Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization
Abstract
:1. Introduction
2. Materials and Methods
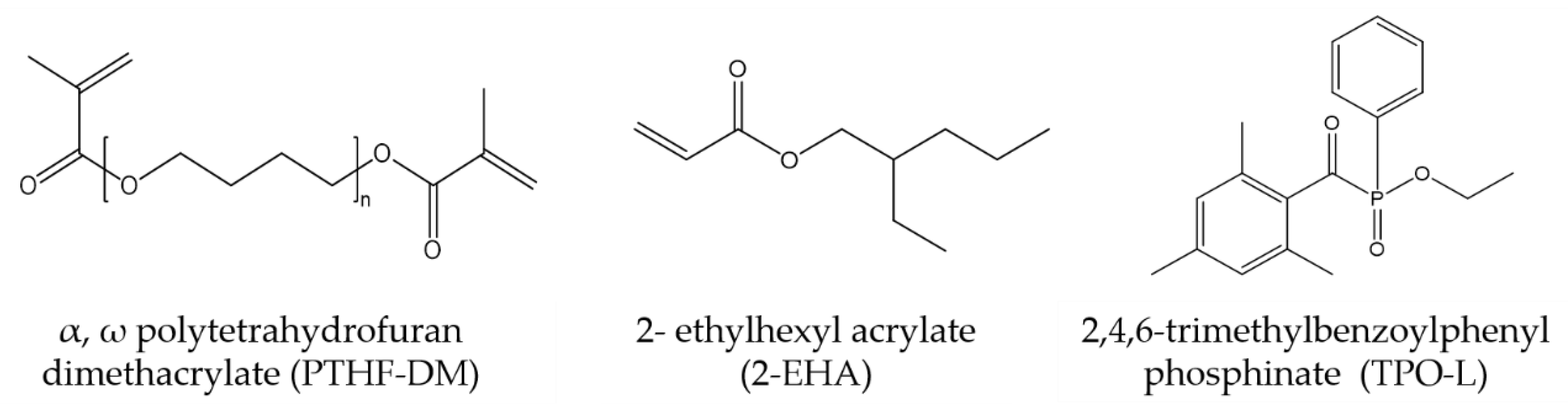
2.1. Materials
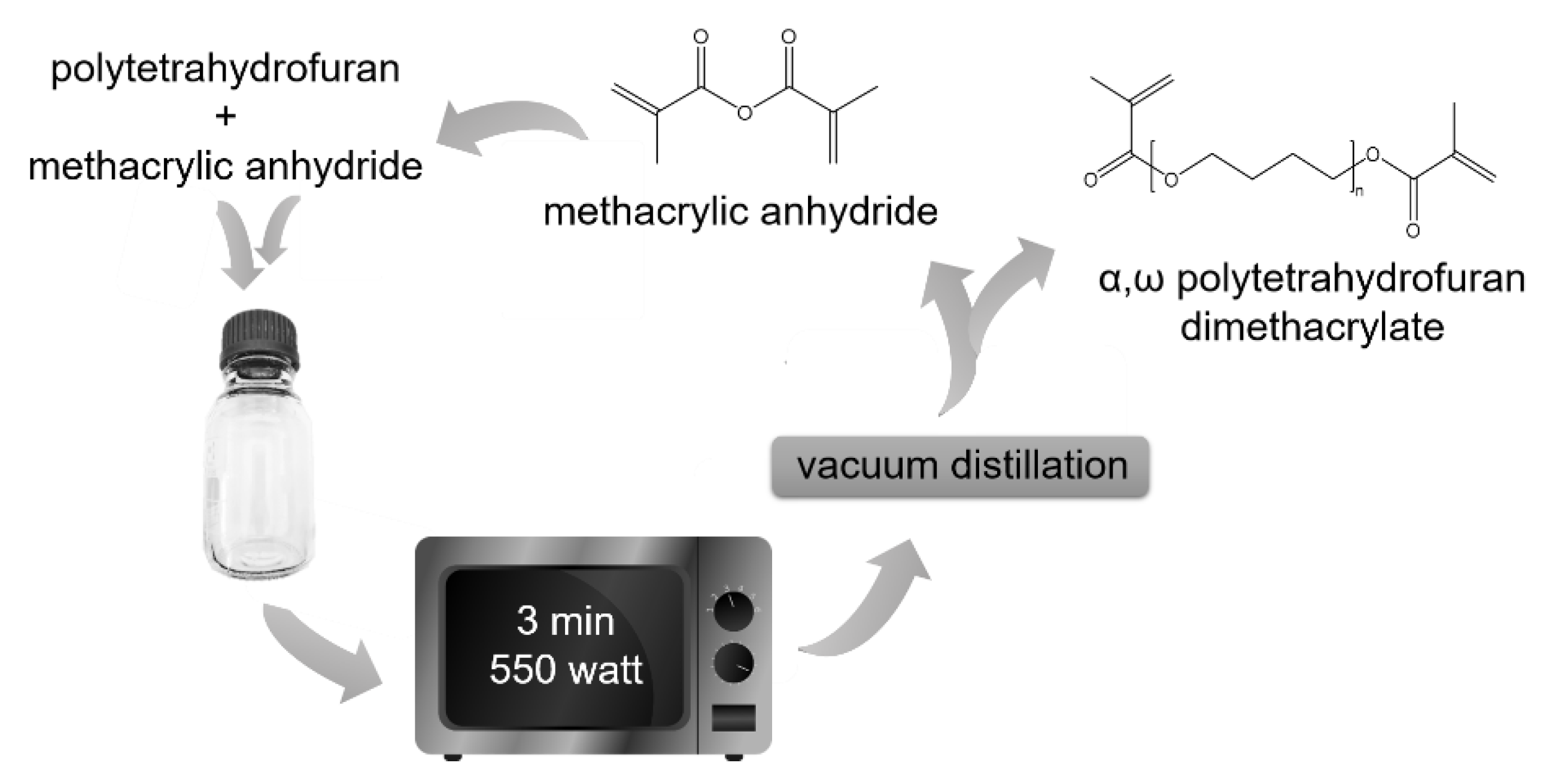
2.2. Polyol Modification
2.3. Preparation of Photobleaching Systems (PBS)
2.4. UV-Vis Spectroscopy and Photobleaching Experiments
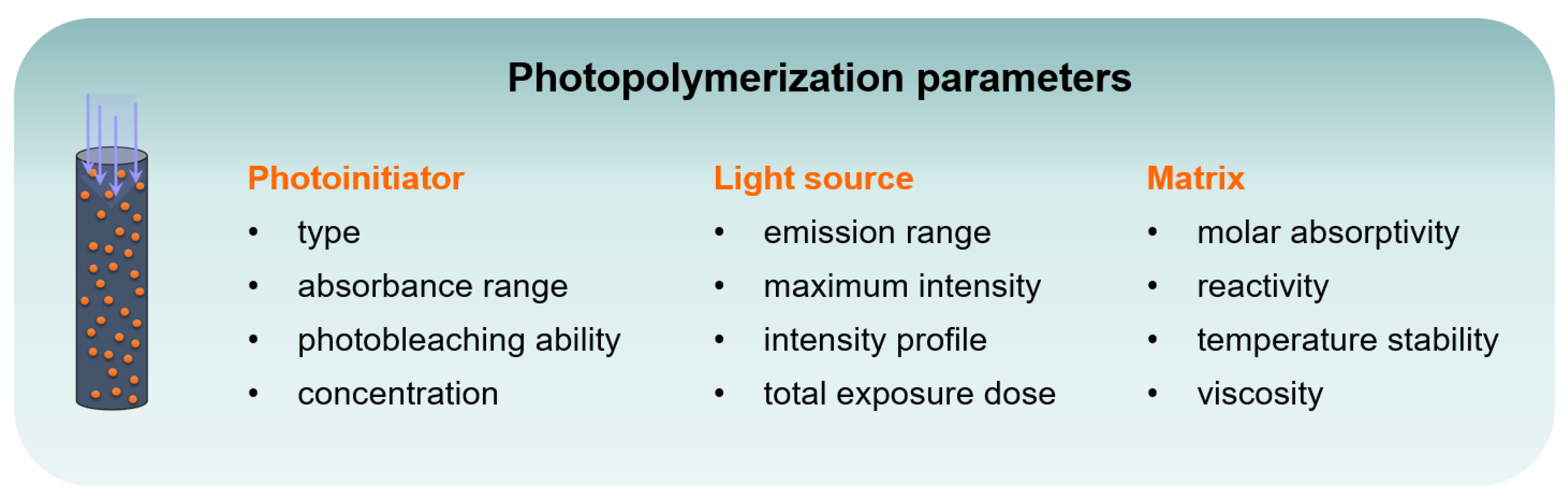
2.5. Photopolymerization and Characterization of Curing Behavior
3. Results and Discussion
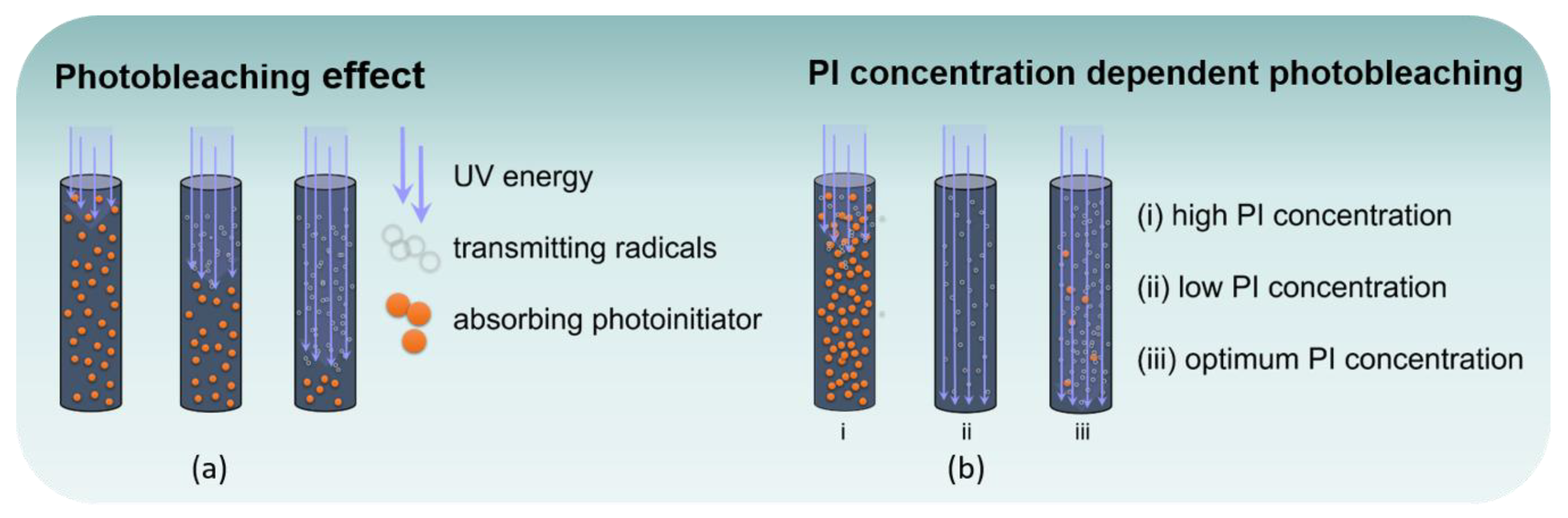
3.1. Optical Characteristics of Monomers and Photobleaching Systems
3.2. Monitoring of Photobleaching
3.3. Polymerization Behavior of Photobleaching Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steyrer, B.; Neubauer, P.; Liska, R.; Stampfl, J. Visible Light Photoinitiator for 3D-Printing of Tough Methacrylate Resins. Materials 2017, 10, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Lorenz, H.; Despont, M.; Fahrni, N.; LaBianca, N.; Renaud, P.; Vettiger, P. SU-8: A low-cost negative resist for MEMS. J. Micromech. Microeng. 1997, 7, 121–124. [Google Scholar] [CrossRef]
- Buonocore, M. Adhesive sealing of pits and fissures for caries prevention, with use of ultraviolet light. J. Am. Dent. Assoc. 1970, 80, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Green, W.A. Industrial photoinitiators. A Technical Guide; Taylor & Francis: Boca Raton, FL, USA, 2010; ISBN 9781439827451. [Google Scholar]
- Zhang, Z.; Corrigan, N.; Bagheri, A.; Jin, J.; Boyer, C. A Versatile 3D and 4D Printing System through Photocontrolled RAFT Polymerization. Angew. Chem. Int. Ed. Engl. 2019, 58, 17954–17963. [Google Scholar] [CrossRef]
- Femmer, T.; Jans, A.; Eswein, R.; Anwar, N.; Moeller, M.; Wessling, M.; Kuehne, A.J.C. High-Throughput Generation of Emulsions and Microgels in Parallelized Microfluidic Drop-Makers Prepared by Rapid Prototyping. ACS Appl. Mater. Interfaces 2015, 7, 12635–12638. [Google Scholar] [CrossRef] [PubMed]
- Männel, M.J.; Fischer, C.; Thiele, J. A Non-Cytotoxic Resin for Micro-Stereolithography for Cell Cultures of HUVECs. Micromachines 2020, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Robertson, I.D.; Yourdkhani, M.; Centellas, P.J.; Aw, J.E.; Ivanoff, D.G.; Goli, E.; Lloyd, E.M.; Dean, L.M.; Sottos, N.R.; Geubelle, P.H.; et al. Rapid energy-efficient manufacturing of polymers and composites via frontal polymerization. Nature 2018, 557, 223–227. [Google Scholar] [CrossRef]
- Pojman, J.A.; Ilyashenko, V.M.; Khan, A.M. Free-radical frontal polymerization: Self-propagating thermal reaction waves. Faraday Trans. 1996, 92, 2825. [Google Scholar] [CrossRef]
- Bomze, D.; Knaack, P.; Koch, T.; Jin, H.; Liska, R. Radical induced cationic frontal polymerization as a versatile tool for epoxy curing and composite production. J. Polym. Sci. A Polym. Chem. 2016, 54, 3751–3759. [Google Scholar] [CrossRef]
- Bomze, D.; Knaack, P.; Liska, R. Successful radical induced cationic frontal polymerization of epoxy-based monomers by C–C labile compounds. Polym. Chem. 2015, 6, 8161–8167. [Google Scholar] [CrossRef]
- Knaack, P.; Klikovits, N.; Tran, A.D.; Bomze, D.; Liska, R. Radical induced cationic frontal polymerization in thin layers. J. Polym. Sci. A Polym. Chem. 2019, 57, 1155–1159. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Li, Z.; Shi, F.; Liu, X. Extremely deep photopolymerization using upconversion particles as internal lamps. Polym. Chem. 2016, 7, 2457–2463. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Li, S.; Liu, S.; Miao, H.; Wu, S. Near-Infrared Light Driven Photopolymerization Based On Photon Upconversion. ChemPhotoChem 2019, 3, 1077–1083. [Google Scholar] [CrossRef]
- Oprych, D.; Schmitz, C.; Ley, C.; Allonas, X.; Ermilov, E.; Erdmann, R.; Strehmel, B. Photophysics of Up-Conversion Nanoparticles: Radical Photopolymerization of Multifunctional Methacrylates Comprising Blue- and UV-Sensitive Photoinitiators. ChemPhotoChem 2019, 3, 1119–1126. [Google Scholar] [CrossRef]
- Pojman, J.A. Frontal Polymerization. Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, NL, USA, 2012; pp. 957–980. ISBN 9780080878621. [Google Scholar]
- Carion, P.; Ibrahim, A.; Allonas, X.; Croutxé-Barghorn, C.; L’Hostis, G. Frontal free-radical photopolymerization of thick samples: Applications to LED-induced fiber-reinforced polymers. J. Polym. Sci. A Polym. Chem. 2019, 57, 898–906. [Google Scholar] [CrossRef]
- Davidenko, N.; García, O.; Sastre, R. The efficiency of titanocene as photoinitiator in the polymerization of dental formulations. J. Biomater. Sci. Polym. Ed. 2003, 14, 733–746. [Google Scholar] [CrossRef]
- Kolczak, U.; Rist, G.; Dietliker, K.; Wirz, J. Reaction Mechanism of Monoacyl- and Bisacylphosphine Oxide Photoinitiators Studied by 31 P-, 13 C-, and 1 H-CIDNP and ESR. J. Am. Chem. Soc. 1996, 118, 6477–6489. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Asmusen, S.; Arenas, G.; Cook, W.D.; Vallo, C. Photobleaching of camphorquinone during polymerization of dimethacrylate-based resins. Dent. Mater. 2009, 25, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Dolinski, N.D.; Page, Z.A.; Callaway, E.B.; Eisenreich, F.; Garcia, R.V.; Chavez, R.; Bothman, D.P.; Hecht, S.; Zok, F.W.; Hawker, C.J. Solution Mask Liquid Lithography (SMaLL) for One-Step, Multimaterial 3D Printing. Adv. Mater. Weinheim. 2018, 30, e1800364. [Google Scholar] [CrossRef] [PubMed]
- Terrones, G.; Pearlstein, A.J. Effects of Optical Attenuation and Consumption of a Photobleaching Initiator on Local Initiation Rates in Photopolymerizations. Macromolecules 2001, 34, 3195–3204. [Google Scholar] [CrossRef]
- Miller, G.A.; Gou, L.; Narayanan, V.; Scranton, A.B. Modeling of photobleaching for the photoinitiation of thick polymerization systems. J. Polym. Sci. A Polym. Chem. 2002, 40, 793–808. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization. Polymers 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, A.K.; Bowman, C.N. Modeling Thermal and Optical Effects on Photopolymerization Systems. Macromolecules 2003, 36, 7777–7782. [Google Scholar] [CrossRef]
- Zhang, Y.; Kranbuehl, D.E.; Sautereau, H.; Seytre, G.; Dupuy, J. Modeling and Measuring UV Cure Kinetics of Thick Dimethacrylate Samples. Macromolecules 2009, 42, 203–210. [Google Scholar] [CrossRef]
- Ivanov, V.V.; Decker, C. Kinetic study of photoinitiated frontal polymerization. Polym. Int. 2001, 50, 113–118. [Google Scholar] [CrossRef]
- Lee, J.H.; Prud’homme, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Hayki, N.; Lecamp, L.; Désilles, N.; Lebaudy, P. Kinetic Study of Photoinitiated Frontal Polymerization. Influence of UV Light Intensity Variations on the Conversion Profiles. Macromolecules 2010, 43, 177–184. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Wilson, B.D.; Benoit, D.S.W. Microwave-assisted functionalization of poly(ethylene glycol) and on-resin peptides for use in chain polymerizations and hydrogel formation. J. Vis. Exp. 2013, e50890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangee, O.D.; Wilson, V.H.; East, G.C.; Holme, I. Antioxidant-induced yellowing of textiles. Polym. Degrad. Stab. 1995, 50, 313–317. [Google Scholar] [CrossRef]


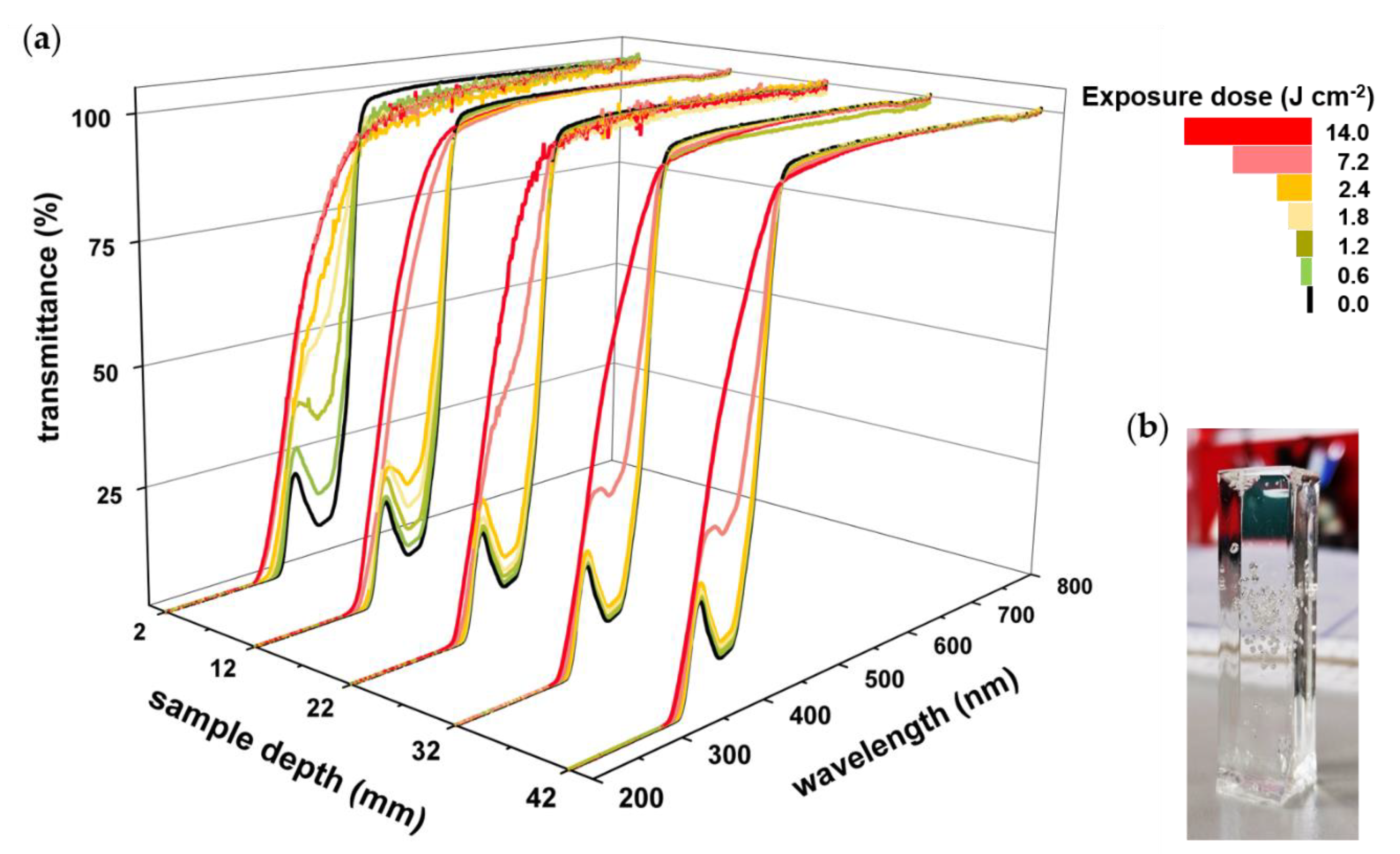


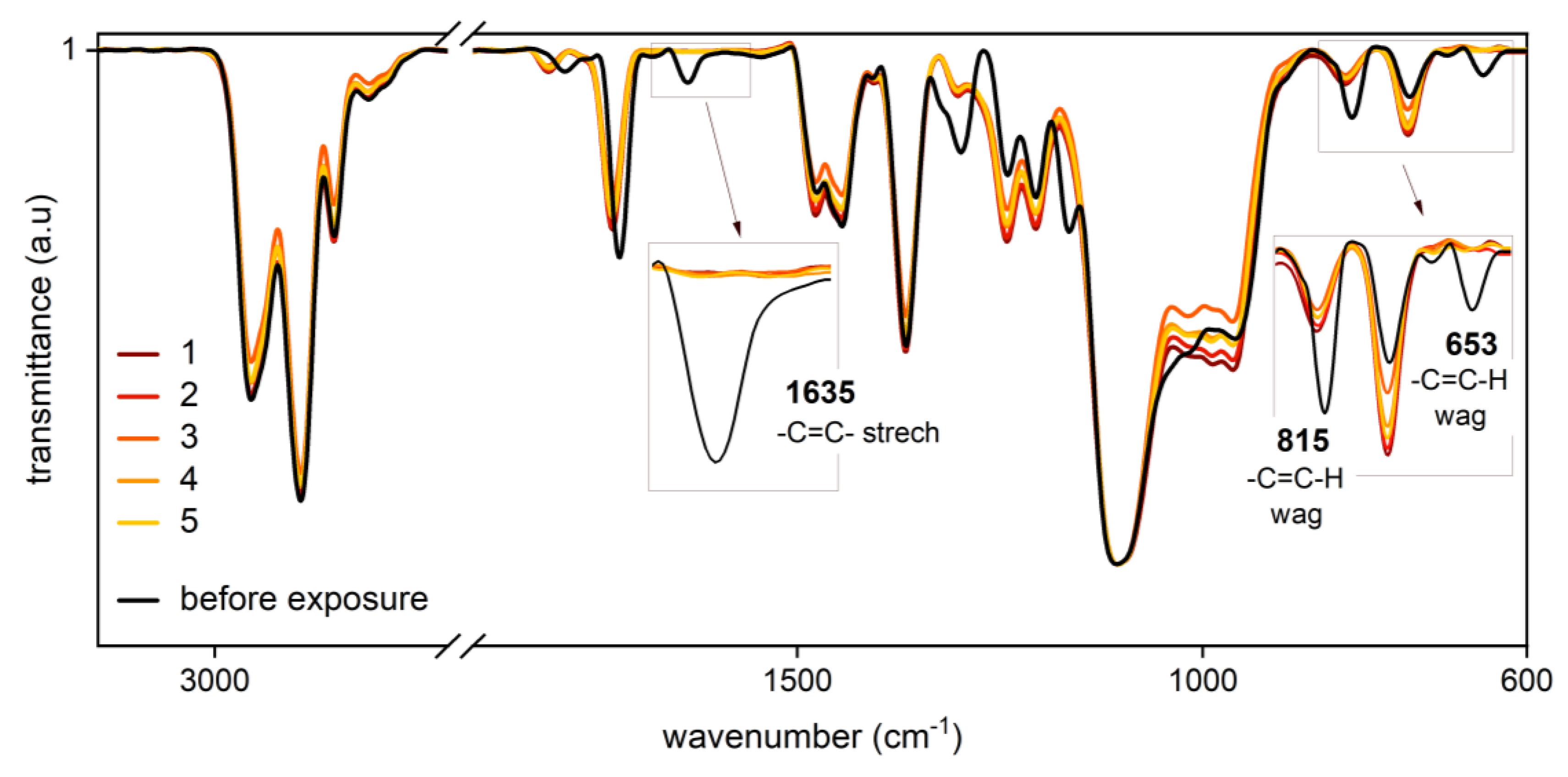
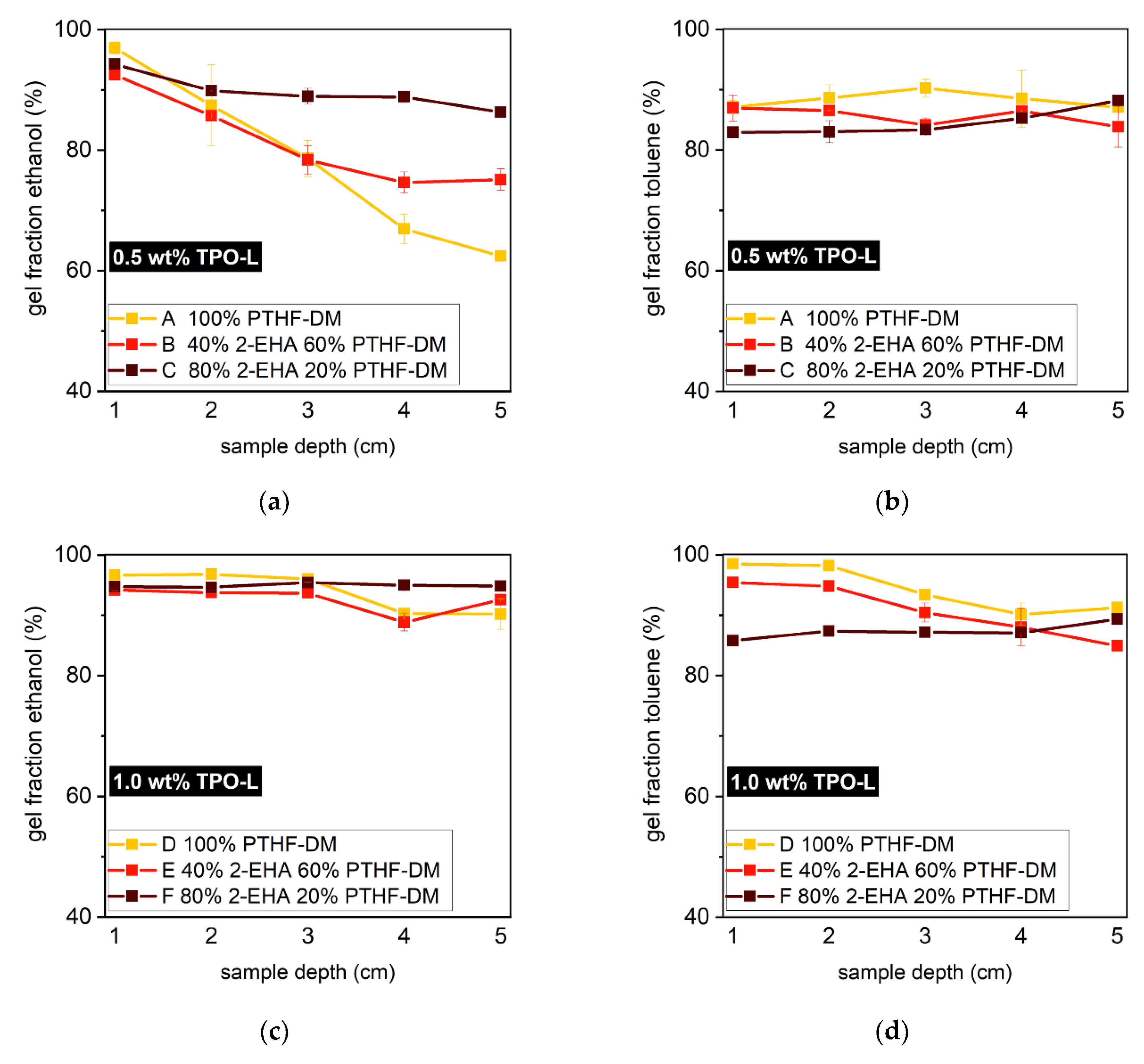
| Cycle | Power Level (%) | Intensity (watt cm−2) | Runs | Exposure Dose (J cm−2) | Total Exposure Dose (J cm−2) |
|---|---|---|---|---|---|
| 1 | 30 | 0.13 | 1 | 0.60 | 0.6 |
| 2 | 30 | 0.13 | 1 | 0.60 | 1.2 |
| 3 | 30 | 0.13 | 1 | 0.60 | 1.8 |
| 4 | 30 | 0.13 | 1 | 0.60 | 2.4 |
| 5 | 50 | 0.34 | 3 | 4.8 | 7.2 |
| 6 | 60 | 0.48 | 3 | 6.8 | 14.0 |
| Cycle | Power Level (%) | Intensity (watt cm−2) | Runs | Exposure Dose (J cm−2) | Total Exposure Dose (J cm−2) |
|---|---|---|---|---|---|
| 1 | 40 | 0.24 | 3 | 3.4 | 3.4 |
| 2 | 60 | 0.48 | 3 | 6.9 | 10.3 |
| 3 | 100 | 0.96 | 3 | 10.5 | 20.8 |
| Photobleaching System | PTHF2900 Dimethacrylate (wt%) | 2-EHA (wt%) | TPO-L (wt%) |
|---|---|---|---|
| A | 100 | 0 | 0.5 |
| B | 100 | 0 | 1.0 |
| C | 60 | 40 | 0.5 |
| D | 60 | 40 | 1.0 |
| E | 20 | 80 | 0.5 |
| F | 20 | 80 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebner, C.; Mitterer, J.; Eigruber, P.; Stieger, S.; Riess, G.; Kern, W. Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization. Polymers 2020, 12, 1291. https://doi.org/10.3390/polym12061291
Ebner C, Mitterer J, Eigruber P, Stieger S, Riess G, Kern W. Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization. Polymers. 2020; 12(6):1291. https://doi.org/10.3390/polym12061291
Chicago/Turabian StyleEbner, Catharina, Julia Mitterer, Paul Eigruber, Sebastian Stieger, Gisbert Riess, and Wolfgang Kern. 2020. "Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization" Polymers 12, no. 6: 1291. https://doi.org/10.3390/polym12061291
APA StyleEbner, C., Mitterer, J., Eigruber, P., Stieger, S., Riess, G., & Kern, W. (2020). Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization. Polymers, 12(6), 1291. https://doi.org/10.3390/polym12061291








