73. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Bai, H.; Tan, Q.; Wang, H.; He, B.; Xiang, Y.; Lu, S. A New High Temperature Polymer Electrolyte Membrane Based on Tri-Functional Group Grafted Polysulfone for Fuel Cell Application. J. Membr. Sci. 2019, 572, 496–503. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Li, R.; Yu, H.; Hong, H.; Lin, H.; Chen, J.; Liao, B.-Q. Physicochemical Correlations between Membrane Surface Hydrophilicity and Adhesive Fouling in Membrane Bioreactors. J. Colloid Interface Sci. 2017, 505, 900–909. [Google Scholar] [CrossRef]
- Eduok, U.; Ohaeri, E.; Szpunar, J. Electrochemical and Surface Analyses of X70 Steel Corrosion in Simulated Acid Pickling Medium: Effect of Poly (N-Vinyl Imidazole) Grafted Carboxymethyl Chitosan Additive. Electrochim. Acta 2018, 278, 302–312. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Z.; Li, X.; Zhao, H.; Hua, M.; Zhang, Y. Synthesis and Performance of a Novel Nanofiltration Membrane with a Crosslinked Sulfonated Polysulfone Separation Layer. Desalin. Water Treat. 2016, 57, 25960–25971. [Google Scholar] [CrossRef]
- Koga, Y.; Meguro, H.; Fujieda, H.; Ueno, Y.; Miwa, K.; Kainoh, M. A New Hydrophilic Polysulfone Hemodialysis Membrane Can Prevent Platelet–Neutrophil Interactions and Successive Neutrophil Activation. Int. J. Artif. Organs 2019, 42, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mi, Z.; Chen, C.; Zhou, H.; Zhao, X.; Wang, D. Preparation of Hydrophilic and Antifouling Polysulfone Ultrafiltration Membrane Derived from Phenolphthalin by Copolymerization Method. Appl. Surf. Sci. 2017, 401, 69–78. [Google Scholar] [CrossRef]
- Hezarkhani, M.; Yilmaz, E. Pullulan Modification via Poly(N-Vinylimidazole) Grafting. Int. J. Boil. Macromol. 2019, 123, 149–156. [Google Scholar] [CrossRef]
- Islam, N.; Khan, M.N.; Mallik, A.K.; Rahman, M.M. Preparation of Bio-Inspired Trimethoxysilyl Group Terminated Poly(1-Vinylimidazole)-Modified-Chitosan Composite for Adsorption of Chromium (VI) Ions. J. Hazard. Mater. 2019, 379, 120792. [Google Scholar] [CrossRef]
- Zou, S.; Smith, E.D.; Lin, S.; Martin, S.M.; He, Z. Mitigation of Bidirectional Solute Flux in Forward Osmosis via Membrane Surface Coating of Zwitterion Functionalized Carbon Nanotubes. Environ. Int. 2019, 131, 104970. [Google Scholar] [CrossRef]
- Magaña, H.; Palomino, K.; Cornejo-Bravo, J.M.; Alvarez- Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-Grafting of Acrylamide onto Silicone Rubber Films for Diclofenac Delivery. Radiat. Phys. Chem. 2015, 107, 164–170. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Concheiro, A.; Jiménez-Páez, V.M.; Bucio, E. Modification of Medical Grade PVC with N-Vinylimidazole to Obtain Bactericidal Surface. Radiat. Phys. Chem. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Ladewig, B.; Al-Shaeli, M.N.Z. Fundamentals of Membrane Bioreactors; Springer Transactions in Civil and Environmental Engineering; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Saito, K.; Fujiwara, K.; Sugo, T. Fundamentals of Radiation-Induced Graft Polymerization in Innovative Polymeric Adsorbents; Springer: Singapore, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Zhuang, S.; Yin, Y.; Wang, J. Removal of Cobalt Ions from Aqueous Solution Using Chitosan Grafted with Maleic Acid by Gamma Radiation. Nucl. Eng. Technol. 2018, 50, 211–215. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Wang, Y.; Peng, J.; Zhao, L.; Du, J.; Zhai, M. Amphoteric Ion Exchange Membranes Prepared by Preirradiation-Induced Emulsion Graft Copolymerization for Vanadium Redox Flow Battery. Polymers 2019, 11, 1482. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent Advancements in Applications of Alkaline Anion Exchange Membranes for Polymer Electrolyte Fuel Cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Grasselli, M.; Marchese, J.; Ochoa, N.A. Preparation, Structural and Functional Characterization of Modified Porous PVDF Membranes by γ-Irradiation. J. Membr. Sci 2012, 389, 91–98. [Google Scholar] [CrossRef]
- Guo, D.; Zhuo, Y.Z.; Lai, A.N.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Interpenetrating Anion Exchange Membranes Using Poly(1-Vinylimidazole) as Bifunctional Crosslinker for Fuel Cells. J. Membr. Sci. 2016, 518, 295–304. [Google Scholar] [CrossRef]
- Wu, H.; Jia, W.; Liu, Y. An Imidazolium-Type Hybrid Alkaline Anion Exchange Membrane with Improved Membrane Stability for Alkaline Fuel Cells Applications. J. Mater. Sci. 2017, 52, 1704–1716. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Xu, Z.-K.; Wang, J.-Q.; Wu, J.; Fu, J.-J. Surface Modification of Polypropylene Microfiltration Membranes by Graft Polymerization of N-Vinyl-2-Pyrrolidone. Eur. Polym. J. 2004, 40, 2077–2087. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, L.-P.; Zhang, P.-B.; Sun, J.; Zhu, B.-K.; Xu, Y.-Y. Molecular Separation by Poly (N-Vinyl Imidazole) Gel-Filled Membranes. J. Mater. Sci. 2016, 497, 472–484. [Google Scholar] [CrossRef]
- Davari, S.; Omidkhah, M.; Abdollahi, M. Improved Antifouling Ability of Thin Film Composite Polyamide Membrane Modified by a PH-Sensitive Imidazole-Based Zwitterionic Polyelectrolyte. J. Mater. Sci. 2018, 564, 788–799. [Google Scholar] [CrossRef]
- Costoya, A.; Velázquez Becerra, L.E.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Mayer, C.; Otero, A.; Concheiro, A.; Bucio, E.; Alvarez-Lorenzo, C. Immobilization of Antimicrobial and Anti-Quorum Sensing Enzymes onto GMA-Grafted Poly(Vinyl Chloride) Catheters. Int. J. Pharm. 2019, 558, 72–81. [Google Scholar] [CrossRef]
- Caner, H.; Yilmaz, E.; Yilmaz, O. Synthesis, Characterization and Antibacterial Activity of Poly(N-Vinylimidazole) Grafted Chitosan. Carbohydr. Polym. 2007, 69, 318–325. [Google Scholar] [CrossRef]
- González-Hernández, G.; Pino-Ramos, V.H.; Islas, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-Grafting of N-Vinylcaprolactam and 2-Hydroxyethyl Methacrylate onto Polypropylene Films to Obtain a Thermo-Responsive Drug Delivery System. Radiat. Phys. Chem. 2019, 157, 6–14. [Google Scholar] [CrossRef]
- Kutcherlapati, S.R.; Koyilapu, R.; Jana, T. Poly(N -Vinyl Imidazole) Grafted Silica Nanofillers: Synthesis by RAFT Polymerization and Nanocomposites with Polybenzimidazole. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 365–375. [Google Scholar] [CrossRef]
- Zavala-Lagunes, E.; Ruiz, J.-C.; Varca, G.H.C.; Bucio, E. Synthesis and Characterization of Stimuli-Responsive Polypropylene Containing N -Vinylcaprolactam and N -Vinylimidazole Obtained by Ionizing Radiation. Mater. Sci. Eng. 2016, 67, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, C.; Yang, Q.; Yin, Y.; Bai, X.; Zhen, S.; Fan, C.; Sun, K. Investigation of Polysulfone Film on High-Performance Anode with Stabilized Electrolyte/Electrode Interface for Lithium Batteries. J. Energy Chem. 2020, 42, 49–55. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, C.; Gao, L.; Xu, Y.; Che, Q.; He, R. Influences of the Structure of Imidazolium Pendants on the Properties of Polysulfone-Based High Temperature Proton Conducting Membranes. J. Membr. Sci. 2015, 493, 80–87. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, L.; Chen, Z.; Wang, Y.; Tusiime, R.; Cheng, C.; Zhou, S.; Liu, Y.; Yu, M.; Zhang, H. Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers 2019, 11, 461. [Google Scholar] [CrossRef]
- Hoffmann, C.; Silau, H.; Pinelo, M.; Woodley, J.M.; Daugaard, A.E. Surface Modification of Polysulfone Membranes Applied for a Membrane Reactor with Immobilized Alcohol Dehydrogenase. Mater. Today Commun. 2018, 14, 160–168. [Google Scholar] [CrossRef]
- Singh, B.; Rajneesh. Gamma Radiation Synthesis and Characterization of Gentamicin Loaded Polysaccharide Gum Based Hydrogel Wound Dressings. J. Drug Deliv. Sci. Technol. 2018, 47, 200–208. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.B.; Burillo, G.; Bucio, E.; Alvarez-Lorenzo, C. Modification of Polyethylene Films by Radiation Grafting of Glycidyl Methacrylate and Immobilization of β-Cyclodextrin. Radiat. Phys. Chem. 2009, 78, 19–24. [Google Scholar] [CrossRef]
- Mehrdad, A.; Noorani, N. Study of CO2 Adsorption onto Poly(1–Vinylimidazole) Using Quartz Crystal Microbalance and Density Functional Theory Methods. J. Mol. Liq. 2019, 291, 111288. [Google Scholar] [CrossRef]
- Li, D.; Niu, X.; Yang, S.; Chen, Y.; Ran, F. Thermo-Responsive Polysulfone Membranes with Good Anti-Fouling Property Modified by Grafting Random Copolymers via Surface-Initiated EATRP. Sep. Purif. Technol. 2018, 206, 166–176. [Google Scholar] [CrossRef]
- Elsharma, E.M.; Saleh, A.S.; Abou-Elmagd, W.S.I.; Metwally, E.; Siyam, T. Gamma Radiation Induced Preparation of Polyampholyte Nanocomposite Polymers for Removal of Co(II). Int. J. Biol. Macromol. 2019, 136, 1273–1281. [Google Scholar] [CrossRef]
- Nguyen, H.T.V.; Ngo, T.H.A.; Do, K.D.; Nguyen, M.N.; Dang, N.T.T.; Nguyen, T.T.H.; Vien, V.; Vu, T.A. Preparation and Characterization of a Hydrophilic Polysulfone Membrane Using Graphene Oxide. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Radiation Grafting of Poly(Methyl Methacrylate) and Poly(Vinylimidazole) onto Polytetrafluoroethylene Films and Silver Immobilization for Antimicrobial Performance. Appl. Surf. Sci. 2019, 473, 951–959. [Google Scholar] [CrossRef]
- Willott, J.D.; Humphreys, B.A.; Murdoch, T.J.; Edmondson, S.; Webber, G.B.; Wanless, E.J. Hydrophobic Effects within the Dynamic PH-Response of Polybasic Tertiary Amine Methacrylate Brushes. Phys. Chem. Chem. Phys. 2015, 17, 3880–3890. [Google Scholar] [CrossRef]
- Miao, A.; Wei, M.; Xu, F.; Wang, Y. Influence of membrane hydrophilicity on water permeability: An experimental study bridging simulations. J. Membr. Sci. 2020, 604, 1–7. [Google Scholar] [CrossRef]
- Pérez-Calixto, M.; González-Pérez, G.; Dionisio, N.; Bucio, E.; Burillo, G.; García-Uriostegui, L. Surface Functionalization of Polypropylene and Polyethylene Films with Allylamine by γ Radiation. MRC Commun. 2019, 9, 264–269. [Google Scholar] [CrossRef]
- Jang, H.; Song, H.; Kim, I.; Kwon, Y. Fouling control through the hydrophilic surface modification of poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2015, 132, 41712. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Zhao, F.; Liu, Y.; Liu, L.; Wang, L.; Geng, C.; Huang, P. Preparation of a hydrophilic and antibacterial dual function ultrafiltration membrane with quaternized graphene oxide as a modifier. J. Colloid Interface Sci. 2019, 562, 182–192. [Google Scholar] [CrossRef]
- Xiang, S.; Guo, Z.; Wang, Y.; Liu, H.; Zhang, J.; Cui, Z.; Wang, H.; Li, J. The microstructure regulation, strengthening, toughening and hydrophilicity of polyamide6 in fabricating poly (vinylidene fluoride)-based flat membrane via the thermally induced phase separation technique. Eur. Polym. J. 2020, 126, 109568. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Bucio, E. Synthesis of a Thermo- and PH-Sensitive Comb-Type Graft Copolymer by Ionizing Radiation. MRS Comm. 2018, 8, 1335–1342. [Google Scholar] [CrossRef]
- Hegazy, E.-S.A.; El-Rehim, H.A.A.; Shawky, H.A. Investigations and Characterization of Radiation Grafted Copolymers for Possible Practical Use in Waste Water Treatment. Radiat. Phys. Chem. 2000, 57, 85–95. [Google Scholar] [CrossRef]
- Abdul Mannan, H.; Mukhtar, H.; Shima Shaharun, M.; Othman, M.; Murugesan, T. Polysulfone/Poly(Ether Sulfone) Blended Membranes for CO2 Separation. J. Appl. Polym. Sci. 2016, 133, 42946. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Zhang, J.; Zhang, J.; Lu, S.; Xiang, Y. High Temperature Polymer Electrolyte Membrane Achieved by Grafting Poly(1-Vinylimidazole) on Polysulfone for Fuel Cells Application. J. Membr. Sci. 2019, 592, 117395. [Google Scholar] [CrossRef]
- Lock, S.S.M.; Lau, K.K.; Mei, I.L.S.; Shariff, A.M.; Yeong, Y.F.; Bustam, A.M. Molecular Simulation and Mathematical Modelling of Glass Transition Temperature Depression Induced by CO2 Plasticization in Polysulfone Membranes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012172. [Google Scholar] [CrossRef]
- Bong, Y.C.; Oh, P.C. The Effect of Amine Substituent Chain Length on POSS/Polysulfone Mixed Matrix Membrane. J. Mech. Eng. Sci. 2018, 12, 3494–3504. [Google Scholar] [CrossRef]
- Aquino, R.R.; Tolentino, M.S.; Esmalde, M.L.; Condol, D.V.B.; Basilia, B.A. Effect of Polysulfone/Organomontmorillonite Blends on Nanocomposite Membrane Properties. Key Eng. Mater. 2019, 801, 331–336. [Google Scholar] [CrossRef]
- Fodor, C.; Bozi, J.; Blazsó, M.; Iván, B. Thermal Behavior, Stability, and Decomposition Mechanism of Poly(N -Vinylimidazole). Macromolecules 2012, 45, 8953–8960. [Google Scholar] [CrossRef]
- Woo, S.-M.; Kim, D.-J.; Nam, S.-Y. Preparation and Properties of Polysulfone–Poly(Ethylene Glycol) Graft Copolymer Membrane. J. Nanosci. Nanotechnol. 2014, 14, 7804–7808. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M. Effect of Glass Transition Temperature in Enhanced Polymeric Blend Membranes. Procedia Eng. 2016, 148, 11–17. [Google Scholar] [CrossRef]
- Riera, F.; Suárez, A.; Muro, C. Nanofiltration of UHT flash cooler condensates from a dairy factory: Characterisation and water reuse potential. Desalination 2013, 309, 52–63. [Google Scholar] [CrossRef]
- Hernández, K.; Muro, C.; Ortega, R.E.; Velazquez, S.; Riera, F. Water recovery by treatment of food industry wastewater using membrane processes. Environ. Technol. 2019, 25, 1–14. [Google Scholar] [CrossRef]
- Deng, B.; Yu, M.; Yang, X.; Zhang, B.; Li, L.; Xie, L.; Li, J.; Lu, X. Antifouling Microfiltration Membranes Prepared from Acrylic Acid or Methacrylic Acid Grafted Poly(Vinylidene Fluoride) Powder Synthesized via Pre-Irradiation Induced Graft Polymerization. J. Membr. Sci. 2010, 350, 252–258. [Google Scholar] [CrossRef]
- Reis, R.; Duke, M.C.; Tardy, B.L.; Oldfield, D.; Dagastine, R.R.; Orbell, J.D.; Dumée, L.F. Charge Tunable Thin-Film Composite Membranes by Gamma-Ray Triggered Surface Polymerization. Sci. Rep. 2017, 7, 4426. [Google Scholar] [CrossRef]




| Experiment 1 | PSU-VIM 50% (v/v) and different gamma irradiation doses (kGy) | |||
| PSU film | 200 | 300 | 400 | |
| Contact angle (o) |  |  |  |  |
| 90.7 ± 1 | 79.2 ± 1.5 | 66 ± 1.5 | 64.3 ± 1 | |
| Experiment 2 | PSU-VIM (30–70% v/v) and 300 kGy irradiation dose | |||
| 30 | 50 | 60 | 70 | |
| Contact angle (o) |  |  |  |  |
| 84 ± 0.5 | 61 ± 0.5 | 11.8 ± 1 | ≈ 0 | |
| Membrane Sample | Thermogravimetric Analysis (TGA) | Differential Scanning Calorimetry (DSC) | ||
|---|---|---|---|---|
| Ts (°C) | Td (°C) | Sample Weight (%) | Tg (°C) | |
| PSU film | 501 | 529 | 26 | 189.15 |
| PSU-VIM (50% v/v) | 401 | 514 | 13.84 | 185.40 |
| PSU-VIM (60% v/v) | 368 | 513 | 0.06 | 186.28 |
| PSU-VIM (70% v/v) | 310 | 510 | 0.08 | 186.58 |
| Grafting in PSU-Film by VIM | Operation Time (s) | Feed Flow (×10−4 mL/cm2·s) | Total Solids Feed Flow (mg/L) | Salt Rejection Flow (×10−4 mL/cm2·s) | Total Solids Rejection Flow (mg/L) | Water Permeation Flow (×10−4 mL/cm2·s) | Total Solids Permeation Flow (mg/mL) |
|---|---|---|---|---|---|---|---|
| PSU film | 120 ± 15 | 3.50 ± 1 | 100 ± 1 | 1.05 ± 0.3 | 73.50 ± 5 | 1.75 ± 0.3 | 5.87 ± 1 |
| PSU-VIM 50% v/v | 900 ± 10 | 24.5 ±3 | 1000 ± 5 | 7.36 ± 7 | 845.00 ± 15 | 15.18 ± 3 | 85.34 ± 5 |
| PSU-VIM 60% v/v | 250 ± 15 | 29.1 ± 5 | 1000 ± 5 | 3.55 ± 5 | 292.15 ± 21 | 16.76 ± 2 | 488.65 ± 5 |
| PSU-VIM 70% v/v | 168 ± 5 | 30.2 ± 10 | 1000 ± 5 | 2.34 ± 0.9 | 125.78 ± 25 | 25.95 ± 10 | 595.21 ± 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, E.; Muro, C.; Illescas, J.; Burillo, G.; Hernández, O.; Rivera, E. Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation. Polymers 2020, 12, 1284. https://doi.org/10.3390/polym12061284
Vázquez E, Muro C, Illescas J, Burillo G, Hernández O, Rivera E. Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation. Polymers. 2020; 12(6):1284. https://doi.org/10.3390/polym12061284
Chicago/Turabian StyleVázquez, Elizabeth, Claudia Muro, Javier Illescas, Guillermina Burillo, Omar Hernández, and Ernesto Rivera. 2020. "Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation" Polymers 12, no. 6: 1284. https://doi.org/10.3390/polym12061284
APA StyleVázquez, E., Muro, C., Illescas, J., Burillo, G., Hernández, O., & Rivera, E. (2020). Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation. Polymers, 12(6), 1284. https://doi.org/10.3390/polym12061284