1. Introduction
The polymer hydrogel is a polymer material composed of a hydrophilic 3D network structure, which allows the polymer hydrogel to absorb a large amount of solvent and swell, and also has good biocompatibility. The forces that affect the water absorption of hydrogels include hydration, penetration, and capillary phenomena. The swelling balance depends on the relative magnitude of these forces, and to a large extent affects some important properties of hydrogels, including diffusion characteristics, internal transportation, and mechanical properties [
1,
2,
3]. Hydrophilic hydrogels have many unique physical and chemical properties, which make hydrogels widely used in the field of biomedical materials. For example, it is used in the controlled release of drugs. Because of the weak hydrophobic force between the hydrogel and the protein, the macromolecule drug can be effectively coated in the polymer matrix of the hydrogel [
4]. Hydrogels are also considered to be biomimetic tissues because they can contain a lot of water inside the structure, and this water can allow small molecules to pass through, promote local and sustained release of drugs, and thus reduce the number of administrations, prevent damage to drugs, and allow relatively low dose delivery. Natural or synthetic polymers can be used to prepare hydrogels [
5,
6,
7]. Natural polymer hydrogels can provide sufficient biocompatibility, biometrics, biodegradability, and other functions, but they cannot provide sufficient mechanical strength. Synthetic polymer hydrogels do not have fixed biological activity characteristics, but the mechanical properties of synthetic polymer hydrogels are better than natural polymer hydrogels. There are many classification methods for hydrogels, mainly depending on their physical properties, swelling properties, polymerization composition, preparation method, ionic charge, crosslinking method, and biodegradability [
8]. Hydrogel materials will change their characteristics due to environmental stimuli. When hydrogels are physically or chemically stimulated, they will swell or shrink (also known as intelligent hydrogels). These environmental factors include temperature, pressure, pH value, magnetic field, electric field, optical radiation, and ultrasonic radiation [
9,
10]. The stimulus-responsive hydrogel will give different feedbacks due to changes in the external environment, such as oxygen permeability response, volume change, mechanical strength, etc. Physical stimuli include temperature, light source, pressure, electric and magnetic fields, etc., changing the intermolecular interactions that occur at the critical point. The chemical stimulus contains the pH value, chemical agents, and ions, and it responds to the interaction between the functional groups contained in the polymer chain and the solvent. The different response behaviors of hydrogels can be divided into the several types [
11], like temperature-sensitive hydrogel [
12,
13,
14,
15,
16,
17,
18], pH-sensitive hydrogel [
6,
19,
20,
21,
22,
23,
24], ionic strength hydrogel [
19], glucose sensing hydrogel [
25], and light-sensitive hydrogel [
26]. A pH-sensitive hydrogel is a polymer hydrogel that can change in volume with changes in the environmental pH and ionic strength. The interaction forces that cause the volume change of the pH-sensitive hydrogel are mainly the forces between ions and hydrogen bonds. Its molecular structure usually has an ionizable acid (carboxy) group (–COOH or –SO
3H) or an amino group. With the change of environmental pH, the group will dissociate, making the hydrogen between the polymer chains in the structure. The bond dissociates, and the force between the ions increases the electrostatic repulsion, resulting in swelling and de-swelling behavior. Generally, pH-sensitive hydrogels can be divided into three different response types, which are anionic, cationic, and zwitterionic [
13,
27]. The sensitive group of the anionic pH-sensitive hydrogel is generally –COO
–. This type of hydrogel is generally in a contracted state in a low-pH environment, because in a low-pH environment, the dissociation degree of the ionizable groups of the hydrogel is low, making the electrostatic repulsion force on the gel swelling not contribute. When the pH value of the environment increases, the electrostatic repulsion between the carboxylate groups increases, and the electrostatic repulsion causes the expansion rate of the hydrogel to increase. The sensitive groups of cationic pH-sensitive hydrogels are generally basic amine groups, and their pH sensitivity mainly comes from the protonation of amino groups. The more amino groups, the stronger the hydration of the hydrogel, the greater the electrostatic repulsion between the protonated amino groups, resulting in a greater degree of equilibrium swelling [
20]. Zwitterionic pH-sensitive hydrogels also have acid–base groups. Its pH sensitivity comes from the ionization of two groups in the hydrogel network. Basic groups will ionize in low-pH environments. The acidic group is ionized in a high-pH environment, so the zwitterionic hydrogel has a relatively large electrostatic repulsion in high- or low-pH environments, so it has a large swelling capacity and has relatively smaller swelling ability in a moderate environment. Therefore, this type of hydrogel has good swelling ability in almost all pH ranges, and it is more sensitive to the changes of ionic strength.
The swelling step of the hydrogel can be divided into two stages. The first step is that the solvent molecules enter the hydrogel and interact with the network polymer chains to form a hydrated layer. Then, the second stage is that the solvent molecules continue to penetrate. At this time, the volume of the hydrogel will increase greatly, and the weight of the hydrogel at this time can reach dozens of times the weight of the dried hydrogel. Because of the dissociation effect of ionic groups (such as –NH2, –COOH, and –SO3H) in the structure, the ionic hydrogel increases the hydrophilicity of the hydrogel, resulting in its strong water absorption capacity. At the same time, the increase in the degree of dissociation further extends the polymer chains in the network and makes sufficient contact with water molecules. The properties of the polymer itself (hydrophilicity/hydrophobicity and crosslinking degree) and the degree of dissociation of hydrogel functional groups and various factors of ionic hydration (environmental factors such as temperature, pH, ionic strength, ionic properties, and swelling medium composition) will affect the swelling ability of the hydrogel.
The water molecules in hydrogel polymers can be divided into three different forms: (1) Free water, also known as intermediate water or free water, is usually distributed on the surface of the polymer and is easily lost. Its thermodynamic behaviors, such as crystallization, melting temperature, and enthalpy change, are similar to those of pure water. (2) Non-freezing bond water, which is water that is bonded to the polar hydrophilic group of the polymer network structure through hydrogen bonding, has no phase change within the temperature range of −100~−50 °C, and its crystallization exothermic or melting endothermic is usually not observed; it is more closely bonded to the polymer matrix. (3) Freezing bond water’s phase transition temperature is usually lower than 0 °C; the water in this state in the hydrogel should be weakly bond water. Non-freezing bond water surrounds the water in the bond water network with a certain orientation, forming a second or third hydration layer. Although the bonding effect of the freezing bond water is slightly weaker than that of the non-freezing bond water, it is not easy to remove [
28,
29,
30,
31,
32].
The bulk water content is the sum of the content of freezing bond water and non-freezing bond water. Bonded water has a great influence on the structure and function of hydrogels and also plays an important role in the process of biological metabolism. Many scholars use Differential Scanning Calorimetry (DSC) to detect the presence of water in different polymers [
28]. By analyzing the melting endothermic peaks of polymer that can be frozen near 0 °C, the ratio of the enthalpy value (ΔH
fm) to the endothermic peak enthalpy value (ΔH
0) of pure water at 0 °C can be used to calculate the polymer’s freezing water content (W
fm). The bulk water content (BWC) of the polymer is the sum of freezing water content (W
fm) and non-freezing water (W
nf), so the amount of water in different states in the polymer can be obtained, as expressed by the following formulas:
where W
wet is the overall water content of the hydrogel when it reaches equilibrium, and W
dry is the weight of the hydrogel polymer when it is dry.
When water is in contact with the hydrogel network structure, the hydrogel polymer chain will interact with the solvent molecules, causing the hydrogel network structure to swell, and this swelling behavior can be calculated by using the Flory–Rehner theory [
33] thermodynamics of the mixing process. It can be proved by combining the theory of rubber elasticity and thermodynamics that the equilibrium swelling is mainly controlled by two opposing forces, which are (1) the mixing force between the hydrogel and the solvent, and (2) the high hydrogel of the contraction force of the molecular chain; when these two opposing forces reach equilibrium, the two forces can cancel each other out [
34,
35]
The interaction constant χ value (interaction force parameter) can be regarded as the interaction index between the polymer gel and the solvent, and it can be roughly regarded as the hydrophilicity and hydrophobicity of the polymer in the solvent. The force parameters are derived from the Flory–Huggins theory [
36]. After subsequent research, the force parameter expression can be obtained from Equation (4), which can be expressed by the effective swelling balance.
where
ϕ2 is the volume fraction of the polymer when it expands,
V1 is the molar volume of the solvent,
υe is the effective crosslink density, and
ƒ−1 is the average number of active elastic chains. The magnitude of the force parameter is related to the contribution of the mixed chemical potential energy, so from a numerical perspective, the elastic contribution only has a slight effect on the force parameter, because the value of
υe in the system is very small, and it can even be ignored, in order to obtain Equation (5).
Finally, the force parameter between the polymer and the water molecule can be calculated by Equation (6).
The behavior of water molecules diffusing into the hydrogel network and the relaxation of the polymer chains can be used to explain the dynamic behavior of the gel during swelling [
37,
38].
Fick’s model is suitable for lamellar hydrogel films. When the water content of the hydrogel film is not large, and the macromolecular chain relaxation rate between the network-like structures is fast, the swelling process of the hydrogel film is mainly controlled by the water molecule diffusion process, which can be obtained by using Fick’s diffusion equation description:
where
Mt is the weight measured under a fixed time interval,
M∞ is the weight measured when swelling reaches equilibrium,
k (characteristic constant) is a constant describing the structure or geometry of the hydrogel,
t is time, and
n (mechanism exponent) indicates the transport mode of the solvent into the gel. By plotting the slope and intercept of log (
Mt/
M∞) vs. log (
t), we obtain the values of
n and
k, respectively.
According to the calculated value of n, it can be divided into three different transmission behaviors:
- (1)
When the calculated value of n is less than or equal to 0.5 (n ≤ 0.5), it is called Fick’s diffusion transmission behavior. In this case, the diffusion rate is much greater than the relaxation rate of the hydrogel polymer chain. In other words, the interaction between the molecules of the hydrogel network structure and the solvent is much larger than the interaction between the molecular chains of the gel network structure, so that the polymer chain of the hydrogel can quickly relax and expand. Thus, this system is a diffusion control process.
- (2)
When the calculated value of n = 1.0 (n = 1.0), it is an extreme transmission behavior. In this case, the relaxation rate of the hydrogel polymer chain is much greater than the diffusion rate, meaning that the interaction between the hydrogel network molecular chain is much greater than the interaction between the hydrogel network molecular chain and the solvent. Therefore, the system is controlled by the speed of hydrogel swelling.
- (3)
When the calculated value of n is in the range of 0.5~1.0 (n = 0.5~1.0), it is called non-Fick’s transmission behavior. It means that, during the swelling process of the hydrogel, the relaxation rate of the polymer chain is equivalent to the diffusion rate of the solvent.
The structure of chitosan has a primary amine group and a first- and second-order hydroxyl group, and its pka is about 6.5. This property allows it to have different swelling and de-swelling behavior in an acid or alkali environment. In other words, chitosan is also one of the pH-sensitive materials. However, although chitosan has good biocompatibility and degradability, when it is used as a drug-release material, it is too sensitive to pH and it is difficult to control. Therefore, it can be physically blended or connected by using a crosslinking agent or a polymer. To enhance mechanical properties to improve shortcomings and increase drug-release time, Chitosan itself contains a hydrophilic hydroxyl group (–OH) and a more reactive nucleophilic amine group (–NH), which is prone to high water swelling, and in an acidic environment, the structure is easily damaged, so a crosslinking agent is often used for crosslinking reaction to increase its mechanical properties and acid resistance [
39,
40]. However, chitosan can provide more application options after physical or chemical crosslinking [
41,
42]. Because chitosan is positively charged, has active functional groups, and has excellent biodegradability and biocompatibility, research on chitosan can be seen in disease treatment and drug release [
43,
44,
45,
46]. There are many other applications, such as external dressing [
47], protein separation [
48], biosensor [
49], tissue engineering [
50], and biochips [
51,
52,
53,
54,
55,
56,
57,
58].
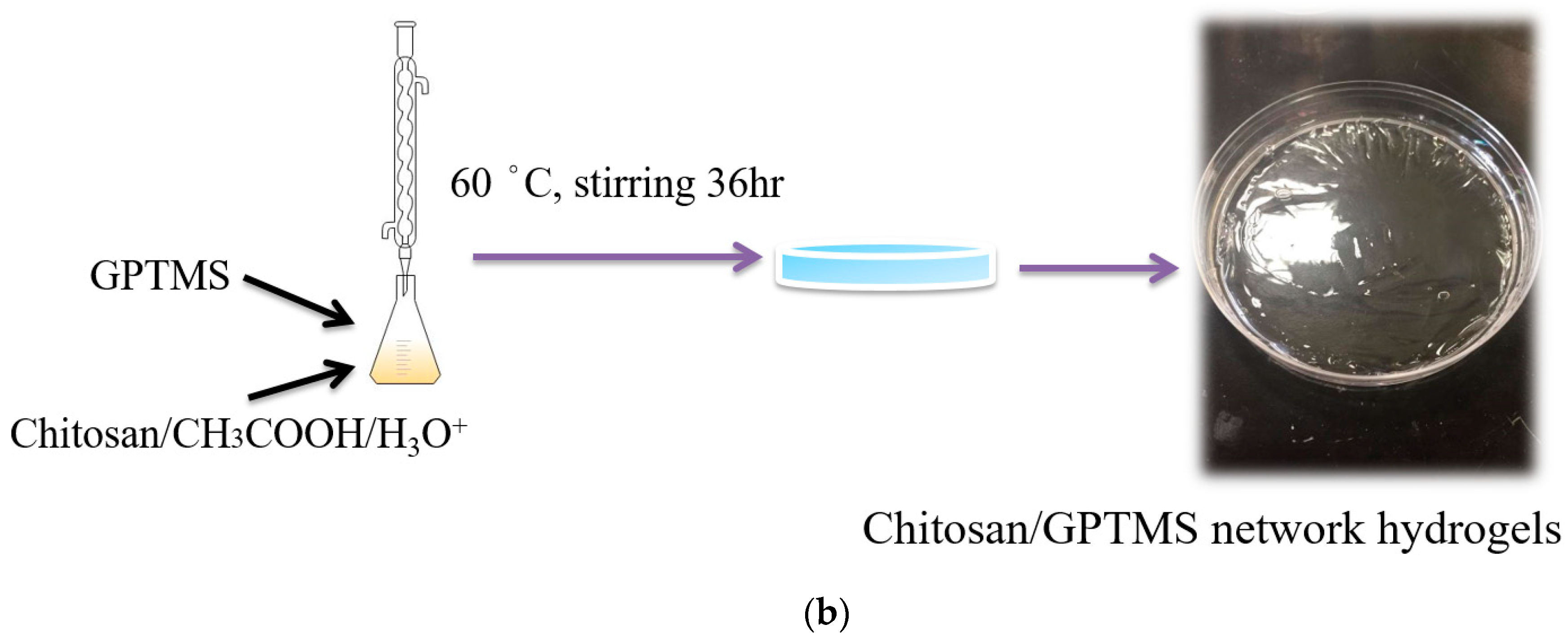
The design of this study mainly uses GPTMS as the crosslinking agent to carry out ring-opening and sol-gel crosslinking reaction with chitosan (
Figure 1). It is hoped that the crosslinking reaction can increase the mechanical properties and acid resistance of chitosan hydrogel. Various characteristic analyses such as water swelling ratio, DSC thermal analysis, pH response test, expansion heat, and kinetics, to analyze the characteristics of different proportions of GPTMS additions, were carried out (
Figure 2). The result shows that Chitosan/GPTMS hybrid hydrogel is suitable for pH-sensitive hydrocolloid materials.