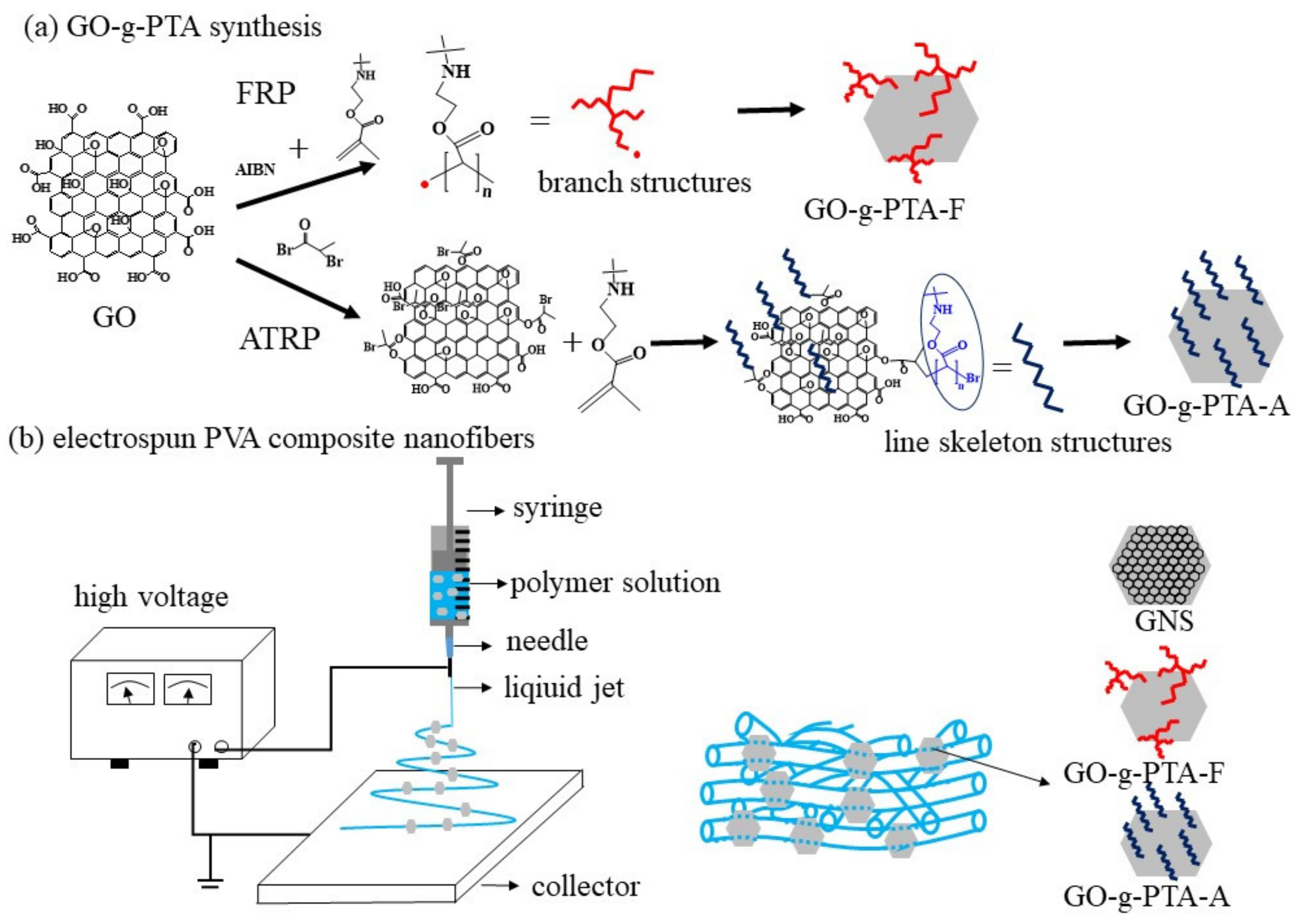
3.1. Filler Characterization
The morphologies of GNS, GO, GO-
g-PTA-F, and GO-
g-PTA-A were characterized by TEM.
Figure 1a shows the deposited GNS structure on the TEM grid prepared from o-DCB solution. GNS was transparent with folding and wrinkling. More than 150 measurements of long and short axes were obtained for GNS, GO, GO-
g-PTA-F, and GO-
g-PTA-A to determine the average long and short axes. The number-average long and short axis lengths were 6.36 ± 2.15 μm and 3.00 ± 1.22 μm, respectively.
Figure 1b,c, and d illustrate the structures of the deposited GO, GO-
g-PTA-F, and GO-
g-PTA-A, respectively, on the TEM grid prepared from the methanol solution. GO appeared transparent, with folding and wrinkling and small amounts of graphene stuck together. The number-average lengths of long and short axes of GO were 2.75 ± 0.94 μm and 1.40 ± 0.88 μm, respectively. GO-
g-PTA-F showed that the GO surface was randomly decorated with PTA wormlike clusters. This observation indicated that PTA was attached onto the GO surface. The number-average lengths of the long and short axes of GO-g-PTA-F were 2.13 ± 1.08 μm and 1.42 ± 0.89 μm, respectively. GO-
g-PTA-A showed that the GO surface was coated with a rough PTA layer. This observation indicated that the PTA brushes grew from the GO surface. The number-average lengths of the long and short axes of GO-
g-PTA-A were 1.63 ± 1.07 μm and 0.91 ± 0.62 μm, respectively. The morphology of GO-
g-PTA-F was different from that of GO-
g-PTA-A. This result indicated that the morphologies of polymer-grafted GO would be different for different grafting methods.
Figure 2 shows the AFM image of GO, GO-
g-PTA-F, and GO-
g-PTA-A, as well as their height profile. The samples were prepared by depositing a drop of GNS/o-DCB solution onto mica, followed by drying. The thickness of GNS, GO-
g-PTA-F, and GO-
g-PTA-A for flat regions was approximately 7.2, 18, and 14 nm, respectively. The height analysis of the minimum GO thickness was measured on 20 different sheets. The determined average minimum thickness of GO, GO-
g-PTA-F, and GO-
g-PTA-A was 7.4 ± 2.4, 36.7 ± 13.2, and 11.0 ± 3.1 nm, respectively. The average thickness of GO was smaller than that of GO-
g-PTA-F, and GO-
g-PTA-A. This finding indicated that the PTA was grafted on the GO surface.
Figure 3a shows the TGA curves of PTA, GO, GO-
g-PTA-F, GO-
g-PTA-A, and GNS in nitrogen atmospheres at 10 °C/min. The 5% thermal degradation in nitrogen atmosphere of PTA, GO, GO-
g-PTA-F, GO-
g-PTA-A, and GNS began at 251.4, 50.6, 161.3, 181.6, and 623.9 °C, respectively. Moreover, the residue of neat PTA, GO, GO-
g-PTA-F, GO-
g-PTA-A, and GNS left at 800 °C was 2.69, 40.5, 20.4, 21.5, and 91.6 wt %, respectively. According to the literature [
31,
32], the weight loss of GO between 25 and 100 °C in the TGA plots resulted from water absorption on the oxygen functional groups of GO, and the weight loss between 150 and 260 °C was due to the loss of oxygen functional groups. The residue of GNS was high, thereby indicating that less oxygen functional groups were available on the GNS surfaces. For PTA, two-step degradation was observed. The weight loss between 250 and 330 °C resulted from the loss of (tert-butylamino)ethyl side groups, and the weight loss between 390 and 440 °C corresponded to polymer main chain scission [
37]. Similar degradation was also observed in GO-
g-PTA-F and GO-
g-PTA-A with increased temperature. The first sharp transitions for GO-
g-PTA-F and GO-
g-PTA-A were detected at 170–280 and 200–290 °C, respectively, and the second sharp transitions for GO-
g-PTA-F and GO-
g-PTA-A were detected at 340–430 and 360–430 °C, respectively.
Figure 3b shows the FTIR spectra of PTA, GO, GO-
g-PTA-F, and GO-
g-PTA-A. For GO, a broad new peak at approximately 3600 to 3000 cm
−1 was attributed to O–H stretching vibration. In addition, the peak intensity at 1740 and 1662 cm
−1 was assigned to C=O in quinone [
38]. The peak at approximately 1610 to 1580 cm
−1 was assigned to C=C from unoxidized graphite domains. The peaks at 1369, 1220, and 1122 cm
−1 were assigned to the stretching vibrations from C–OH, epoxy, and C=O, respectively [
39,
40,
41]. When GO was grafted with PTA via FRP, the absorbance bands at 3000–2800 cm
−1 were detected with the asymmetric stretching of the alkyl groups (C–H of CH
3 and CH
2). Moreover, the absorbance bands at 1230 and1150 cm
−1 were assigned to C–N bending signals of PTA [
37,
42]. Moreover, similar FTIR results were obtained in the GO-g-PTA-A powders.
The Raman spectra of GO, GO-
g-PTA-F, and GO-
g-PTA-A are shown in
Figure 3c. The well-known
G band at approximately 1583 cm
−1 and
D band at approximately 1350 cm
−1 suggested the characteristic sp
2 bonds in graphene and sp
3 bonds caused by oxidation, respectively, [
43,
44].
ID/
IG reveals the structural information of a carbon material with different ratio of sp
3/sp
2, where
ID is the intensity area of
D band, and
IG is the intensity area of
G band. The
ID/
IG value of GO was 2.3. By contrast, the
ID/
IG value of GO-
g-PTA-F and GO-
g-PTA-A was 2.8 and 2.5, respectively. The
ID/
IG value of GO-
g-PTA-F was significantly higher than those of GO and GO-
g-PTA-A, because the PTA growing radical was added to the double bonds of GO.
Figure 3d shows the XPS spectra of GO, GO-
g-PTA-F, and GO-
g-PTA-A. C 1s at ~285 eV and O 1s at 532 eV signals were observed for GO, GO-
g-PTA-F, and GO-
g-PTA-A. By contrast, N 1s at ~399 eV signal was only observed for GO-
g-PTA-F and GO-
g-PTA-A samples. These findings indicated that PTA could be successfully grafted on the GO surface via FRP and ATRP. The carbon, oxygen, and nitrogen contents in GO, GO-
g-PTA-F, and GO-
g-PTA-A were determined, as shown in
Table 1. The oxygen content of GNS, GO, GO-
g-PTA-F, and GO-
g-PTA-A were approximately 4.0, 26.3, 18.1, and 17.7 at %, respectively. Because the GNSs were obtained through GO thermal reduction, a small amount of oxygen functional groups was still in the GNS. Moreover, the nitrogen contents of GO, GO-g-PTA-F, and GO-g-PTA-A were 0.4, 3.4, and 3.0 at %, respectively. A very small amount of nitrogen was detected in GO, which indicated a small amount of impurity in the precursor, natural graphite powder. Based on the nitrogen content of the XPS results, the PTA percentage for GO-
g-PTA-F and GO-
g-PTA-A was 25.7 and 22.3 wt %, respectively.
Figure S1 (Supplementary Materials) also shows the high magnification C 1s spectra of GO, GO-
g-PTA-F, and GO-
g-PTA-A. The C 1s spectra of GO could be fitted into four fitted curves, which were assigned to C–C (sp
2 and sp
3), C–O, C=O, and O=C–O at 284.5, 286.4, 287.2, and 288.8 eV, respectively [
45,
46,
47]. In addition, the C 1s spectra of GO-g-PTA-F and GO-
g-PTA-A could be fitted into five fitted curves, which were assigned to C–C (sp
2 and sp
3), C–O, C=O, O=C–O, and C–N, at 284.5, 286.4, 287.2, 288.8, and 285.6 eV, respectively [
25,
48]. The relative intensity of the C–N peak of GO-
g-PTA-F and GO-
g-PTA-A samples significantly increased, and the relative intensity of the C–O peak of GO-
g-PTA-F and GO-
g-PTA-A samples decreased. The relative intensity of the C–O peak of GO-
g-PTA-F decreased more than that of GO-
g-PTA-A. This finding suggested that the loss of epoxy groups of GO-
g-PTA-F was higher than that of GO-
g-PTA-A. Therefore, for GO-
g-PTA-F, the PTA growing radical was not only added to the double bonds of GO but also to the epoxy groups of GO.
To realize the antimicrobial activity of GNS, GO-
g-PTA-F, and GO-
g-PTA-A, a viable cell-counting method was also utilized to evaluate the antimicrobial activity of GNS, GO-
g-PTA-F, and GO-
g-PTA-A. The method obtained the MBC values for the tested biocidal agents or fillers against various bacteria. MBC is the minimum concentration of tested biocidal agents or fillers in bacteria medica at which 99.9% of the initial bacterial colony is killed [
49]. Because of difference in the cell structure of bacteria, such as the Gram-positive bacteria,
S. aureus, and the Gram-negative bacteria,
E. coli, the antimicrobial activities of GNS, GO-
g-PTA-F, and GO-
g-PTA-A may be expected to differ among different bacterial species. The most common bacteria found in chronic wounds is
S. aureus [
50,
51,
52]. Moreover, PTA has shown better antimicrobial ability against
S. aureus than against
E. coli [
30]. Thus, in this study, we selected
S. aureus to examine the antimicrobial activity of GO-
g-PTA. GNS, GO, GO-
g-PTA-F, and GO-
g-PTA-A were added to
S. aureus-containing media.
Figure 4 shows the CFU/mL versus the concentration of GNS, GO, GO-
g-PTA-F, and GO-
g-PTA-A against
S. aureus. The number of bacterial colonies of GO-
g-PTA-A against
S. aureus was 0 CFU/mL at a GO-
g-PTA-A concentration of 8 mg/mL. These results indicated that all the initial
S. aureus colonies were killed by GO-
g-PTA-A at 8 mg/mL. Thus, the MBC value of GO-
g-PTA-A was 8 mg/mL. However, the number of bacterial colonies of GNS, GO, and GO-
g-PTA-F at 8 mg/mL against
S. aureus was 5.57 × 10
7, 2.90 × 10
8, and 1.14 × 10
8 CFU/mL, respectively. Notably, despite the increase in the filler concentrations to 10 mg/mL, the number of bacterial colonies of GNS, GO, and GO-
g-PTA-F against
S. aureus was only reduced to 4.50 × 10
6, 2.26 × 10
8, and 7.24 × 10
7 CFU/mL. This result implied that GNS, GO, and GO-
g-PTA-F were unable to completely kill the initial bacterial colonies but effectively inhibited the growth of
S. aureus. GO-
g-PTA-A had higher biocidal efficacy against
S. aureus than GNS, GO, and GO-
g-PTA-F. The PTA polymer structures of GO-
g-PTA-A and GO-
g-PTA-F were different because GO-
g-PTA-A and GO-
g-PTA-F had different grafted method on the GO surface. The grafted PTA polymer structures of GO-
g-PTA-F are branch structures along the polymer backbone, whereas the grafted PTA polymer structures of GO-
g-PTA-A are line skeleton structures. Therefore, although GO-
g-PTA-F and GO-
g-PTA-A have the same chemical monomer structure, the different polymer grafting method caused the different grafted PTA polymer structures and surface morphologies of GO-
g-PTA-F and GO-
g-PTA-A. The PTA polymer of GO-
g-PTA-A were uniformly grafted on the GO surface compared with that of GO-
g-PTA-F. Moreover, the cations of the line skeleton structure of PTA had higher probability to contact bacterial than that of the branch structures of PTA. These findings further affected the antimicrobial activity of GO-
g-PTA-F and GO-
g-PTA-A.
3.2. Effect of Filler Concentration on Electrospinning and As-Spun Fiber Morphology
Different amounts of GNS, GO-
g-PTA-F, and GO-
g-PTA-A were added into the 7 wt % PVA solution, which was used to obtain smooth PVA nanofibers via electrospinning. The details of this observation are discussed in the following section.
Figure 5 shows the conductivity of the PVA solution filled with GNS, GO-
g-PTA-F, and GO-
g-PTA-A. For the neat PVA solution, the measured
κ was 0.92 mS/cm. Given that GNSs are conductive fillers, adding GNSs into the PVA solutions significantly improved
κ values. After adding 10 wt % GNSs to the PVA solution, the measured
κ is 0.99 mS/cm, which was higher than that of neat PVA solution. This result indicated that the addition of GNS in the PVA solution increased the conductivity of the PVA solution. The conductivities of the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions similarly increased as the GO-g-PTA-F and GO-g-PTA-A contents increased, respectively. The conductivities of the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions were significantly higher than those of the PVA/GNS solutions with the same filler content. This discrepancy may be attributed to the grafted cationic PTA on the GO surface. The cationic polymer enhanced the conductivities of the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions. The
κ values of PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions were 1.22 and 1.29 mS/cm at 10 wt % GO-g-PTA-F and GO-g-PTA-A, respectively. Thus, the
κ value of the PVA/GO-g-PTA-A solutions was higher than that of the PVA/GO-g-PTA-F solutions. This discrepancy could be attributed to the different grafted cationic PTA structures of GO-g-PTA-A and GO-g-PTA-F. Although the grafted PTA ratio of GO-g-PTA-A was smaller than that of GO-g-PTA-F, the cation dissociation of the line skeleton structure of GO-g-PTA-A was higher than that of the branch structures of GO-g-PTA-F.
Figure S2 (Supplementary Materials) shows the functioning domain for electrospinning 7 wt % PVA solution with various GNS, GO-g-PTA-F, and GO-g-PTA-A contents. Functioning domains [
35,
36] are the operating windows of electric field and flow rates that are required for a stable cone-jet mode. The lower- and upper-bound electric fields are denoted as V
s and V
us, respectively. Given the volatility of the water solvent, a working distance (H) of 14 cm was used. At a given Q, the operating windows (V
us − V
s) are the variations in the PVA solution filled with GNS, GO-g-PTA-F, and GO-g-PTA-A contents. Based on the functioning domain for electrospinning PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A solutions (
Figure S2, Supplementary Materials), a common but limited processing window exists to determine the effect of GNS, GO-g-PTA-F, and GO-g-PTA-A. Therefore, the determined Q and V were 0.4 mL/h and 13 kV, respectively, in electrospinning the PVA/GNS solution to demonstrate the effects of GNS on fiber diameter. Similar results were obtained in electrospinning the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions. The determined Q and V were 0.4 mL/h and 12 kV, respectively, in electrospinning the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A solutions to demonstrate the effects of GO-g-PTA-F, and GO-g-PTA-A on fiber diameter.
To determine the effect of graphene-based fillers addition on fiber morphology and diameter, we electrospun and compared 7 wt % PVA solutions filled with 1–10 wt % GNS, GO-g-PTA-F, and GO-g-PTA-A.
Figure 6 shows the SEM images of the nanofiber collected from electrospinning 7 wt % PVA solutions with various amounts of GNS at conditions of Q = 0.4 mL/h, H = 14 cm, and V = 13 kV. The arrows indicate the GNS positions.
Figure S3 (Supplementary Materials) shows the SEM images of the PVA fiber products collected from the electrospinning of the 7 wt % PVA solutions, and bead-free neat PVA fibers were obtained. The SEM images in
Figure 6 shows that the PVA composite nanofibers became less smooth and formed an increasingly irregular structure along the fiber with increasing amount of GNS. Several larger and irregular structures, which increased with increased GNS contents, could be observed. The TEM images in
Figure 7, GNSs are identifiable in the TEM images. The GNS particles protrude from the smooth PVA nanofiber. The lateral dimension of GNS was larger than the diameter of the electrospun PVA fibers. When the GNS content in the PVA solution increased, several GNSs aggregated during electrospinning and numerous protrusions of these aggregates were observed on the fiber surface. At 5 wt % GNS content, GNSs became distinctly distributed in the PVA fiber, and the inter-GNS distance decreased. When the GNS content increased to 7 wt %, the GNSs were more distinctly distributed in the PVA nanofiber, and the distance between GNSs became smaller. Although the addition of SDBS in the prepared PVA/GNS solution prevented GNS aggregation, several GNSs were still slightly aggregated in the PVA/GNS composite nanofibers with 10 wt % GNS content.
Similar results were obtained in the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A composite nanofibers.
Figure 6 shows the SEM images of the nanofibers collected from electrospinning 7 wt % PVA solutions with various amounts of GO-g-PTA-F and GO-g-PTA-A at conditions of Q = 0.4 mL/h, H = 14 cm, and V = 12 kV. The arrows indicate the GO-g-PTA-F and GO-g-PTA-A positions. The PVA composite nanofibers also became less smooth and formed an increasingly irregular structure along the fiber with increasing amounts of GO-g-PTA-F and GO-g-PTA-A. Several larger and irregular structures, which increased with increased GNS contents, could be observed. The TEM images in
Figure 7 show that GO-g-PTA-F and GO-g-PTA-A were identifiable and that they were embedded in the PVA composite fibers. The lateral dimension of GO-g-PTA-F and GO-g-PTA-A were also larger than the diameter of the electrospun PVA fibers, and the GO-g-PTA-F or GO-g-PTA-A particles protruded from the smooth PVA fiber. At an increased GO-g-PTA-F or GO-g-PTA-A content in the PVA composite fibers, numerous GO-g-PTA-F or GO-g-PTA-A protrusions were observed on the fiber surface, with some GO-g-PTA-F or GO-g-PTA-A appearing curly in the PVA/GO-g-PTA-F or PVA/GO-g-PTA-A composite fibers. At 7 wt % GO-g-PTA-F and GO-g-PTA-A content, GO-g-PTA-F and GO-g-PTA-A became distinctly distributed in the PVA fiber, and the inter-GO-g-PTA-F and inter-GO-g-PTA-A distances decreased.
Notably, the amounts of GNS in the PVA/GNS composite nanofibers were higher than those of GO-g-PTA-F in the PVA/GO-g-PTA-F and GO-g-PTA-A in the PVA/GO-g-PTA-A composite nanofibers at the same filler weight percentage. The amounts of GO-g-PTA were smaller than that of GNS at the same weight because PTA was grafted on the GO surface and the oxygen functional groups were retained in GO. Grafted PTA and oxygen functional groups existed on the GO-g-PTA-F and GO-g-PTA-A surfaces, but no PTA percentage was found on GO and GNS. Therefore, the volume or the number of sheets of GNS, GO-g-PTA-F, and GO-g-PTA-A were different under the same weight. In other words, the densities of GNS, GO-g-PTA-F, and GO-g-PTA-A were different. The XPS results indicated that the grafted PTA percentages on the GO-g-PTA-F and GO-g-PTA-A surfaces were approximately 25.7 and 22.3 wt %, respectively, and the amount of oxygen functional groups on the GO surface was approximately 26.3 wt %. Assuming that the amount of oxygen atoms on the GO surface—which was grafted with PTA via FRP or ATRP—would not change is reasonable. Therefore, the weight of GO-g-PTA-F and GO-g-PTA-A sheet was approximately 1.85- and 1.79-fold higher than that of GNS, respectively. For example, the GNS, GO-g-PTA-F, and GO-g-PTA-A volume percentage of the PVA/GNS, PVA/GO-g-PTA-F, and PVA-g-PTA-A composite nanofiber mats with 7 wt % filler content was 4.05, 2.23, and 2.31 vol %, respectively.
Electrospun PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite fiber diameters were measured using a collection of over 200 fibers.
Figure 8a shows the fiber diameter (
df) of PVA composite nanofibers filled with GNS at the same electrospinning conditions of Q = 0.4 mL/h, H = 14 cm, and V = 13 kV. For neat PVA nanofibers, the measured
df was 315 ± 42 nm. The
df of PVA/GNS composite nanofibers gradually decreased as the GNS content increased to 5 wt %, and then increased as the GNS content increased to 10 wt %. In addition, the measured
df of PVA/GNS 95/5 nanofibers was 199 ± 51 nm. Similar results were obtained in electrospun PVA/GO-g-PTA-F and PVA/GO-g-PTA-A composite fiber diameters.
Figure 8b,c show the
df of PVA composite nanofibers filled with GO-g-PTA-F and GO-g-PTA-A at the same electrospinning conditions of Q = 0.4 mL/h, H = 14 cm, and V = 12 kV. The
df of PVA/GO-g-PTA-F composite nanofibers gradually decreased as the GO-g-PTA-F content increased to 5 wt %, and then increased as the GO-g-PTA-F content increased to 10 wt %. Moreover, the
df of PVA/GO-g-PTA-A composite nanofibers initially decreased as the GO-g-PTA-A content increased to 1 wt %, and then gradually increased as the GO-g-PTA-A content increased to 10 wt %. This finding is consistent with our previous electrospun PVA/GNS-H [
10] and PTT/GNS [
53] composite nanofibers. In electrospinning, solution viscosity and conductivity are important factors for determining the
df of electrospun fibers. In previous studies [
54,
55],
df decreased with decreasing solution viscosity and increasing solution conductivity. The results in
Figure 5 and
Figure S4 indicated that the conductivity and viscosity of the PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A solutions increased as the GNS, GO-g-PTA-F, and GO-g-PTA-A content increased, respectively. The initial change in
df was attributed to a greater increase in solution conductivity than in solution viscosity, and the final change in
df was attributed to a greater increase in solution viscosity than in solution conductivity.
3.3. Crystallization Behavior of Electrospun PVA Composite Fibers
Figure S5a–c (Supplementary Materials) show the DSC heating traces of PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite fibers. Melting peak temperature and endothermic enthalpy are denoted as
Tm and
ΔHm, respectively, as listed in
Table 2. The
Tm of neat PVA was 216.0 °C. The
Tm of the PVA/GNS composite fiber slightly shifted to low temperature with increased GNS content. When the GNS content increased to 10 wt %, the
Tm slightly decreased to 216.3 °C. This phenomenon indicated that the presence of high GNS contents in the PVA solution retarded PVA crystallization and yielded PVA lamellae with small thicknesses in the fibers during electrospinning and thermal heating. To compare the amount of melting crystals,
ΔHm was normalized with the PVA content to derive
ΔH’m = [
ΔHm/(1 −
φw)]. The
ΔH’m of the PVA/GNS nanofiber slightly increased with increasing GNS from 71.7 J/g for the neat PVA nanofiber to 71.9 J/g for the PVA nanofibers filled with 1 wt % GNS.
ΔH’m decreased for GNS higher than 1 wt %. The
ΔH’m results are consistent with those of our previous study [
10]. The
Tm of the PVA/GO-g-PTA-F composite fiber remained unchanged at approximately 219.0 °C with increased GO-g-PTA-F content. Moreover, the
ΔH’m of the PVA/GO-g-PTA-F composite nanofibers increased slightly with increasing GO-g-PTA-F content. This phenomenon indicated that the presence of GO-g-PTA-F contents in the PVA solution would not affect the PVA lamellae thicknesses and increase the crystallinity in PVA fibers during electrospinning and thermal heating. The
Tm of the PVA/GO-g-PTA-A composite fibers decreased to 212.7 °C with 3 wt % GO-g-PTA-A content, and then gradually increased to 216.3 °C with 7 wt % GO-g-PTA-A content. The
Tm of the PVA/GO-g-PTA-A composite nanofibers finally decreased to 215.8 °C with 10 wt % GO-g-PTA-A content. The
ΔH’m of the PVA/GO-g-PTA-A composite nanofibers significantly decreased to 56.1 J/g with 3 wt % GO-g-PTA-A content and then increased to 82.4 J/g with 7wt % GO-g-PTA-A content. This phenomenon indicated that the presence of GO-g-PTA-A contents in the PVA solution retarded the PVA crystallization more during electrospinning and thermal heating than GNS dose.
Figure S5d–f (Supplementary Materials) show the subsequent cooling curves, which reveal the nucleating effects of GNS, GO-g-PTA-F, and GO-g-PTA-A on the crystallization of PVA from the melt state. Crystallization peak temperature and exothermic enthalpy are denoted as
Tc and
ΔHc, respectively, as listed in
Table 2. Compared with the neat PVA nanofibers melt-crystallized at 177.9 °C, the PVA composite nanofiber melt filled with 1 wt % GNS increased to 182.4 °C (
Figure S5d) and then gradually decreased to 174.2 °C with 10 wt % GNS content. To compare the amount of PVA crystallization from the melt state,
ΔHc was normalized with the PVA content to derive
ΔH’c = [
ΔHc/(1 −
φw)]. The
ΔH’c of the PVA/GNS nanofiber decreased with increasing GNS from 45.9 J/g for the neat PVA nanofiber to 37.6 J/g for nanofibers filled with 7 wt % GNS. Then,
ΔH’c increased for GNS higher than 7 wt %. Thus, the melted crystallization rate and crystallinity of PVA chains in the GNS-filled composite nanofibers from the melt state decreased with increasing GNS content. The
Tc of the PVA/GO-g-PTA-F composite fibers gradually increased to 196.0 °C with 10 wt % GO-g-PTA-F content. In addition, the
ΔH’c of the PVA/GO-g-PTA-F composite fibers decreased to 35.8 J/g with 1 wt % GO-g-PTA-F content and then increased to 54.6 J/g with 10 wt % GO-g-PTA-F content. Therefore, the melted crystallization rate and crystallinity of PVA chains in the PVA/GO-g-PTA-F composite nanofibers from the melt state increased with high GO-g-PTA-F content. Furthermore, the
Tc of the PVA/GO-g-PTA-A composite fibers increased to 184.2 °C with 1 wt % GO-g-PTA-A content and then significantly decreased to 162.7 °C with 3 wt % GO-g-PTA-F content. The
Tc of the PVA/GO-g-PTA-A composite fibers remained unchanged at approximately 163.8 °C with increased GO-g-PTA-A content. The
ΔH’c of the PVA/GO-g-PTA-A composite fibers also decreased to 28.3 J/g with 3 wt % GO-g-PTA-A content and then slightly increased to 28.7 J/g with 10 wt % GO-g-PTA-A content. Therefore, the melted crystallization rate and crystallinity of PVA chains in the PVA/GO-g-PTA-A from the melt state composite nanofiber decreased with the GO-g-PTA-A content.
The
Tm and
ΔH’m of the PVA/GO-g-PTA-F composite nanofibers were similar to that in our previous study on electrospun PVA/GNS-H composite nanofibers [
10]. Moreover, the
Tc of the PVA/GO-g-PTA-F composite fibers increased with high GO-g-PTA-F content. These findings were attributed to the PVA nucleation ability of GO-g-PTA-F, which resulted from hydrogen bond interactions between PVA and GO-g-PTA-F. In addition, the overgrown PVA crystals on the GO-g-PTA-F surface of the PVA/GO-g-PTA-F composite nanofibers may result in the retardation alignment of PVA crystals in the PVA/GO-g-PTA-F composite nanofibers under quiescent heating with high GO-g-PTA-F content.
These results indicated that the influences of GNS, GO-g-PTA-F, and GO-g-PTA-A on the induced PVA crystallization during electrospinning or nucleating effects from melt state are different. The induced PVA crystallization during electrospinning and the melted crystallization of the PVA composite fibers was enhanced by GO-g-PTA-F, but retarded by GNS and GO-g-PTA-A. According to our previous study on electrospun PVA/GNS-H composite nanofibers [
10], the PVA crystal could be formed during PVA electrospinning. GNS-H functions as an effective nucleating agent in PVA/GNS-H composite nanofibers during the melted crystallization process, thereby increasing the degree of crystallinity and the crystallization rate. The polar and hydrogen bond interactions between PVA and GNS were less than that between PVA and GO or PVA and GNS-H because fewer polar oxygen functional groups existed in the GNS than in the GO or GNS-H. The hydrophobic property of PTA also resulted in less hydrogen bond interactions between PVA and PTA. Thus, GO-g-PTA should have a similar result, and the melted crystallization of the PVA composite fibers could be retarded by GO-g-PTA. However, the melted crystallization of the PVA composite fibers was enhanced by GO-g-PTA-F. This finding was attributed to the different morphologies between GO-g-PTA-F and GO-g-PTA-A. For GO-g-PTA-F, the branch of PTA growing radicals were randomly added on the GO surface, and the partial oxygen functional groups of GO were covered by the grafted PTA polymers. However, for GO-g-PTA-A, the line skeleton PTA polymers were grown from the hydroxy group on the GO surface, and all oxygen functional groups of GO were covered by the grafted PTA polymers. Therefore, the polar and hydrogen bond interactions between PVA and GO-g-PTA-A were less than that between PVA and GO-g-PTA-F.
3.4. Antimicrobial Abilities and Cell Viability of PVA Composite Fibers
According to our previous study [
10], thermally treated PVA nanofibers can stabilize fiber structures against disintegration in water and exhibit better performances for 3T3 cells in terms of adhesion and proliferation. Therefore, all PVA composite nanofiber mats for antimicrobial and cell viability tests were heated to 190 °C at 10 °C/min and held at 190 °C for 15 min to crystallize the PVA chains in the hot stage to achieve better performances for cell adhesion and proliferation. The nanofibers were subsequently cooled to room temperature at 10 °C/min. The antimicrobial activity of PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite nanofiber mats against
S. aureus, was assessed through a broth microdilution assay and a viable cell counting method [
56]. The MIC of PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite nanofiber mats against
S. aureus, can be determined using the broth microdilution method. MIC is the minimal concentration of tested antimicrobial agents or fillers filled in PVA composite nanofiber mats at which the bacterial growth amount after 18 h incubation is lower than two times the corresponding initial bacterial amount at 0 h [
57]. The optical densities (OD
600) of the original untreated (the control group) and PVA composite nanofiber mat-added bacterial suspensions were monitored at fixed time intervals. The antimicrobial ability of PVA composite nanofiber mats was evaluated by a broth microdilution assay because the OD
600 value is positively correlated with the number of growing bacteria.
Figure 9a shows the bacterial growth curves of the PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite nanofibers mats versus the filler weight percentage determined by monitoring the OD
600 of
S. aureus for 18 h. After a 24 h incubation period, the OD
600 values of the bacterial suspension at PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite nanofiber mats with 3 wt % filler content were 0.08, 0.69, and 0.47, respectively. Therefore, the antimicrobial ability of the PVA/GO-g-PTA-F composite nanofiber mats was lower than that of the PVA/GNS and PVA/GO-g-PTA-A composite nanofiber mats at the same filler weight percentage. Moreover, the OD
600 value of the bacterial suspension at the PVA/GNS composite nanofiber mats with 3wt % GNS content was less than twice the OD
600 value (1.04) of the corresponding bacterial suspension at 0 h. As a result, the MIC of the PVA/GNS composite nanofiber mats against
S. aureus was 3 wt % GNS content. Using the same method, the MIC values of the PVA/GO-g-PTA-A composite nanofiber mats against
S. aureus was 5 wt %. The PVA/GO-g-PTA-F composite nanofiber mats only showed a limited antimicrobial ability against
S. aureus despite an improved antimicrobial ability relative to neat PVA nanofiber mats.
Figure 9b shows the bacterial growth curves of the PVA/GNS, PVA/GO-g-PTA-F, and PVA/GO-g-PTA-A composite nanofibers mats versus the filler weight percentage determined by monitoring the log (CFU/mL) of
S. aureus for 24 h. The number of bacterial colonies of the neat PVA nanofiber mats against
S. aureus was 6.55 × 10
8 CFU/mL. No significant antimicrobial ability was observed from the PVA/GO-g-PTA-F composite nanofiber mats. When the GNS content was higher than 7 wt % and the GO-g-PTA-A content was higher than 10 wt %, the PVA/GNS and PVA/GO-g-PTA-A composite nanofiber mats had no evident biocidal effects. All initial bacterial colonies were completely killed by the PVA/GNS and PVA/GO-g-PTA-A composite nanofiber mats at 7 wt % GNS and 10 wt % GO-g-PTA-A contents, respectively. MBC in this section is the minimum concentration of tested biocidal agents or fillers filled in PVA composite nanofiber mats at which 99.9% of the initial bacterial colony is killed. As a result, the MBC values for the PVA/GNS and PVA/GO-g-PTA-A composite nanofiber mats were 7 wt % GNS and 10 wt % GO-g-PTA-A contents, respectively. Therefore, the PVA/GNS and PVA/GO-g-PTA-A composite nanofiber mats exhibited antimicrobial ability against
S. aureus. Notably, based on the antimicrobial ability results of GNS, GO-g-PTA-F, and GO-g-PTA-A powders, the GO-g-PTA-A powders showed the best antimicrobial ability against
S. aureus, but the antimicrobial ability of the PVA/GNS composite nanofiber mats was higher than that of the PVA/GO-g-PTA-F and PVA/GO-g-PTA-A composite nanofiber mats at the same weight filler content. However, because the amounts of GNS in the PVA/GNS composite nanofiber mats were higher than those of GO-g-PTA-A in the PVA/GO-g-PTA-A composite nanofiber mats at the same filler weight percentage, the GNS in the PVA/GNS composite nanofiber mats had higher probability to contact
S. aureus than GO-g-PTA-A in the PVA/GO-g-PTA-A composite nanofiber mats. If the addition of filler to the PVA composite nanofiber mats was presented in volume percentages, then the MBC vol % value of PVA/GNS and PVA/GO-g-PTA-A was 4.05 and 3.37 vol %, respectively (
Figure 9c). The MBC vol % value of the PVA/GNS composite nanofiber mats was higher than that of the PVA/GO-g-PTA-A composite nanofiber mats. Therefore, the PVA/GO-g-PTA-A composite nanofiber mats had better antimicrobial ability against
S. aureus than the PVA/GNS and PVA/GO-g-PTA-F composite nanofiber mats. This phenomenon was due to the synergistic effects of cationic polymer and conductive graphene in GO-g-PTA-A.
As a result, fibroblast NIH-3T3 cells were selected as model cells for evaluating the cell viability of the PVA composite nanofiber mats. The results indicated that the PVA composite nanofiber mats significantly enhanced the antimicrobial abilities with filler higher than 3 wt %. Thus, the neat PVA nanofiber mats as the control group and the presence of filler loading content of 5, 7, and 10 wt % in the PVA composite nanofiber mats were assigned as the sample selection for experimental groups to evaluate their cell viability via a ISO10993-5 standard test method.
Figure 10 shows the cell viability of the treated 3T3 cells for the PVA composite nanofiber mats after 1, 3, 7, and 10 days of incubation. The differential response of cell viability to the various amounts of filler in groups of the neat PVA, PVA/GNS, and PVA/GO-g-PTA-A composite nanofiber mats showed no statistically significant difference in cell viabilities due to the large standard deviation. Moreover, the trends in cell proliferation in different nanofiber mats of neat PVA, PVA/GNS, and PVA/GO-g-PTA-A exhibited similar results. Therefore, the PVA/GO-g-PTA-A composite nanofiber mats had no cytotoxicity under the controlled conditions in this experiment.