Green Highly Clay-Filled Polyethylene Composites as Coating Materials for Cable Industry—A New Application Route of Non-Organophilised Natural Montmorillonites in Polymeric Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- An ethylene-butyl acrylate copolymer (EBA) with melting temperature ca. 95 °C and density 0.92 g/cm3 (Lucalen A2700M; LyondellBasell, Rotterdam, The Netherlands);
- An ethylene-vinyl acetate copolymer (EVA) with melting temp. ca. 73 °C, density 0.95 g/cm3 and 28 wt. % of VAc (Elvax 265; DuPont, Wilmington, DE, USA);
- An ethylene-vinyl acetate copolymer modified with maleic anhydride (m-EVA) with melting temp. ca. 71 °C and density 0.96 g/cm3 (Fusabond C250; DuPont, Wilmington, DE, USA).
- A powdered natural calcium montmorillonite (C–Ca) with an average particle size (d50) of 10 μm (Cloisite Ca++; BYK-Chemie, Wesel, Germany);
- A powdered montmorillonite (CW9) organophilised with dimethyl benzyl hydrogenated tallow ammonium salt and with an average particle size (d50) of 20 μm (Dellite CW9; Laviosa Chimica Mineraria, Livorno, Italy).
2.2. Composites Preparation
2.3. Methods
3. Results
3.1. Selection of MMT Dispersing Agents
3.2. Properties of Highly MMT-Filled PE Composites
4. Conclusions
- All of the tested aluminosilicates were not fully exfoliated in the PE matrix, however, slight intercalation of the clays was observed by the XRD technique.
- C–Ca and CW9 pre-dispersions did not affect the crystallinity of PE matrix (the XRD analyses) as well as did not unpredictably change thermal features of the material during thermal processing (DSC analyses at a heating/cooling mode).
- The incorporation of the MMT pre-dispersion (all of the tested types) into PE reduced tensile strength and elongation at break of the compression-moulded samples. On the other hand, both fillers spectacularly increased Young’s modulus values of the composites, however, the highest doses of the EVA/C–Ca or mEVA/CW9 pre-dispersions diminished this phenomenon. The hardness of the reference PE material was not markedly affected by the fillers.
- The C–Ca addition markedly increased limited oxygen index (LOI) value from 18% O2 (PE) up to 22.0% O2 (the PEVA/Ca-7.5 composite with the EVA/C–Ca pre-dispersion, 7.5 wt. % of the natural clay). An insignificantly higher LOI value (22.2% O2) was noted for the system with 10 wt. % of CW9. Materials filled with this clay exhibited the highest temperature values at 10% and 50% mass loss during their heating in the air. Nevertheless, the amount of calcination residue (at 900 °C) nonlinearly increased with the increasing initial concentration of the C–Ca filler (in relation to the sample containing 5 wt. % of this clay). In the case of the CW9 additive, this relation was reversed (the higher filler content, the lower efficiency of the calcination residue formation process).
- Due to the medium content of the unmodified montmorillonite, the high LOI value as well as the acceptable mechanical features, the PEVA/Ca-7.5 composite seems to be the most interesting material for further study.
Author Contributions
Funding
Conflicts of Interest
References
- British Standard no. B7211. Specification for Thermosetting Insulated Cables for Electrical Power and Lighting with Low Emission of Smoke and Corrosive Gases When Affected by Fire; BSI Group: London, UK, 1998. [Google Scholar]
- British Standard no. BS 7655. Specification for Insulating and Sheathing Materials for Cables; BSI Group: London, UK, 1997. [Google Scholar]
- Verusca de Oliveira, S.; dos Santos Filho, E.; Araújo, E.; Correia Pereira, C.; Passador, F. Preparation and flammability properties of polyethylene/organoclay nanocomposites. Diff. Found. 2018, 20, 92–105. [Google Scholar] [CrossRef]
- Ubowska, A.; Kowalczyk, K.; Krala, G. Injection molding of transparent polymeric materials with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide for enhanced fire retardancy. Polimery 2018, 63, 57–62. [Google Scholar] [CrossRef]
- Krala, G.; Ubowska, A.; Kowalczyk, K. Mechanical and thermal analysis of injection molded poly(methyl methacrylate) modified with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) fire retarder. Polym. Eng. Sci. 2014, 54, 1030–1037. [Google Scholar] [CrossRef]
- Purser, D. The evolution of toxic effluents in fires and the assessment of toxic hazard. Toxicol. Lett. 1992, 64–65, 247–255. [Google Scholar] [CrossRef]
- Wu, K.; Shen, M.; Hu, Y. Combustion behavior and thermal oxidative degradation of EVA containing intumescent flame retardant. Polym-Plast. Technol. Eng. 2010, 49, 1527–1533. [Google Scholar] [CrossRef]
- Cross, M.; Cusack, P.; Hornsby, P. Effects of tin additives on the flammability and smoke emission characteristics of halogen-free ethylene-vinyl acetate copolymer. Polym. Degrad. Stabil. 2003, 79, 309–318. [Google Scholar] [CrossRef]
- Lu, S.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Spychaj, T.; Ubowska, A.; Schmidt, B. Industrially applicable methods of poly(methyl methacrylate)/organophilic montmorillonite nanocomposites preparation: Processes and cast materials characterisation. Appl. Clay Sci. 2014, 97–98, 96–103. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer-nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L. Clay-Containing Polymeric Nanocomposites (Vol. 1); Rapra Technology Limited: Shawbury, UK, 2004. [Google Scholar]
- Alexandre, M.; Dubois, P.; Sun, T.; Garces, J.; Jerome, R. Polyethylene-layered silicate nanocomposites prepared by the polymerisation-filling technique: Synthesis and mechanical properties. Polymer 2002, 43, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Rong, J.; Jing, Z.; Sheng, M. A polyethylene nanocomposite prepared via in situ polymerisation. Macromol. Rapid Commun. 2001, 22, 329–334. [Google Scholar] [CrossRef]
- Heinemann, J.; Reichert, P.; Thomann, R.; Muelhaupt, R. Polyolefin nanocomposites formed by melt compounding and transition metal catalyzed ethene homo- and copolymerisation in the presence of layered silicates. Macromol. Rapid Commun. 1999, 20, 423–430. [Google Scholar] [CrossRef]
- Zhang, J.; Wilkie, C. Preparation and flammability properties of polyethylene-clay nanocomposites. Polym. Degrad. Stab. 2003, 80, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Qin, H.; Gong, F.; Feng, M.; Zhang, S.; Yang, M. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym. Degrad. Stab. 2005, 87, 183–189. [Google Scholar] [CrossRef]
- Lenża, J.; Merkel, K.; Rydarowski, H. Comparison of the effect of montmorillonite, magnesium hydroxide and a mixture of both on the flammability properties and mechanism of char formation of HDPE composites. Polym. Degrad. Stab. 2012, 97, 2581–2593. [Google Scholar] [CrossRef]
- Morawiec, J.; Pawlak, A.; Slouf, M.; Gałęski, A.; Piorkowska, E.; Krasnikowa, N. Preparation and properties of compatibilised LDPE/organo-modified montmorillonite nanocomposites. Eur. Polym. J. 2005, 41, 1115–1122. [Google Scholar] [CrossRef]
- Tomczak, M.; Łopiński, J.; Kowalczyk, K.; Schmidt, B.; Rokicka, J. Vinyl intumescent coatings modified with platelet-type nanofillers. Prog. Org. Coat. 2019, 126, 97–105. [Google Scholar] [CrossRef]
- Tjong, S. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Iyer, R.; Suin, S.; Shrivastava, N.; Maiti, S.; Khatua, B. Compatibilisation mechanism of nanoclay in immiscible PS/PMMA blend using unmodified nanoclay. Polym-Plast. Technol. Eng. 2013, 52, 514–524. [Google Scholar] [CrossRef]
- Lu, F.; Shen, M.; Xue, Y.; Zeng, S.; Chen, S.; Hao, L.; Yang, L. Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites. e-Polymers 2019, 19, 92–102. [Google Scholar] [CrossRef]
- Zhang, J.; Manias, E.; Wilkie, C.A. Polymerically modified layered silicates: An effective route to nanocomposites. J. Nanosci. Nanotechnol. 2008, 8, 1597–1615. [Google Scholar] [CrossRef] [Green Version]
- La Mantia, F.; Ceraulo, M.; Mistretta, M.; Sutera, F.; Ascione, L.; Nasillo, H. Effect of elongational flow and polarity of organomodified clay on morphology and mechanical properties of a PLA based nanobiocomposite. Int. Polym. Proc. 2016, 31, 541–547. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Zare, Y. The roles of nanoparticles accumulation and interphase properties in properties of polymer particulate nanocomposites by a multistep methodology. Compos. A Appl. Sci. Manuf. 2016, 91, 127–132. [Google Scholar] [CrossRef]
- IEC 60502-2:2005-03 Standard, Part 2. Cables for Rated Voltages from 6 kV (Um = 7.2 kV) up to 30 kV (Um = 36 kV); IEC: Geneva, Switzerland, 2005. [Google Scholar]
- Mahmoudian, S.; Wahit, M.; Yussuf, A.; Nematzadeh, N. Preparation and thermal properties of cellulose/layered silicate montmorillonite nanocomposites prepared via ionic liquids. Key Eng. Mater. 2011, 471–472, 786–791. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallisation behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Liang, G.; Xu, J.; Bao, S.; Xu, W. Polyethylene/maleic anhydride grafted polyethylene/organic montmorillonite nanocomposites. I. Preparation, microstructure, and mechanical properties. J. Appl. Polym. Sci. 2010, 91, 3974–3980. [Google Scholar] [CrossRef]
- Çağlayan, T.; Güven, O. Preparation and characterisation of poly(ethylene-vinyl acetate) based nanocomposites using radiation-modified montmorillonite. Radiat. Phys. Chem. 2020, 169, 107844. [Google Scholar] [CrossRef]
- Liu, S. Flame retardant and mechanical properties of polyethylene/ magnesium hydroxide/ montmorillonite nanocomposites. J. Ind. Eng. Chem. 2014, 20, 2401–2408. [Google Scholar] [CrossRef]
- Ara’ujo, E.; Barbosa, R.; Rodrigues, A.; Melo, T.; Ito, E. Processing and characterisation of polyethylene/Brazilian clay nanocomposites. Mater. Sci. Eng. A 2007, 445–446, 141–147. [Google Scholar] [CrossRef]
- Alothman, O. Processing and characterisation of high density polyethylene/ ethylene vinyl acetate blends with different VA contents. Adv. Mater. Sci. Eng. 2012, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mathur, G. Proceedings of Recent Advances in Polymers and Composites; Society for Polymer Science: New Delphi, India, 2000. [Google Scholar]
- Ashraf, M.; Peng, W.; Zare, Y.; Rhee, K. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Querol, S.; Yáñez-Pacios, A.; Martín-Martínez, J. New binary blends of ethylene-co-n-butyl acrylate (EBA) copolymer and low molecular weight rosin ester resin with potential as pressure sensitive adhesives. Materials 2018, 11, 2037. [Google Scholar] [CrossRef] [Green Version]
- Skauge, A.; Fuller, N.; Hepler, L. Specific heats of clay minerals: Sodium and calcium kaolinites, sodium and calcium montmorillonites, illite, and atiapulgite. Thermochim. Acta 1983, 61, 139–145. [Google Scholar] [CrossRef]
- Zazoum, B.; David, E.; Ngô, A. LDPE/HDPE/clay nanocomposites: Effects of compatibilizer on the structure and dielectric response. J. Nanotechnol. 2013, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A. Plastic properties and testing. In Introduction to Plastics Engineering; Elsevier: Chennai, India, 2018. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Sultan, B.; Sörvik, E. Thermal degradation of EVA and EBA—A comparison. II. Changes in unsaturation and side group structure. J. Appl. Polym. Sci. 1991, 43, 1747–1759. [Google Scholar] [CrossRef]
- Ray, S.; Okamoto, M. Polymer/ layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Attharangsan, S.; Saikrasun, S. Comparative performance of carbon nanotube and organo montmorillonite as a thermo-oxidative-stabilizing modifier for polypropylene- and polyethylene-based thermoplastic composites. J. Thermoplas. Compos. Mater. 2015, 28, 1423–1444. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J. Polymer/montmorillonite nanocomposites with improved thermal properties. Part 1. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta 2007, 453, 75–96. [Google Scholar] [CrossRef] [Green Version]

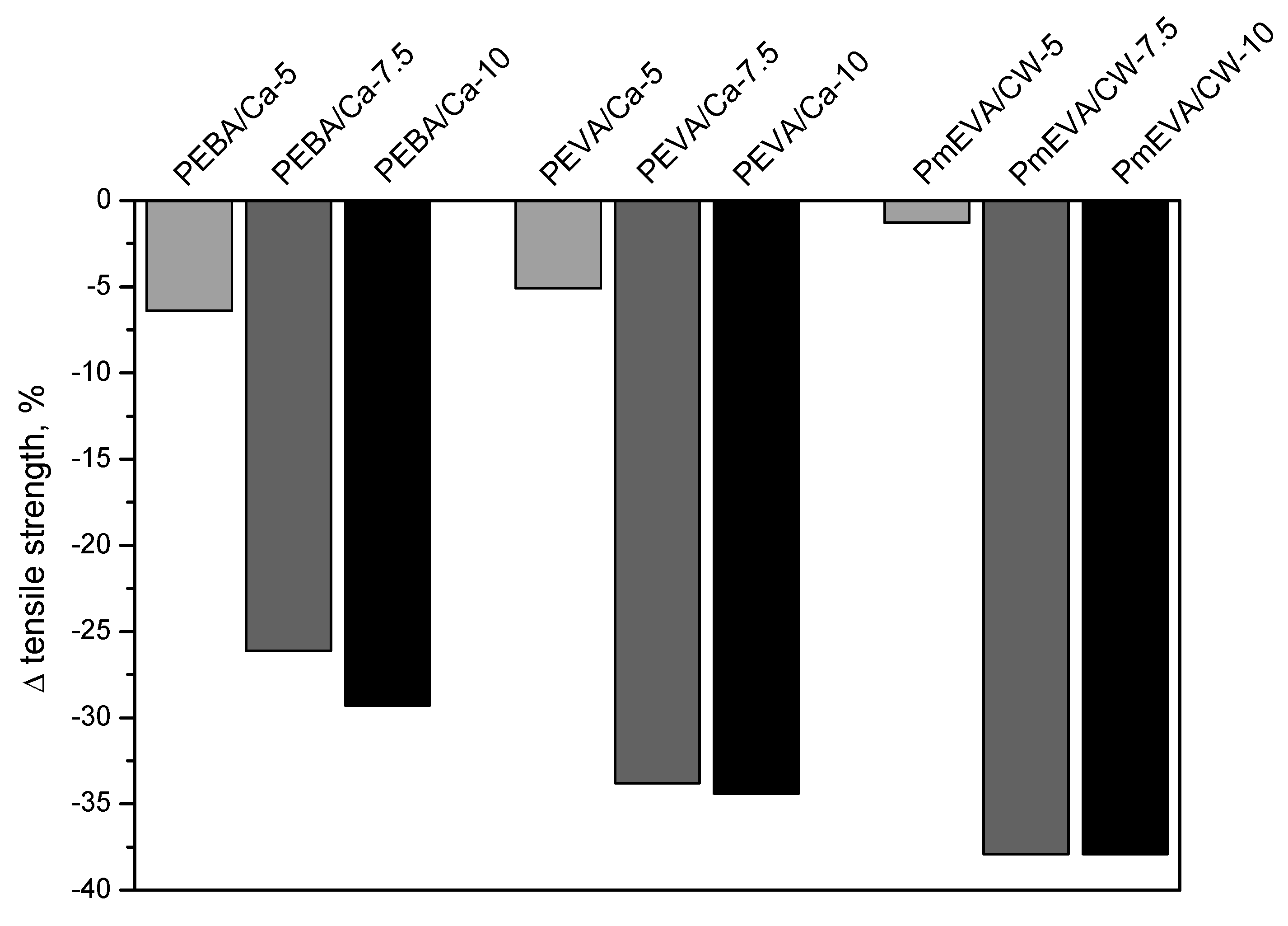
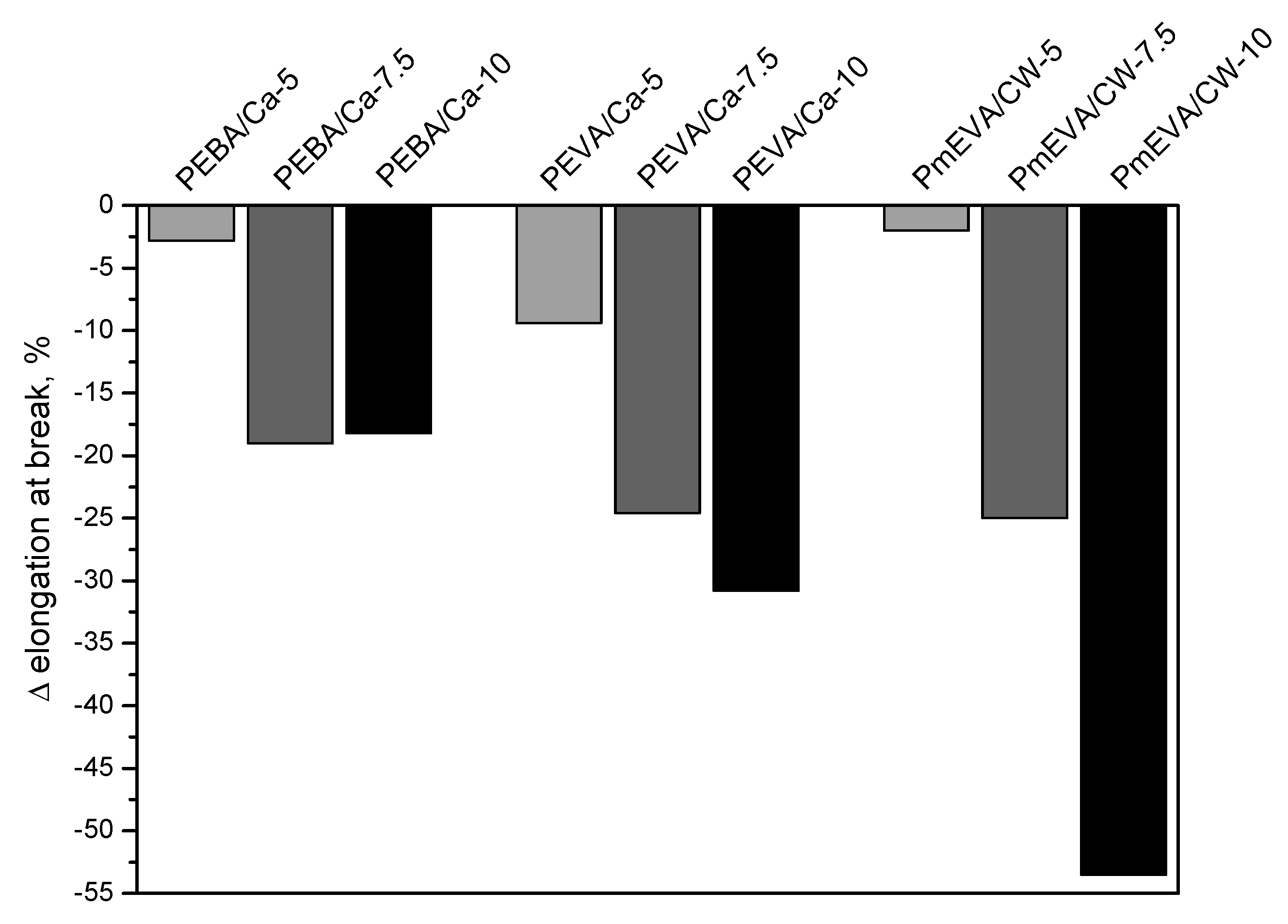
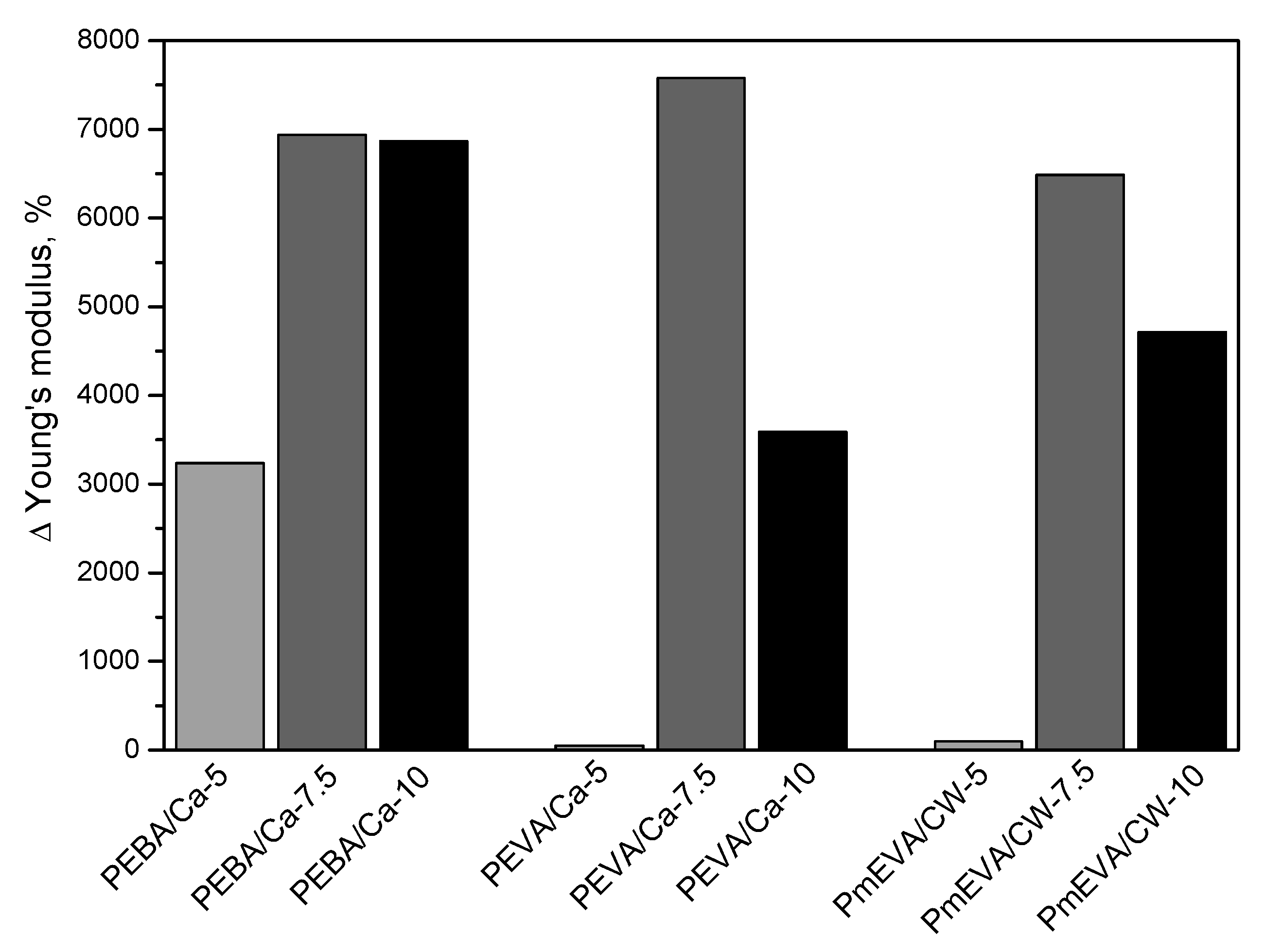
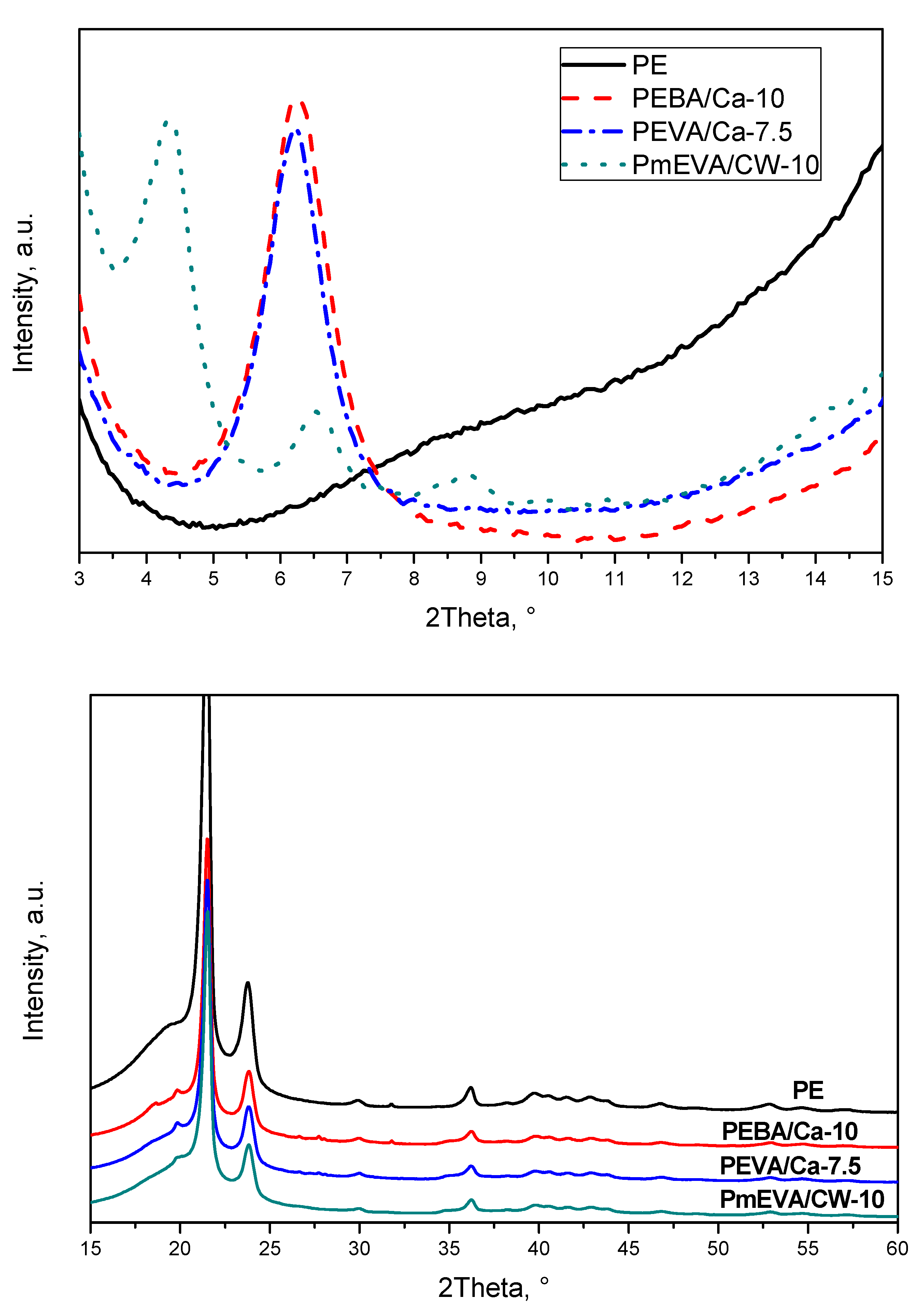



| Sample Symbol | Dispersing Agent | MMT Type | MMT Content a | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|
| PE | — | — | 0 | 15.7 ± 0.5 | 785 ± 85 | 273 ± 55 |
| PEBA/CW-5 | EBA | CW9 | 5 | 10.6 ± 0.8 | 578 ± 84 | 4677 ± 350 |
| PEBA/Ca-5 | EBA | C–Ca | 5 | 14.7 ± 2.4 | 763 ± 58 | 9093 ± 681 |
| PEBA/Ca-7.5 | EBA | C–Ca | 7.5 | 11.6 ± 1.7 | 636 ± 127 | 19,207 ± 195 |
| PEBA/Ca-10 | EBA | C–Ca | 10 | 11.1 ± 1.3 | 642 ± 23 | 19,022 ± 981 |
| PEVA/CW-5 | EVA | CW9 | 5 | 9.7 ± 0.3 | 116 ± 75 | 284 ± 189 |
| PEVA/Ca-5 | EVA | C–Ca | 5 | 14.9 ± 0.4 | 711 ± 26 | 279 ± 44 |
| PEVA/Ca-7.5 | EVA | C–Ca | 7.5 | 10.4 ± 0.7 | 592 ± 83 | 20,964 ± 410 |
| PEVA/Ca-10 | EVA | C–Ca | 10 | 10.3 ± 1.1 | 543 ± 232 | 10,068 ± 792 |
| PmEVA/Ca-5 | m-EVA | C–Ca | 5 | 10.9 ± 0.9 | 606 ± 16 | 221 ± 27 |
| PmEVA/CW-5 | m-EVA | CW9 | 5 | 15.5 ± 0.5 | 769 ± 46 | 347 ± 86 |
| PmEVA/CW-7.5 | m-EVA | CW9 | 7.5 | 9.9 ± 0.3 | 589 ± 122 | 17,982 ± 412 |
| PmEVA/CW-10 | m-EVA | CW9 | 10 | 9.9 ± 0.2 | 365 ± 130 | 13,139 ± 625 |
| Sample Symbol | Hardness (°ShD) | TGA Results | Oxygen Index (% O2) | ||
|---|---|---|---|---|---|
| T10 (°C) a | T50 (°C) a | CR (wt. %) b | |||
| PE | 38 ± 2 | 363 | 413 | 0 | 18.0 |
| PEBA/Ca-5 | 36 ± 3 | 354 | 434 | 4.3 | 21.0 |
| PEBA/Ca-7.5 | 38 ± 2 | 365 | 448 | 5.3 | 21.5 |
| PEBA/Ca-10 | 36 ± 2 | 386 | 452 | 7.6 | 21.5 |
| PEVA/Ca-5 | 37 ± 1 | 378 | 441 | 4.6 | 21.5 |
| PEVA/Ca-7.5 | 37 ± 2 | 393 | 448 | 5.6 | 22.0 |
| PEVA/Ca-10 | 38± 2 | 361 | 435 | 7.9 | 21.5 |
| PmEVA/CW-5 | 38 ± 2 | 404 | 456 | 3.1 | 21.5 |
| PmEVA/CW-7.5 | 36 ± 1 | 421 | 461 | 4.4 | 21.8 |
| PmEVA/CW-10 | 36 ± 2 | 392 | 460 | 5.2 | 22.2 |
| Sample Symbol | Heating | Cooling | ||||
|---|---|---|---|---|---|---|
| TH-0 (°C) a | TH-max (°C) b | QH (J/g) c | TC-0 (°C) a | TC-max (°C) b | QC (J/g) c | |
| PE | 39.7 | 123.9 | 116.1 | 110.8 | 103.9 | 110.0 |
| PEBA/Ca-5 | 42.1 | 123.4 | 107.2 | 112.2 | 106.0 | 103.7 |
| PEBA/Ca-7.5 | 42.9 | 123.6 | 99.9 | 112.7 | 106.3 | 96.3 |
| PEBA/Ca-10 | 38.6 | 123.8 | 95.7 | 113.5 | 106.6 | 89.7 |
| PEVA/Ca-5 | 47.4 | 123.1 | 95.0 | 111.6 | 105.1 | 98.3 |
| PEVA/Ca-7.5 | 46.4 | 123.8 | 92.2 | 111.4 | 104.3 | 91.1 |
| PEVA/Ca-10 | 41.4 | 122.7 | 87.9 | 111.1 | 105.3 | 87.4 |
| PmEVA/CW-5 | 45.8 | 123.5 | 101.5 | 114.3 | 103.9 | 98.0 |
| PmEVA/CW-7.5 | 41.3 | 123.7 | 97.0 | 113.5 | 103.6 | 93.8 |
| PmEVA/CW-10 | 38.6 | 124.2 | 95.6 | 112.2 | 104.6 | 93.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, S.; Kowalczyk, K.; Paszkiewicz, S.; Figiel, P.; Piesowicz, E. Green Highly Clay-Filled Polyethylene Composites as Coating Materials for Cable Industry—A New Application Route of Non-Organophilised Natural Montmorillonites in Polymeric Materials. Polymers 2020, 12, 1399. https://doi.org/10.3390/polym12061399
Wysocki S, Kowalczyk K, Paszkiewicz S, Figiel P, Piesowicz E. Green Highly Clay-Filled Polyethylene Composites as Coating Materials for Cable Industry—A New Application Route of Non-Organophilised Natural Montmorillonites in Polymeric Materials. Polymers. 2020; 12(6):1399. https://doi.org/10.3390/polym12061399
Chicago/Turabian StyleWysocki, Stanisław, Krzysztof Kowalczyk, Sandra Paszkiewicz, Paweł Figiel, and Elżbieta Piesowicz. 2020. "Green Highly Clay-Filled Polyethylene Composites as Coating Materials for Cable Industry—A New Application Route of Non-Organophilised Natural Montmorillonites in Polymeric Materials" Polymers 12, no. 6: 1399. https://doi.org/10.3390/polym12061399






