Pretreatment Affects Activated Carbon from Piassava
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of the Piassava Fibers
2.3. Electrical Discharge Pretreatment
2.4. Extraction Pretreatment
2.5. Chemical Analysis of Untreated Precursor Material
2.6. Activated Carbon (AC) Preparation
2.7. Elemental Analysis
2.8. Infrared Spectroscopy (FTIR)
2.9. Adsorption Tests and Modeling
2.10. Iodine Number (IN) and Surface Area with Methylene Blue–SMB
2.11. Estimation of the Brunauer, Emmett and Teller (BET) Surface Area
3. Results and Discussion
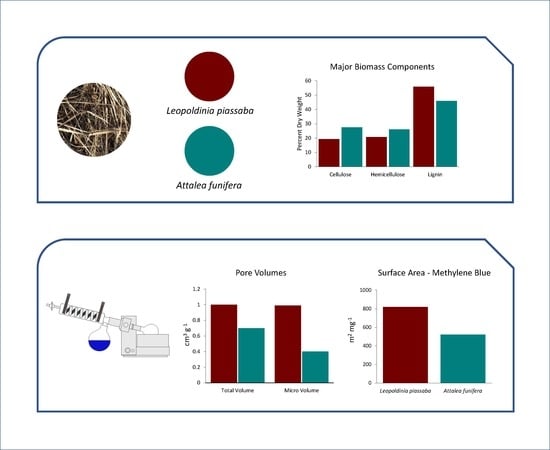
3.1. Chemical Composition of Fibers
3.2. Elemental Composition
3.3. Infrared Spectroscopy (FTIR)
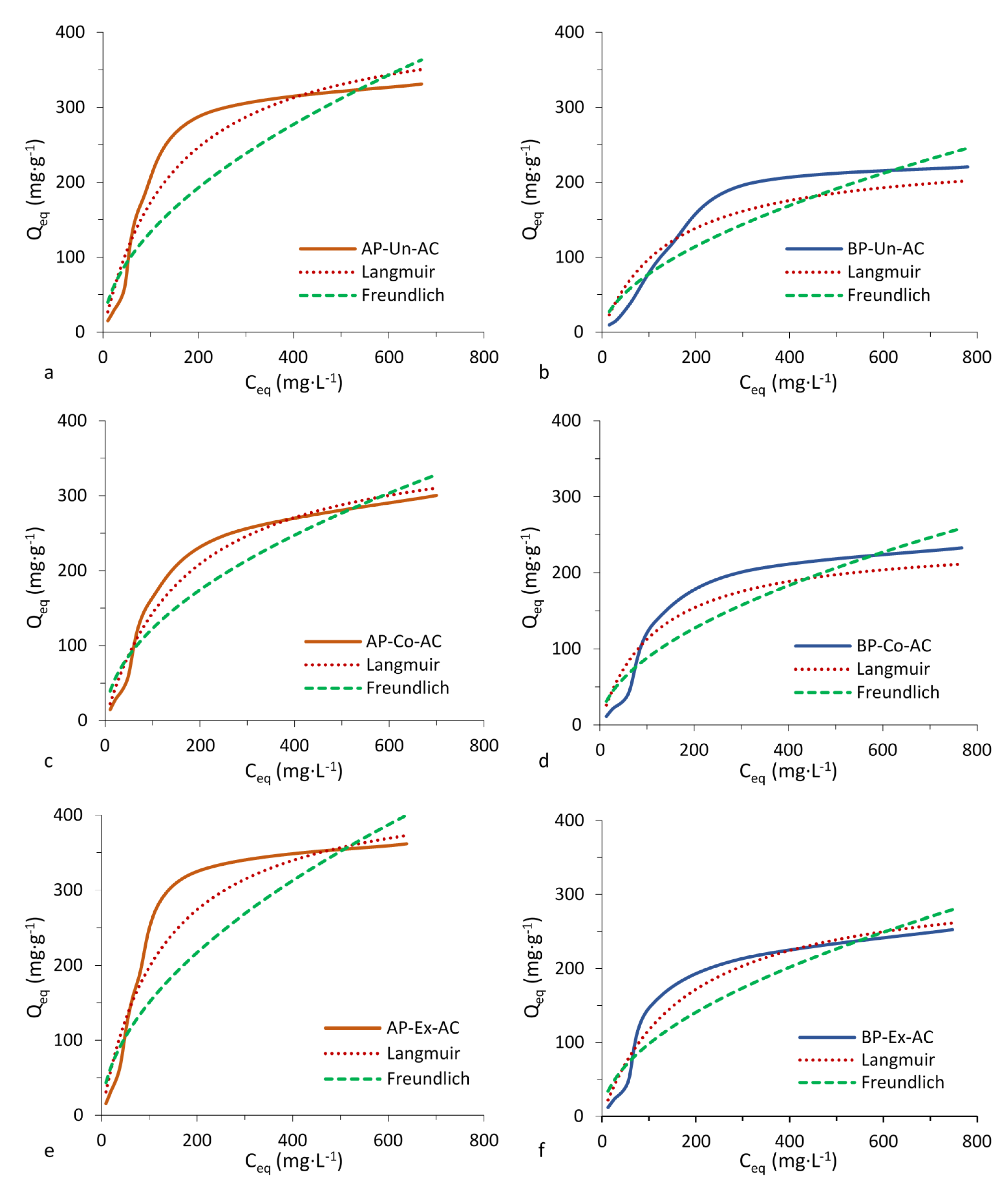
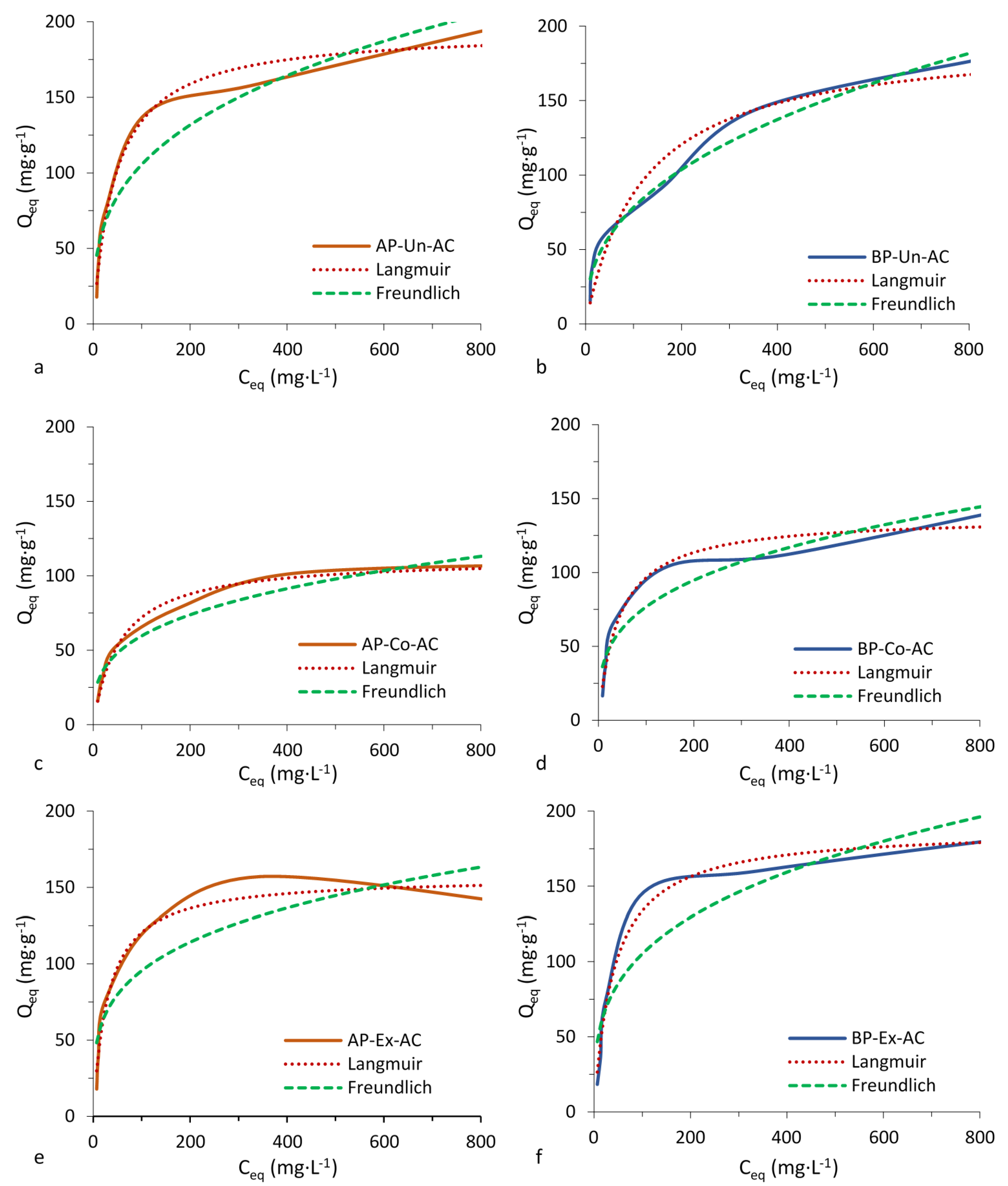
3.4. Adsorption Tests
3.5. Surface Area of the ACs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
EEO Statement
References
- Avelar, F.F.; Bianchi, M.L.; Goncalves, M.; da Mota, E.G. The use of piassava fibers (Attalea funifera) in the preparation of activated carbon. Bioresour. Technol. 2010, 101, 4639–4645. [Google Scholar] [CrossRef]
- Dabioch, M.; Skorek, R.; Kita, A.; Janoska, P.; Pytlakowska, K.; Zerzucha, P.; Sitko, R. A study on adsorption of metals by activated carbon in a large-scale (municipal) process of surface water purification. Cent. Eur. J. Chem. 2013, 11, 742–753. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Kaghazchi, T. Adsorption of gold ions from industrial wastewater using activated carbon derived from hard shell of apricot stones–An agricultural waste. Bioresour. Technol. 2008, 99, 5374–5383. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Laginhas, C.E.C.; Carrott, P.; Carrott, M.R. Production of activated carbons from almond shell. Fuel Process. Technol. 2011, 92, 234–240. [Google Scholar] [CrossRef]
- Castro, J.P.; Nobre, J.R.C.; Bianchi, M.L.; Trugilho, P.F.; Napoli, A.; Chiou, B.-S.; Williams, T.G.; Wood, D.F.; Avena-Bustillos, R.J.; Orts, W.J. Activated carbons prepared by physical activation from different pretreatments of amazon piassava fibers. J. Nat. Fibers 2019, 16, 961–976. [Google Scholar] [CrossRef]
- Martins, A.F.; Cardoso, A.D.L.; Stahl, J.A.; Diniz, J. Low temperature conversion of rice husks, eucalyptus sawdust and peach stones for the production of carbon-like adsorbent. Bioresour. Technol. 2007, 98, 1095–1100. [Google Scholar] [CrossRef]
- Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization), Madeira: Determinação do Material Solúvel em Etanol-Tolueno e em Diclorometano e em Acetona: Método de Ensaio; Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization): Rio de Janeiro, Brazil, 2010; p. 3.
- Browning, B.L. The Chemistry of Wood. In The Chemistry of Wood; Interscience: New York, NY, USA, 1963; p. 574. [Google Scholar]
- Kennedy, J.F.; Phillips, G.O.; Williams, P.A. Wood and Cellulosics: Industrial Utilisation, Biotechnology, Structure and Properties; Ellis Horwood Limited: Hemel Hempstead, UK, 1987. [Google Scholar]
- Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization), Pasta Celulósica e Madeira—Determinação de Lignina Insolúvel em Ácido: Método de Ensaio; Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization): Rio de Janeiro, Brazil, 2010; p. 6.
- Goldschmid, O. Ultraviolet spectra. In Lignins: Occurrence, Formation, Structure and Reactions; John Wiley & Sons: New York, NY, USA, 1971; pp. 241–298. [Google Scholar]
- Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization), Papel, Cartão, Pastas Celulósicas e Madeira—Determinação do Resíduo (Cinza) Após a Incineração a 525 °C: Método de Ensaio; Associação Brasileira de Normas Técnicas (Brazilian National Standards Organization): Rio de Janeiro, Brazil, 2003; p. 4.
- Paula, L.E.d.R.; Trugilho, P.F.; Napoli, A.; Bianchi, M.L. Characterization of residues from plant biomass for use in energy generation. Cerne 2011, 17, 237–246. [Google Scholar] [CrossRef]
- Kumar, K.V.; Sivanesan, S. Equilibrium data, isotherm parameters and process design for partial and complete isotherm of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 134, 237–244. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Determination of Iodine Number of Activated Carbon; ASTM D4607-14; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Stavropoulos, G.; Zabaniotou, A. Production and characterization of activated carbons from olive-seed waste residue. Microporous Mesoporous Mater. 2005, 82, 79–85. [Google Scholar] [CrossRef]
- Nunes, C.A.; Guerreiro, M.C. Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers. Química Nova 2011, 34, 472–476. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wu, Y.; Zheng, M. A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J. Hazard. Mater. 2014, 280, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Browne, F.L. Theories of the Combustion of Wood and Its Control: A Survey of the Literature; Forest Products Laboratory, Forest Service, USA Department of Agriculture: Madison, WI, USA, 1958. [Google Scholar]
- Schuchardt, U.; Bianchi, M.; Gonçalves, A.; Curvelo, A.; Biscolla, F.; Peres, L. Piassava fibers (Attalea funifera). 1. Chemical analysis, extraction and reactivity of its lignin. Cellul. Chem. Technol. 1995, 29, 705–712. [Google Scholar]
- Sekirifa, M.L.; Hadj-Mahammed, M.; Pallier, S.; Baameur, L.; Richard, D.; Al-Dujaili, A.H. Preparation and characterization of an activated carbon from a date stones variety by physical activation with carbon dioxide. J. Anal. Appl. Pyrolysis 2013, 99, 155–160. [Google Scholar] [CrossRef]
- Nogueira, B.R.; Chinellato, A.; Ortiz, A.V.; Parveen, A.; Rangari, V.K.; Moura, E.A. Thermal and morphological behavior of EVOH/piassava fiber composites. In Characterization of Minerals, Metals, and Materials; The Minerals, Metals, & Materials Society: Warrendale, PA, USA, 2012; pp. 373–380. [Google Scholar]
- d’Almeida, J.R.M.; Aquino, R.C.M.P.; Monteiro, S.N. Dynamic Mechanical Behavior of Piassava Fibers (Attalea funifera) Reinforced Polyester Composites. Int. J. Polym. Mater. 2007, 56, 397–403. [Google Scholar] [CrossRef]
- Nascimento, D.C.O.; Ferreira, A.S.; Monteiro, S.N.; Aquino, R.C.M.; Kestur, S.G. Studies on the characterization of piassava fibers and their epoxy composites. Compos. Part A 2012, 43, 353–362. [Google Scholar] [CrossRef]
- Elzubair, A.; Bonelli, C.M.C.; Suarez, J.C.M.; Mano, E.B. Morphological, Structural, Thermal and Mechanical Characterization of Piassava Fibers. J. Nat. Fibers 2007, 4, 13–31. [Google Scholar] [CrossRef]
- Bui, N.Q.; Fongarland, P.; Rataboul, F.; Dartiguelongue, C.; Charon, N.; Vallée, C.; Essayem, N. FTIR as a simple tool to quantify unconverted lignin from chars in biomass liquefaction process: Application to SC ethanol liquefaction of pine wood. Fuel Process. Technol. 2015, 134, 378–386. [Google Scholar] [CrossRef]
- Nishimiya, K.; Hata, T.; Imamura, Y.; Ishihara, S. Analysis of chemical structure of wood charcoal by X-ray photoelectron spectroscopy. J. Wood Sci. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Saka, S. Chemical composition and distribution. In Wood and Cellulosic Chemistry; Marcel Dekker Ltd.: New York, NY, USA, 2000; pp. 51–82. [Google Scholar]
- Sadeghnejad, A.; Aroujalian, A.; Raisi, A.; Fazel, S. Antibacterial nano silver coating on the surface of polyethylene films using corona discharge. Surf. Coat. Technol. 2014, 245, 1–8. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Activated carbons prepared from Thymelaea hirsuta wood: Sustainable adsorbents for polyvinyl alcohol. Environ. Prog. Sustain. Energy 2016, 35, 70–79. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Gao, J.-J.; Qin, Y.-B.; Zhou, T.; Cao, D.-D.; Xu, P.; Hochstetter, D.; Wang, Y.-F. Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: Kinetics, equilibrium, and thermodynamics studies. J. Zhejiang Univ. Sci. B 2013, 14, 650–658. [Google Scholar] [CrossRef]
- Abussaud, B.; Asmaly, H.A.; Saleh, T.A.; Gupta, V.K.; Atieh, M.A. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar] [CrossRef]
- Feng, J.; Qiao, K.; Pei, L.; Lv, J.; Xie, S. Using activated carbon prepared from Typha orientalis Presl to remove phenol from aqueous solutions. Ecol. Eng. 2015, 84, 209–217. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, W.; Li, R.; Feng, C.; Liu, Y.; Ma, N.; Deng, J.; Xing, L.; Gao, Y.; Chen, N. Mineralization of phenol by ozone combined with activated carbon: Performance and mechanism under different pH levels. Environ. Sci. Ecotechnol. 2020, 1, e100005. [Google Scholar] [CrossRef]
- Beker, U.; Ganbold, B.; Dertli, H.; Gülbayir, D.D. Adsorption of phenol by activated carbon: Influence of activation methods and solution pH. Energy Convers. Manag. 2010, 51, 235–240. [Google Scholar] [CrossRef]
- LibreTexts. Infrared Spectroscopy Absorption Table. Available online: https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Parameters/Infrared_Spectroscopy_Absorption_Table (accessed on 25 June 2020).
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Sathishkumar, K. Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J. Chem. Eng. 2011, 28, 149–155. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. [Google Scholar] [CrossRef]
- Pezoti Jr, O.; Cazetta, A.L.; Souza, I.P.; Bedin, K.C.; Martins, A.C.; Silva, T.L.; Almeida, V.C. Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 2014, 20, 4401–4407. [Google Scholar] [CrossRef]
- Miao, Q.; Tang, Y.; Xu, J.; Liu, X.; Xiao, L.; Chen, Q. Activated carbon prepared from soybean straw for phenol adsorption. J. Taiwan Inst. Chem. Eng. 2013, 44, 458–465. [Google Scholar] [CrossRef]
- Zhang, D.; Huo, P.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.-H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Fillat, Ú.; Martín, J.A.; Aranda, P.; Ruiz-Hitzky, E.; Ibarra, D.; Wicklein, B. Biorefinery of Lignocellulosic Biomass from an Elm Clone: Production of Fermentable Sugars and Lignin-Derived Biochar for Energy and Environmental Applications. Energy Technol. 2019, 7, 277–287. [Google Scholar] [CrossRef]

| Fiber Source | Chemical Composition (%) | ||||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Extract | Ash | |
| AP | 19.30 ± 0.08 | 20.83 * | 55.86 ± 0.36 | 3.41 ± 0.23 | 0.59 ± 0.02 |
| BP | 27.51 ± 0.08 | 26.03 * | 45.93 ± 1.49 | 1.48 ± 0.09 | 0.76 ± 0.08 |
| Samples. | C (%) | O (%) | N (%) | H (%) |
|---|---|---|---|---|
| AP-Un | 53.13 | 40.07 | 1.57 | 5.22 |
| AP-Co | 52.24 | 41.24 | 1.37 | 5.14 |
| AP-Ex | 52.77 | 40.66 | 1.34 | 5.22 |
| BP-Un | 50.61 | 42.85 | 1.15 | 5.39 |
| BP-Co | 52.28 | 41.18 | 1.05 | 5,49 |
| BP-Ex | 52.76 | 40.74 | 1.01 | 5.49 |
| AP-Un-Ch | 78.22 | 16.76 | 2.42 | 2.57 |
| AP-Co-Ch | 74.34 | 20.51 | 2.34 | 2.79 |
| AP-Ex-Ch | 73.37 | 21.24 | 2.79 | 2.57 |
| BP-Un-Ch | 69.44 | 26.69 | 1.38 | 2.47 |
| BP-Co-Ch | 79.50 | 16.27 | 1.47 | 2.75 |
| BP-Ex-Ch | 78.23 | 17.63 | 1.41 | 2.72 |
| AP-Un-AC | 82.03 | 13.53 | 2.52 | 1.82 |
| AP-Co-AC | 84.48 | 11.86 | 1.85 | 1.77 |
| AP-Ex-AC | 82.66 | 13.51 | 1.96 | 1.85 |
| BP-Un-AC | 83.38 | 13.66 | 1.39 | 1.52 |
| BP-Co-AC | 81.37 | 15.65 | 1.37 | 1.51 |
| BP-Ex-AC | 82.45 | 14.79 | 1.40 | 1.27 |
| Compound | Activated Carbons (AC) | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | 1/n | R2 | ||
| Methylene blue | AP-Un-AC | 427 | 0.007 | 0.96 | 12 | 0.526 | 0.87 |
| AP-Co-AC | 385 | 0.006 | 0.98 | 12 | 0.504 | 0.92 | |
| AP-Ex-AC | 446 | 0.008 | 0.96 | 13 | 0.527 | 0.86 | |
| BP-Un-AC | 239 | 0.007 | 0.93 | 6 | 0.560 | 0.90 | |
| BP-Co-AC | 243 | 0.009 | 0.93 | 8 | 0.529 | 0.90 | |
| BP-Ex-AC | 324 | 0.006 | 0.96 | 9 | 0.521 | 0.90 | |
| Phenol | AP-Un-AC | 194 | 0.022 | 0.99 | 24 | 0.319 | 0.93 |
| AP-Co-AC | 112 | 0.018 | 0.99 | 14 | 0.307 | 0.95 | |
| AP-Ex-AC | 156 | 0.033 | 0.98 | 29 | 0.258 | 0.83 | |
| BP-Un-AC | 192 | 0.008 | 0.96 | 12 | 0.405 | 0.98 | |
| BP-Co-AC | 137 | 0.023 | 0.97 | 19 | 0.304 | 0.93 | |
| BP-Ex-AC | 188 | 0.025 | 0.99 | 26 | 0.300 | 0.88 | |
| Activated Carbon | SAM (mg2 g−1) | IN (mg g−1) | Est-SBET (mg2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) |
|---|---|---|---|---|---|
| AP-Un-AC | 824 | 508 | 611 ± 67 | 0.99 ± 0.13 | 0.98 ± 0.16 |
| AP-Co-AC | 743 | 514 | 597 ± 65 | 0.91 ± 0.12 | 0.79 ± 0.13 |
| AP-Ex-AC | 861 | 572 | 679 ± 74 | 0.98 ± 0.13 | 0.95 ± 0.15 |
| BP-Un-AC | 461 | 525 | 539 ± 59 | 0.64 ± 0.08 | 0.31 ± 0.05 |
| BP-Co-AC | 469 | 539 | 552 ± 60 | 0.65 ± 0.08 | 0.32 ± 0.05 |
| BP-Ex-AC | 625 | 575 | 619 ± 68 | 0.81 ± 0.10 | 0.57 ± 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.P.; Nobre, J.R.C.; Napoli, A.; Trugilho, P.F.; Tonoli, G.H.D.; Wood, D.F.; Bianchi, M.L. Pretreatment Affects Activated Carbon from Piassava. Polymers 2020, 12, 1483. https://doi.org/10.3390/polym12071483
Castro JP, Nobre JRC, Napoli A, Trugilho PF, Tonoli GHD, Wood DF, Bianchi ML. Pretreatment Affects Activated Carbon from Piassava. Polymers. 2020; 12(7):1483. https://doi.org/10.3390/polym12071483
Chicago/Turabian StyleCastro, Jonnys Paz, João Rodrigo C. Nobre, Alfredo Napoli, Paulo Fernando Trugilho, Gustavo H. D. Tonoli, Delilah F. Wood, and Maria Lucia Bianchi. 2020. "Pretreatment Affects Activated Carbon from Piassava" Polymers 12, no. 7: 1483. https://doi.org/10.3390/polym12071483
APA StyleCastro, J. P., Nobre, J. R. C., Napoli, A., Trugilho, P. F., Tonoli, G. H. D., Wood, D. F., & Bianchi, M. L. (2020). Pretreatment Affects Activated Carbon from Piassava. Polymers, 12(7), 1483. https://doi.org/10.3390/polym12071483