Chemoenzymatic Synthesis of D-Glucitol-Based Non-Ionic Amphiphilic Architectures as Nanocarriers
Abstract
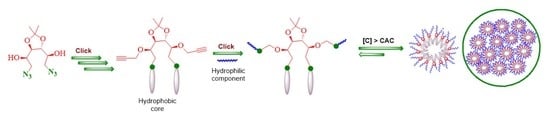
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Methods
2.2.1. NMR, IR Spectroscopy, and GPC Analysis
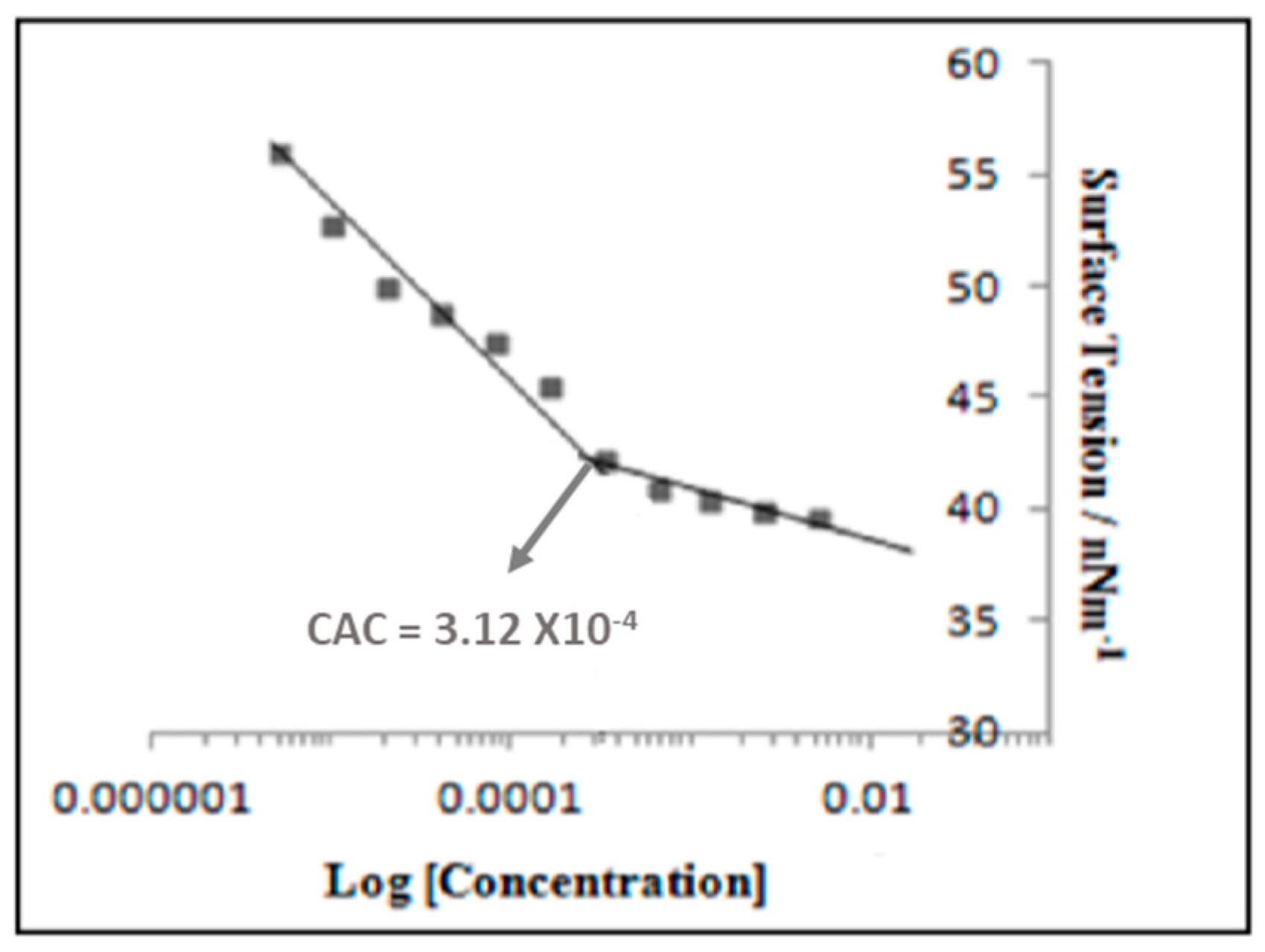
2.2.2. Critical Aggregation Concentration (CAC)
2.2.3. Dynamic Light Scattering (DLS) Measurement
2.2.4. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.2.5. Guest Encapsulation and Quantification
2.2.6. Cytotoxicity Study
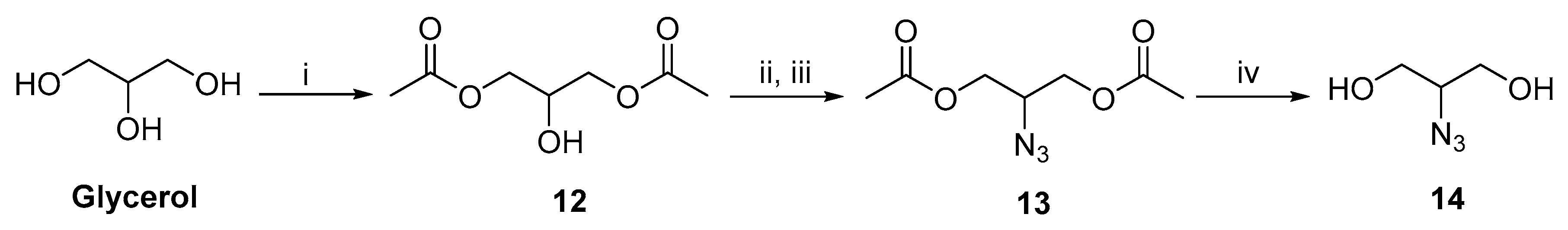
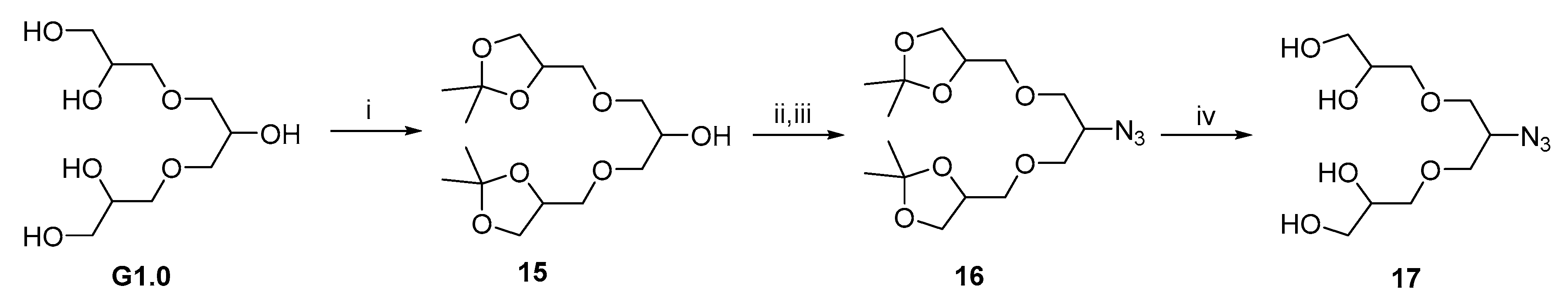
2.3. Synthetic Procedures for Hydrophobic Backbone and Amphiphilic Architectures
2.3.1. D-Glucitol Tris-acetonide (2)
2.3.2. D-Glucitol-3,4-Acetonide (3)
2.3.3. D-Glucitol-3,4-Diacetonide-5,6-Diazide (5)
2.3.4. Compound 6
2.3.5. 1-(Prop-2-yn-1-yloxy)naphthalene 9
2.3.6. Compound 10
2.4. General Procedure for Synthesis of Compounds 7 and 11
2.5. General Procedure for the Synthesis of Amphiphiles (20–25)
2.5.1. Synthesis of Amphiphile 20
2.5.2. Synthesis of Amphiphile 21
2.5.3. Synthesis of Amphiphile 22
2.5.4. Synthesis of Amphiphile 23
2.5.5. Synthesis of Amphiphile 24
2.5.6. Synthesis of Amphiphile 25
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Critical Aggregation Concentration (CAC) Measurement
3.3. Hydrophilic–Lipophilic Balance Determination
3.4. Dynamic Light Scattering and Cryo-TEM Analysis
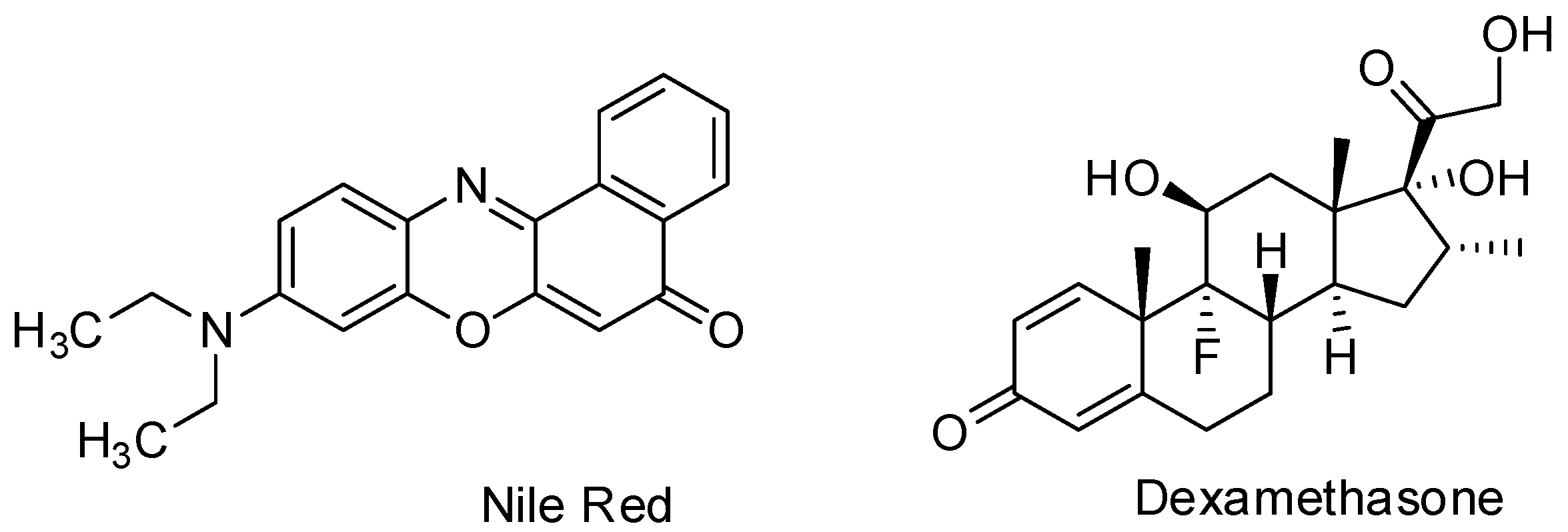
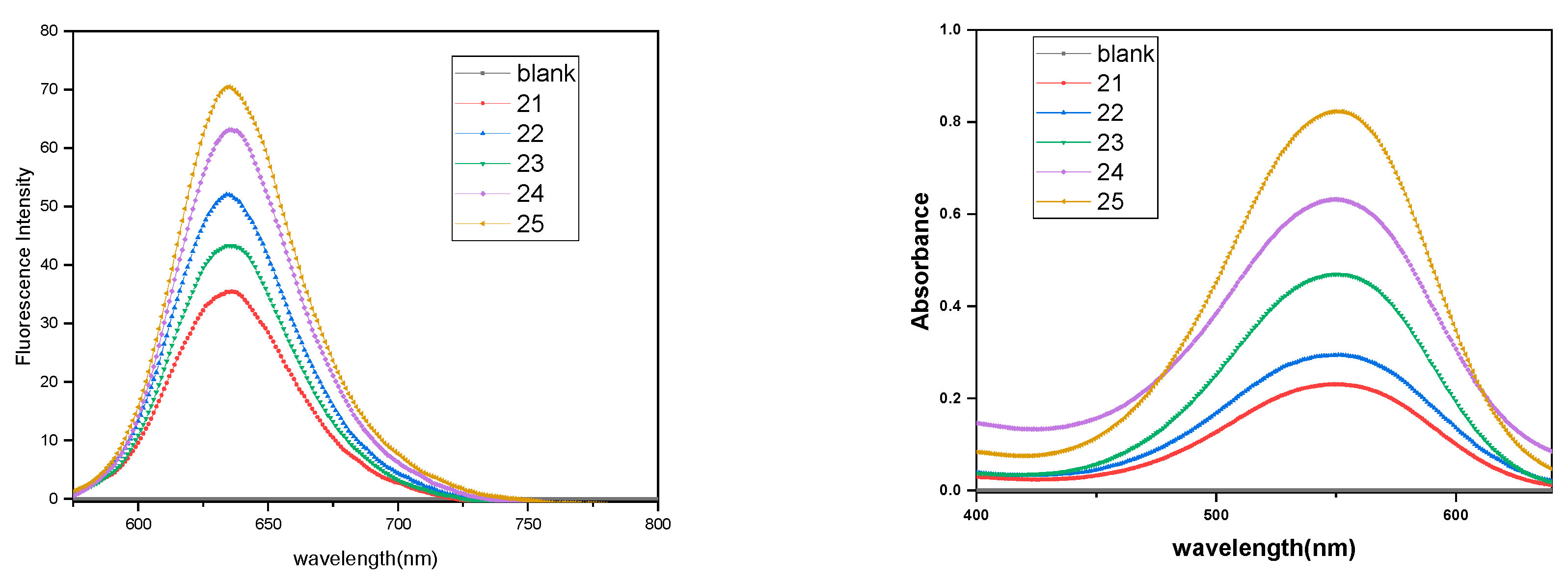
3.5. Encapsulation Study
3.5.1. Nile Red Encapsulation Study
3.5.2. Dexamethasone Encapsulation
3.6. Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korolkov, V.V.; Allen, S.; Roberts, C.J. Faraday Discuss. Surface mediated L-phenylalanyl-Lphenylalanine assembly into large dendritic structures. Faraday Discuss. 2013, 166, 257–267. [Google Scholar] [CrossRef]
- Boncheva, M.; Whitesides, G.M. Making things by self-assembly. MRS Bull. 2005, 30, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Thota, B.N.S.; Urner, L.H.; Haag, R. Supramolecular architectures of dendritic amphiphiles in water. Chem. Rev. 2015, 116, 2079–2102. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of two organic dyes by cationic ester-containing gemini surfactants. J. Colloid Interface Sci. 2012, 376, 112–118. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation behaviour of gemini surfactants and their interaction with macromolecules in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Bhat, P.A.; Dar, A.A.; Rather, G.M. Solubilization Capabilities of some cationic, anionic, and nonionic surfactants toward the poorly water-soluble antibiotic drug erythromycin. J. Chem. Eng. Data 2008, 53, 1271–1277. [Google Scholar] [CrossRef]
- Thota, B.N.S.; Berlepsch, H.V.; Böttcher, C.; Haag, R. Towards engineering of self-assembled nanostructures using non-ionic dendritic amphiphiles. ChemComm 2015, 51, 8648–8651. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Tyagi, R.; Parmar, V.S.; Sharma, S.K.; Haag, R. Polyether based amphiphiles for delivery of active components. Polymer 2012, 53, 3053–3078. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Thota, B.N.S.; Schade, B.; Achazi, K.; Khan, A.; Böttcher, C.; Sharma, S.K.; Haag, R. Aggregation behavior of non-ionic twinned amphiphiles and their application as biomedical nanocarriers. Chem. Asian J. 2017, 12, 1796–1806. [Google Scholar] [CrossRef]
- Atwood, J.L.; Steed, J.W. Encyclopedia of Supramolecular Chemistry; CRC Press: New York, NY, USA, 2004; Volume 2. [Google Scholar]
- Khandare, J.; Calderón, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C. Supramolecular amphiphiles. Chem. Soc. Rev. 2011, 40, 94–101. [Google Scholar] [CrossRef]
- Malmsten, M. Soft drug delivery systems. Soft Matter 2006, 2, 760–769. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-Molecular-weight gelators as base materials for ointments. Gels 2016, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, R. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2020; p. 40. [Google Scholar]
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes consisting of tween-60 and cholesterol improve the chemical stability and antioxidant activity of (−)-epigallocatechin gallate under intestinal tract conditions. J. Agric. Food Chem. 2016, 64, 9180–9188. [Google Scholar] [CrossRef]
- Yang, T.; Cui, F.D.; Choi, M.K.; Cho, J.W.; Chung, S.J.; Shim, C.K.; Kim, D.D. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. 2007, 338, 317–326. [Google Scholar] [CrossRef]
- Fan, K.; Yang, J.; Wang, X.; Song, J. Rational construction of gel-based supramolecular logic gates by using a functional gelator with multiple-stimuli responsive properties. Soft Matter 2014, 10, 8370–8375. [Google Scholar] [CrossRef]
- Fan, K.; Song, J.; Li, J.; Guan, X.; Tao, N.; Tong, C.; Shen, H.; Niu, L. Copper(II)-responsive gel–sol phase transition in supramolecular gel systems of salen-appended sorbitol. J. Mater. Chem. C 2013, 1, 7479–7482. [Google Scholar] [CrossRef]
- Okesola, B.O.; Suravaram, S.K.; Parkin, A.; Smith, D.K. Selective extraction and in situ reduction of precious metal salts from model waste to generate hybrid gels with embedded electrocatalytic nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 183–187. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self—Assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.; Kong, H.; Wang, X.; Yang, X.; Song, J. Tunable self-assembly of two-component gels from novel sorbitol-appended compounds. RSC Adv. 2016, 6, 80934–80938. [Google Scholar] [CrossRef]
- Schuur, B.; Wagenaar, A.; Heeres, A.; Heeres, E.H.J. A synthetic strategy for novel nonsymmetrical bola amphiphiles based on carbohydrates. Carbohydr. Res. 2004, 339, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, A.; Engberts, J.B.F.N. Synthesis of nonionic reduced-sugar based bola amphiphiles and gemini surfactants with an α, ω-diamino-(oxa)alkyl spacer. Tetrahedron 2007, 63, 10622–10629. [Google Scholar] [CrossRef]
- Zhu, Y.; Durand, M.; Molinier, V.; Aubry, J.M. Isosorbide as a novel polar head derived from renewable resources. Application to the design of short-chain amphiphiles with hydrotropic properties. Green Chem. 2008, 10, 532–540. [Google Scholar] [CrossRef]
- Borisch, K.; Diele, S.; Goring, P.; Muller, H.; Tschierske, C. Amphiphilic N-benzoyl-1-amino-1-deoxy-Dglucitol derivatives forming thermotropic lamellar, columnar and different types of cubic mesophases. Liq. Cryst. 1997, 22, 427–443. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.; Achazi, K.; Böttcher, C.; Khandare, J.; Sharma, S.K.; Haag, R. Dendronized multifunctional amphiphilic polymers as Efficient nanocarriers for biomedical applications. Macromol. Rapid Commun. 2015, 36, 254–261. [Google Scholar] [CrossRef]
- Onorato, A.; Pavlik, C.; Invernale, M.A.; Berghorn, I.D.; Sotzing, G.A.; Morton, M.D.; Smith, M.B. Polymer-mediated cyclodehydration of alditols and ketohexoses. Carbohydr. Res. 2011, 346, 1662–1670. [Google Scholar] [CrossRef]
- De Oliveira, P.S.M.; Ferreira, V.F.; de Souza, M.V.N.; de Carvalho, E.M. Química Synthesis of aminoalcohols from D-mannitol. Nova 2008, 31, 776–780. [Google Scholar]
- Arya, A.; Mathur, D.; Tyagi, A.; Kumar, R.; Kumar, V.; Olsen, C.E.; Saxena, R.K.; Prasad, A.K. Chemoenzymatic Synthesis of 3′-Deoxy-3′-(4-Substituted-Triazol-1-YL)-5-Methyluridine. Nucleos. Nucleot. Nucl. 2013, 32, 646–659. [Google Scholar] [CrossRef]
- Gupta, S.; Schade, B.; Kumar, S.; Böttcher, C.; Sharma, S.K.; Haag, R. Non-ionic dendronized multiamphiphilic polymers as nanocarriers for biomedical applications. R. Small 2013, 9, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, M.; Haag, R. A Convergent approach to biocompatible polyglycerol “click” dendrons for the synthesis of modular core–shell architectures and their transport behavior. Chem. Eur. J. 2008, 14, 9202–9214. [Google Scholar] [CrossRef] [PubMed]
- Van Eldijk, M.B.; Smits, F.C.; Vermue, N.; Debets, M.F.; Schoffelen, S.; Van Hest, J.C. Synthesis and self-assembly of well-defined elastin-like polypeptide−poly(ethylene glycol) conjugates. Biomacromolecules 2014, 15, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C.J. Calculations of HLB values of non-ionic surfactants. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Fleige, E.; Ziem, B.; Grabolle, M.; Haag, R.; Resch-Genger, U. Aggregation phenomena of host and guest upon the loading of dendritic core-multishell nanoparticles with solvatochromic dyes. Macromolecules 2012, 45, 9452–9459. [Google Scholar] [CrossRef]



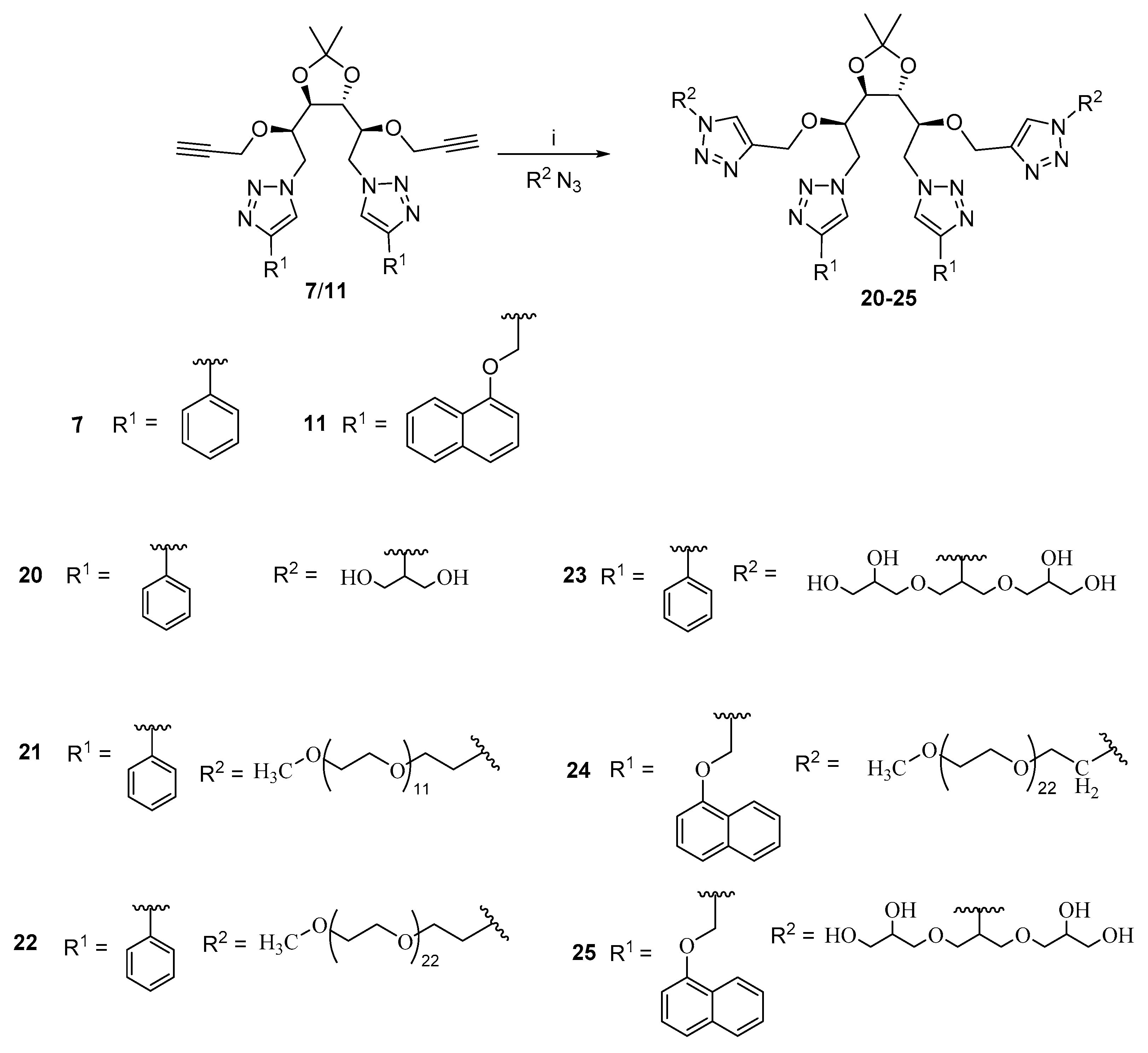




| Amphiphile | CAC (M) |
|---|---|
| 21 | 3.75 × 10−3 |
| 22 | 9.25 × 10−4 |
| 23 | 6.57 × 10−4 |
| 24 | 3.12 × 10−4 |
| 25 | 6.25 × 10−4 |
| Amphiphile | Composition | HLB | |
|---|---|---|---|
| R1 | R2 | ||
| 20 |  |  | 7.83 |
| 21 | 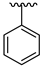 | 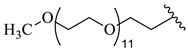 | 14.49 |
| 22 | 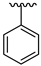 | 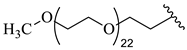 | 16.62 |
| 23 | 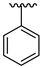 | 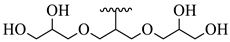 | 11.16 |
| 24 | 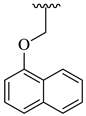 | 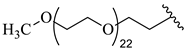 | 15.74 |
| 25 | 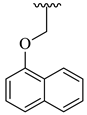 | 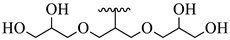 | 9.72 |
| Amphiphile | DLS (Dh, nm) | ||
|---|---|---|---|
| I | V | N | |
| 21 | 147 | 93 | 51 |
| 22 | 138 | 75 | 39 |
| 23 | 94 | 46 | 30 |
| 24 | 15 279 | 12 | 10 |
| 25 | 6 166 | 5 | 4 |
| Amphiphile | Loading Capacity (Guest/Amphiphile) (mmol/mol) | Loading Efficiency (Guest/Amphiphile) (mg/g) | ||
|---|---|---|---|---|
| Nile red | Dexamethasone | Nile Red | Dexamethasone | |
| 21 | 3.46 | 18.25 | 0.64 | 4.16 |
| 22 | 6.53 | 50.91 | 0.8 | 7.68 |
| 23 | 4.48 | 23.84 | 1.32 | 8.64 |
| 24 | 15.44 | 103.59 | 1.78 | 14.72 |
| 25 | 27.2 | 17.22 | 6.97 | 5.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchanda, P.; Achazi, K.; Verma, D.; Böttcher, C.; Haag, R.; Sharma, S.K. Chemoenzymatic Synthesis of D-Glucitol-Based Non-Ionic Amphiphilic Architectures as Nanocarriers. Polymers 2020, 12, 1421. https://doi.org/10.3390/polym12061421
Manchanda P, Achazi K, Verma D, Böttcher C, Haag R, Sharma SK. Chemoenzymatic Synthesis of D-Glucitol-Based Non-Ionic Amphiphilic Architectures as Nanocarriers. Polymers. 2020; 12(6):1421. https://doi.org/10.3390/polym12061421
Chicago/Turabian StyleManchanda, Priyanka, Katharina Achazi, Diksha Verma, Christoph Böttcher, Rainer Haag, and Sunil K. Sharma. 2020. "Chemoenzymatic Synthesis of D-Glucitol-Based Non-Ionic Amphiphilic Architectures as Nanocarriers" Polymers 12, no. 6: 1421. https://doi.org/10.3390/polym12061421