Effect of Dielectric Barrier Discharge (DBD) Treatment on the Dielectric Properties of Poly(vinylidene fluoride)(PVDF)-Based Copolymer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prateek; Thakur, V.K.; Gupta, R.K. Recent progress on ferroelectric polymer-based nanocomposites for high energy density capacitors: Synthesis, dielectric properties, and future aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.J.; Zhou, X.; Ren, K.L.; Neese, B.; Lin, M.R.; Wang, Q.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Huan, T.D.; Boggs, S.; Tegssedre, G.; Laurent, C.; Cakmak, M.; Kumar, S.; Ramprasak, R. Advanced polymeric dielectrics for high energy density applications. Prog. Mater. Sci. 2016, 83, 236–269. [Google Scholar] [CrossRef]
- Li, W.P.; Jiang, L.; Zhang, X.; Shen, Y.; Nan, C.W. High-energy-density dielectric films based on polyvinylidene fluoride and aromatic polythiourea for capacitors. J. Mater. Chem. A 2014, 2, 15803–15807. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q. Novel ferroelectric polymers for high energy density and low loss dielectrics. Macromolecules 2012, 45, 2937–2954. [Google Scholar] [CrossRef]
- Ranjani, M.; Yoo, D.J.; Kumar, G.G. Sulfonated Fe3O4@SiO2 nanorods incorporated sPVdF nanocomposite membranes for DMFC applications. J. Membr. Sci. 2018, 555, 497–506. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Chen, Q.; Chu, B.J.; Zhang, Q.M. Recent development of high energy density polymers for dielectric capacitors. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1036–1042. [Google Scholar] [CrossRef]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembreb, L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J. Colloid Interface Sci. 2008, 328, 412–420. [Google Scholar] [CrossRef]
- Chu, B.J.; Neese, B.; Lin, M.R.; Lu, S.G.; Zhang, Q.M. Enhancement of dielectric energy density in the poly (vinylidene fluoride)-based terpolymer/copolymer blends. Appl. Phys. Lett. 2008, 93, 152903. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, B.J.; Neese, B.; Lin, M.R.; Zhang, Q.M. Electrical energy density and discharge characteristics of a poly (vinylidene fluoride-chlorotrifluoroethylene) copolymer. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1133–1138. [Google Scholar] [CrossRef]
- Xia, W.M.; Li, J.J.; Zhang, Z.C.; Xu, Z. Poly (vinylidene fluoride-chlorotrifluoroethylene)/BaTiO3 composites with high electrical energy density. Ferroelectrics 2010, 407, 125–133. [Google Scholar] [CrossRef]
- Jow, T.R.; Cygan, P.J. Investigation of dielectric breakdown of polyvinylidene fluoride using AC and DC methods. In Proceedings of the Conference Record of the 1992 IEEE International Symposium on Electrical Insulation, Baltimore, MD, USA, 7–10 June 1992; pp. 181–184. [Google Scholar] [CrossRef]
- O’Dwyer, J.J. Theory of Dielectric Breakdown in Solids. J. Electrochem. Soc. 1969, 116, 239–242. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, B.J.; Zhou, X.; Zhang, Q.M. Effect of metal-polymer interface on the breakdown electric field of poly (vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer. Appl. Phys. Lett. 2007, 91, 062907. [Google Scholar] [CrossRef]
- Han, K.; Li, Q.; Chanthad, C.; Gadinski, M.R.; Zhang, G.; Wang, Q. A Hybrid Material Approach Toward Solution-Processable Dielectrics Exhibiting Enhanced Breakdown Strength and High Energy Density. Adv. Funct. Mater. 2015, 25, 3505–3513. [Google Scholar] [CrossRef]
- Tang, J.; Duan, Y.X.; Zhao, W. Characterization and mechanism studies of dielectric barrier discharges generated at atmospheric pressure. Appl. Phys. Lett. 2010, 96, 191503. [Google Scholar] [CrossRef]
- Xu, X.J. Dielectric barrier discharge—Properties and applications. Thin Solid Film. 2001, 390, 237–242. [Google Scholar] [CrossRef]
- Eliasson, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D 1987, 20, 1421–1437. [Google Scholar] [CrossRef]
- Shao, T.; Long, K.H.; Zhang, C.; Yan, P.; Zhang, S.C.; Pan, R.Z. Experimental study on repetitive unipolar nanosecond-pulse dielectric barrier discharge in air at atmospheric pressure. J. Phys. D Appl. Phys. 2008, 41, 215203. [Google Scholar] [CrossRef]
- Liu, S.H.; Neiger, M. Excitation of dielectric barrier discharges by unipolar submicrosecond square pulses. J. Phys. D Appl. Phys. 2001, 34, 1632. [Google Scholar] [CrossRef]
- Shao, T.; Wang, R.X.; Zhang, C.; Yan, P. Atmospheric-pressure pulsed discharges and plasmas: Mechanism, characteristics and applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Shao, T.; Liu, F.; Hai, B.; Ma, Y.F.; Wang, R.X.; Ren, C.Y. Surface modification of epoxy using an atmospheric pressure dielectric barrier discharge to accelerate surface charge dissipation. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1557–1565. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Ren, X.; Fan, C.; Zhou, W.; Wang, F.; Ding, W. Experimental study on surface modification of PET films under bipolar nanosecond-pulse dielectric barrier discharge in atmospheric air. Appl. Surf. Sci. 2014, 313, 53–59. [Google Scholar] [CrossRef]
- Shao, T.; Sun, G.S.; Yan, P.; Wang, J.; Yuan, W.Q.; Sun, Y.H.; Zhang, S.C. An experimental investigation of repetitive nanosecond-pulse breakdown in air. J. Phys. D Appl. Phys. 2006, 39, 2192. [Google Scholar] [CrossRef] [Green Version]
- Jurczuk, K.; Galeski, A.; Mackey, M.; Hiltner, A.; Baer, E. Orientation of PVDF α and γ crystals in nanolayered films. Colloid Polym. Sci. 2015, 293, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.; Jin, J.; Khanchaitit, P.; Wang, J.; Wang, Q. Effect of crystal structure on polarization reversal and energy storage of ferroelectric poly (vinylidene fluoride-co-chlorotrifluoroethylene) thin films. Polymer 2012, 53, 1277–1281. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Hu, X.P.; Chu, B.J.; Chen, S.T.; Salem, D. Flexoelectric effect in PVDF-based polymers. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 727–731. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lu, H.W.; Shen, Z.W.; Li, Z.L.; Shen, Q.D. Cooling rate controlled microstructure evolution through flash DSC and enhanced energy density in P (VDF–CTFE) for capacitor application. J. Polym. Sci. Pol. Phys. 2017, 55, 1245–1253. [Google Scholar] [CrossRef]
- Khanchaitit, P.; Han, K.; Gadinski, M.R.; Li, Q.; Wang, Q. Ferroelectric polymer networks with high energy density and improved discharged efficiency for dielectric energy storage. Nat. Commun. 2013, 4, 2845. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, C.; Liu, X.; Xie, Q.; Yan, P.; Shao, T. Microsecond pulse driven Ar/CF4 plasma jet for polymethylmethacrylate surface modification at atmospheric pressure. Appl. Surf. Sci. 2015, 328, 509–515. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Bodas, D.S.; Mandale, A.B.; Gangal, S.A. Deposition of PTFE thin films by RF plasma sputtering on<1 0 0> silicon substrates. Appl. Surf. Sci. 2005, 245, 202–207. [Google Scholar] [CrossRef]
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge University Press: Cambridge, UK, 1998; pp. 47–87. [Google Scholar] [CrossRef]
- Tong, W.; Lu, C.; Cai, Y.; Huang, Y. Surface Modification of Fluororubber Using Atmospheric Pressure Dielectric Barrier Discharge (DBD). Plasma Sci. Technol. 2007, 9, 296–300. [Google Scholar] [CrossRef]
- Károly, Z.; Klébert, S.; Al-Maliki, H.; Pataki, T. Comparison of NPIII and DBD Plasma Treatment in Terms of Wettability of PTFE and PA6. Sci. Bull. Ser. C 2016, 30, 47–50. [Google Scholar]
- Wang, S.F.; Li, J.; Suo, J.P.; Luo, T.Z. Surface modification of porous poly (tetrafluoraethylene) film by a simple chemical oxidation treatment. Appl. Surf. Sci. 2010, 256, 2293–2298. [Google Scholar] [CrossRef]
- Ru, L.; Jie-Rong, C. Studies on wettability of medical poly (vinyl chloride) by remote argon plasma. Appl. Surf. Sci. 2006, 252, 5076–5082. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, C.; Long, K.H.; Zhang, D.D.; Wang, J.; Yan, P.; Zhou, Y.X. Surface modification of polyimide films using unipolar nanosecond-pulse DBD in atmospheric air. Appl. Surf. Sci. 2010, 256, 3888–3894. [Google Scholar] [CrossRef]
- Kim, K.B.; Tak, Y.H.; Han, Y.S.; Baik, K.H.; Yoon, M.H.; Lee, M.H. Relationship between surface roughness of indium tin oxide and leakage current of organic light-emitting diode. Jpn. J. Appl. Phys. 2003, 42, L438. [Google Scholar] [CrossRef]
- Hariprasad, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. SPVdF-HFP/SGO nanohybrid proton exchange membrane for the applications of direct methanol fuel cells. J. Dispers. Sci. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. Ternary hybrid (SPEEK/SPVdF-HFP/GO) based membrane electrolyte for the applications of fuel cells: Profile of improved mechanical strength, thermal stability and proton conductivity. RSC Adv. 2016, 6, 108851–108863. [Google Scholar] [CrossRef]
- Mizutani, T. Space charge measurement techniques and space charge in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 923–933. [Google Scholar] [CrossRef]
- IEDA, M. Dielectric breakdown process of polymers. IEEE Trans. Dielectr. Electr. Insul. 1980, 3, 206–224. [Google Scholar] [CrossRef]
- Park, Y.W.; Inagaki, N. Surface modification of poly (vinylidene fluoride) film by remote Ar, H2, and O2 plasmas. Polymer 2003, 44, 1569–1575. [Google Scholar] [CrossRef]
- Du, B.X.; Liu, H.J.; Liu, Y. Effects of gamma-ray irradiation on dielectric surface breakdown of polybutylene polymers. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 696–701. [Google Scholar] [CrossRef]
- Ohmi, T.; Miyashita, M.; Itano, M.; Imaoka, T.; Kawanabe, I. Dependence of thin-oxide films quality on surface microroughness. IEEE Trans. Electron. Devices 1992, 39, 537–545. [Google Scholar] [CrossRef]

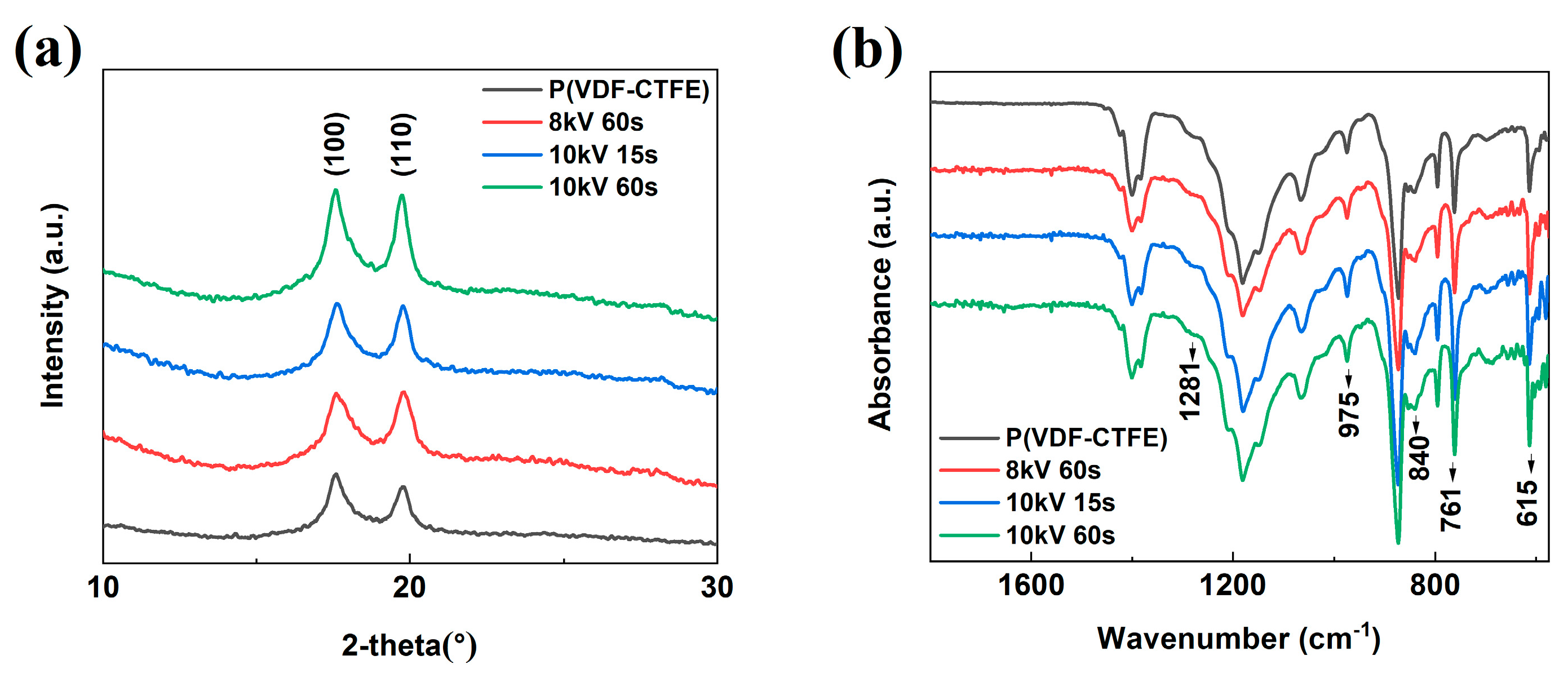
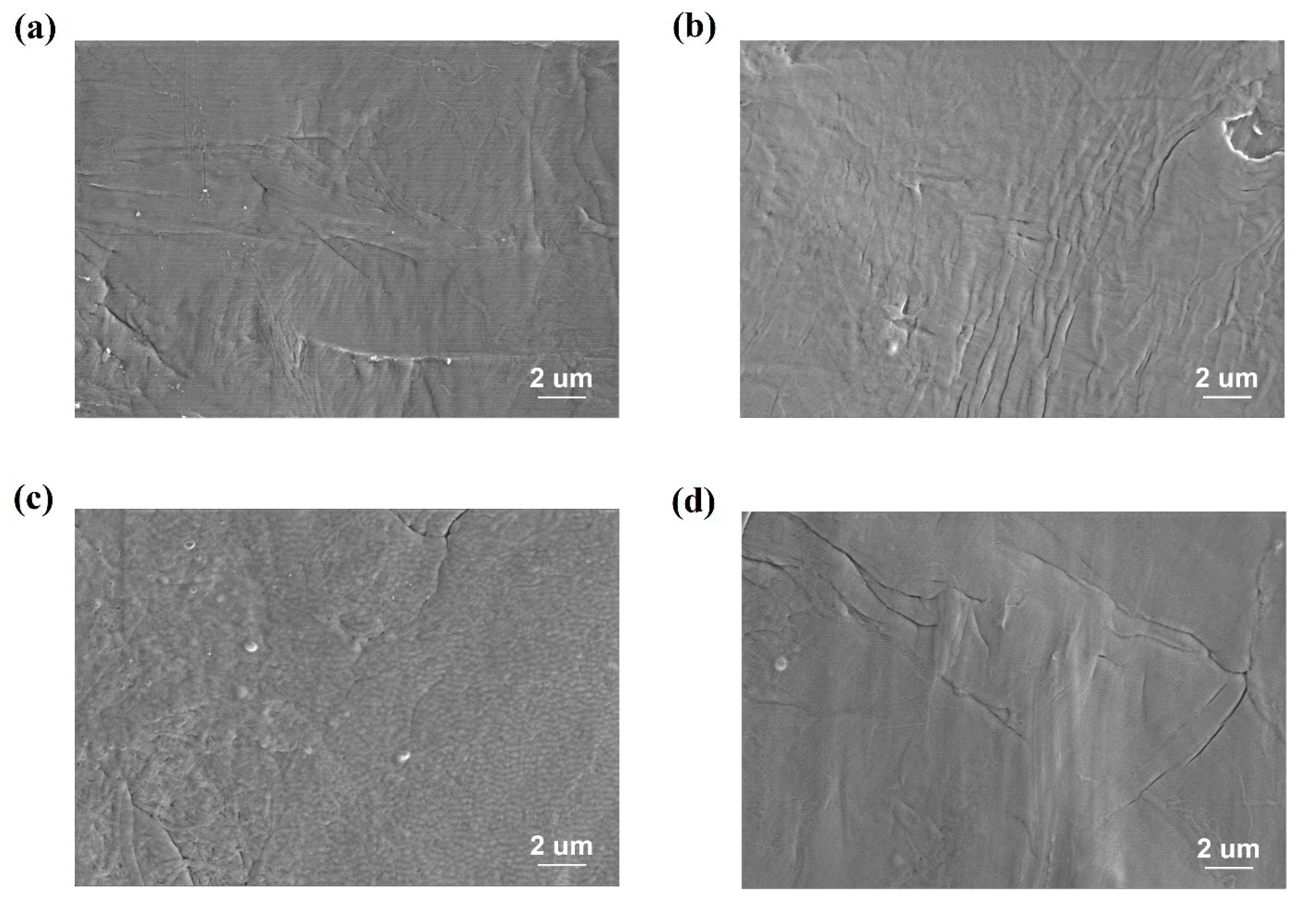
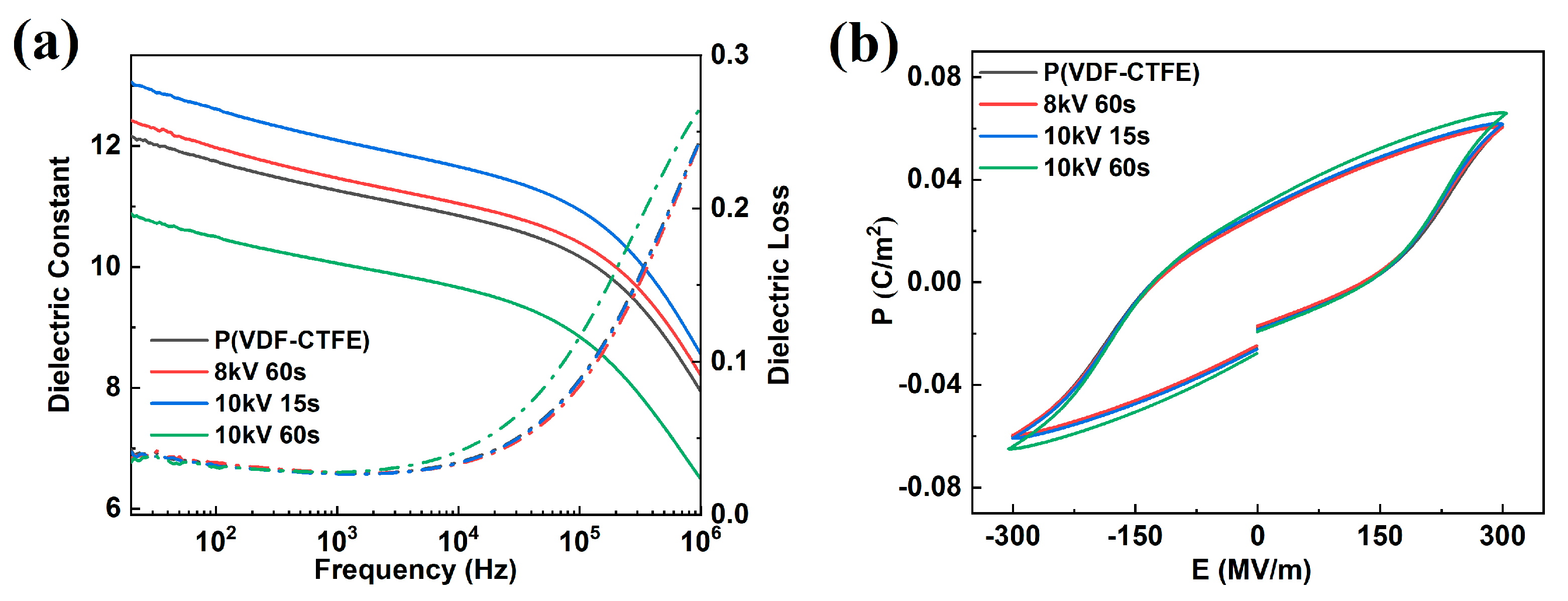
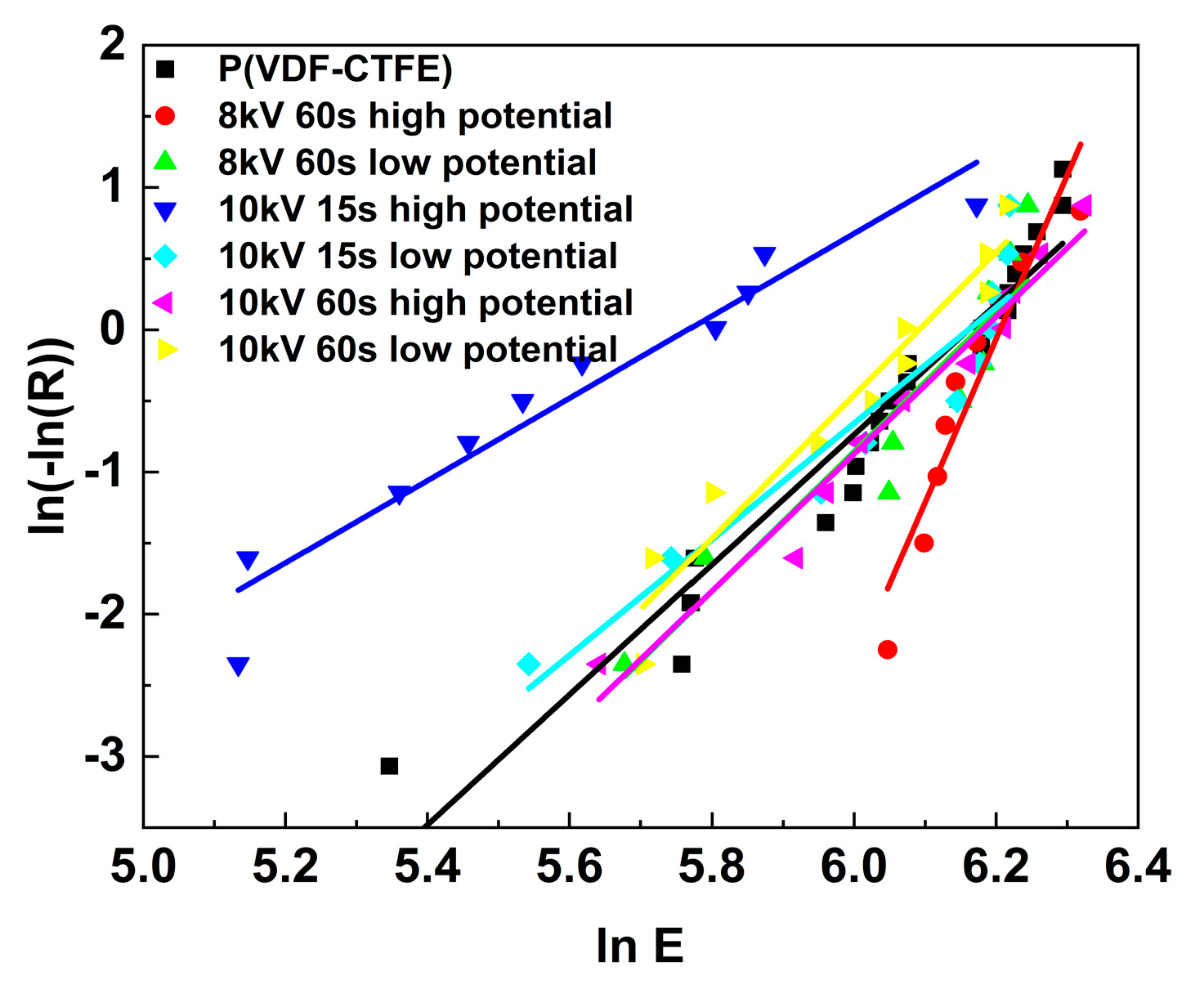


| Treatment Conditions | P(VDF-CTFE) | 8 kV 60 s | 10 kV 15 s | 10 kV 60 s |
|---|---|---|---|---|
| Treated surface connected to low potential side | 473.97 | 480.67 | 473.95 | 483.47 |
| Treated surface connected to high potential side | 473.97 | 495.58 | 319.36 | 442.07 |
| Elements | F | Cl | O | C |
|---|---|---|---|---|
| P(VDF-CTFE) | 24.08% | 1.17% | 0.78% | 73.97% |
| 8 kV 60 s | 22.61% | 1.09% | 1.55% | 74.75% |
| 10 kV 15 s | 14.56% | 0.72% | 3.18% | 81.54% |
| 10 kV 60 s | 13.47% | 0.40% | 4.75% | 81.38% |
| Treatment Conditions | P(VDF-CTFE) | 8 kV 60 s | 10 kV 15 s | 10 kV 60 s |
|---|---|---|---|---|
| Ra | 22.4 | 25.3 | 87.7 | 30.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhou, Y.; Yi, K.; Zhang, S.; Shao, T.; Zhang, C.; Chu, B. Effect of Dielectric Barrier Discharge (DBD) Treatment on the Dielectric Properties of Poly(vinylidene fluoride)(PVDF)-Based Copolymer. Polymers 2020, 12, 1370. https://doi.org/10.3390/polym12061370
Liu J, Zhou Y, Yi K, Zhang S, Shao T, Zhang C, Chu B. Effect of Dielectric Barrier Discharge (DBD) Treatment on the Dielectric Properties of Poly(vinylidene fluoride)(PVDF)-Based Copolymer. Polymers. 2020; 12(6):1370. https://doi.org/10.3390/polym12061370
Chicago/Turabian StyleLiu, Jie, Yang Zhou, Kewang Yi, Shihai Zhang, Tao Shao, Cheng Zhang, and Baojin Chu. 2020. "Effect of Dielectric Barrier Discharge (DBD) Treatment on the Dielectric Properties of Poly(vinylidene fluoride)(PVDF)-Based Copolymer" Polymers 12, no. 6: 1370. https://doi.org/10.3390/polym12061370






