Comparative Study of Aromatic and Cycloaliphatic Isocyanate Effects on Physico-Chemical Properties of Bio-Based Polyurethane Acrylate Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
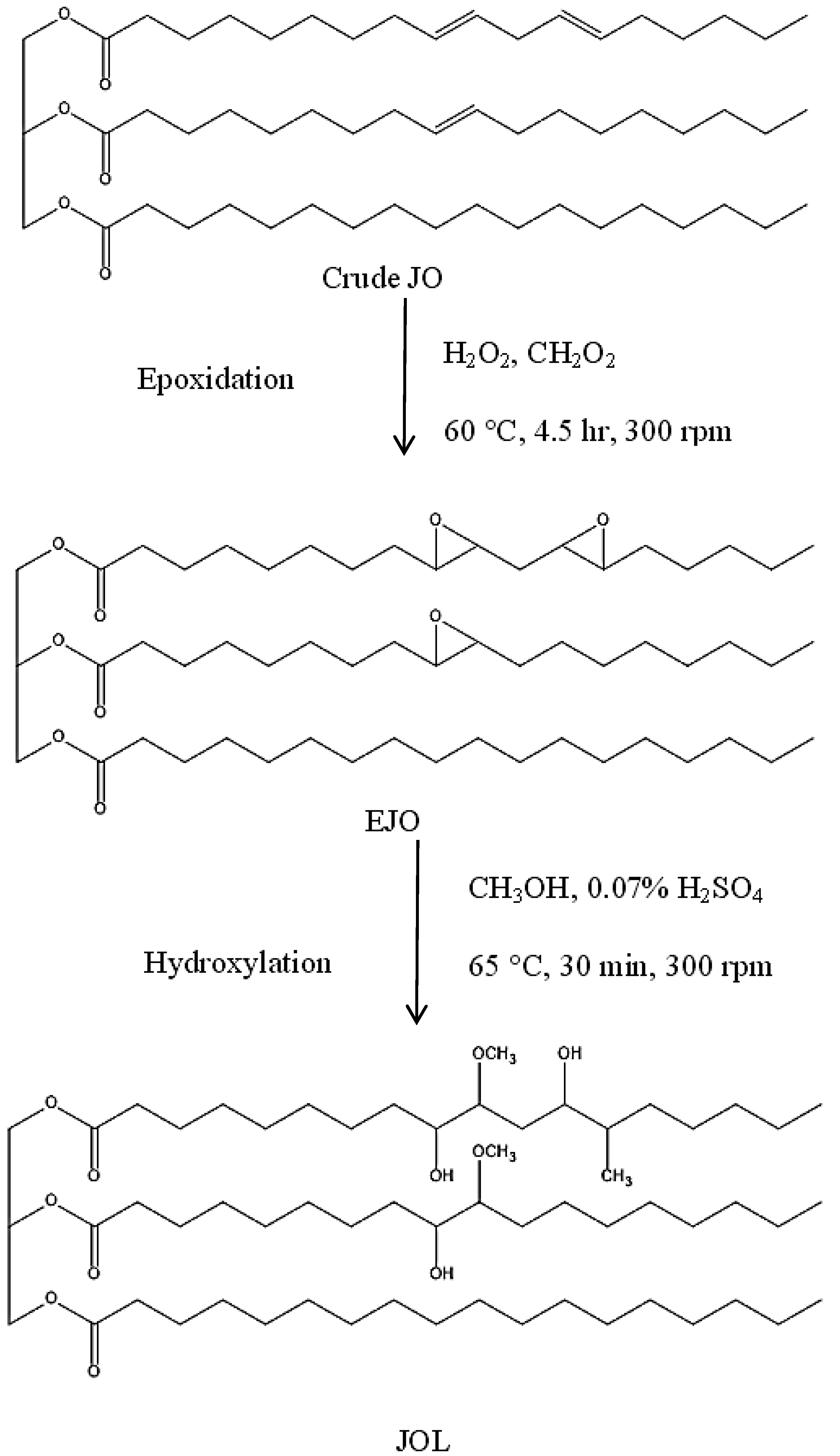
2.2. Synthesis of JOL
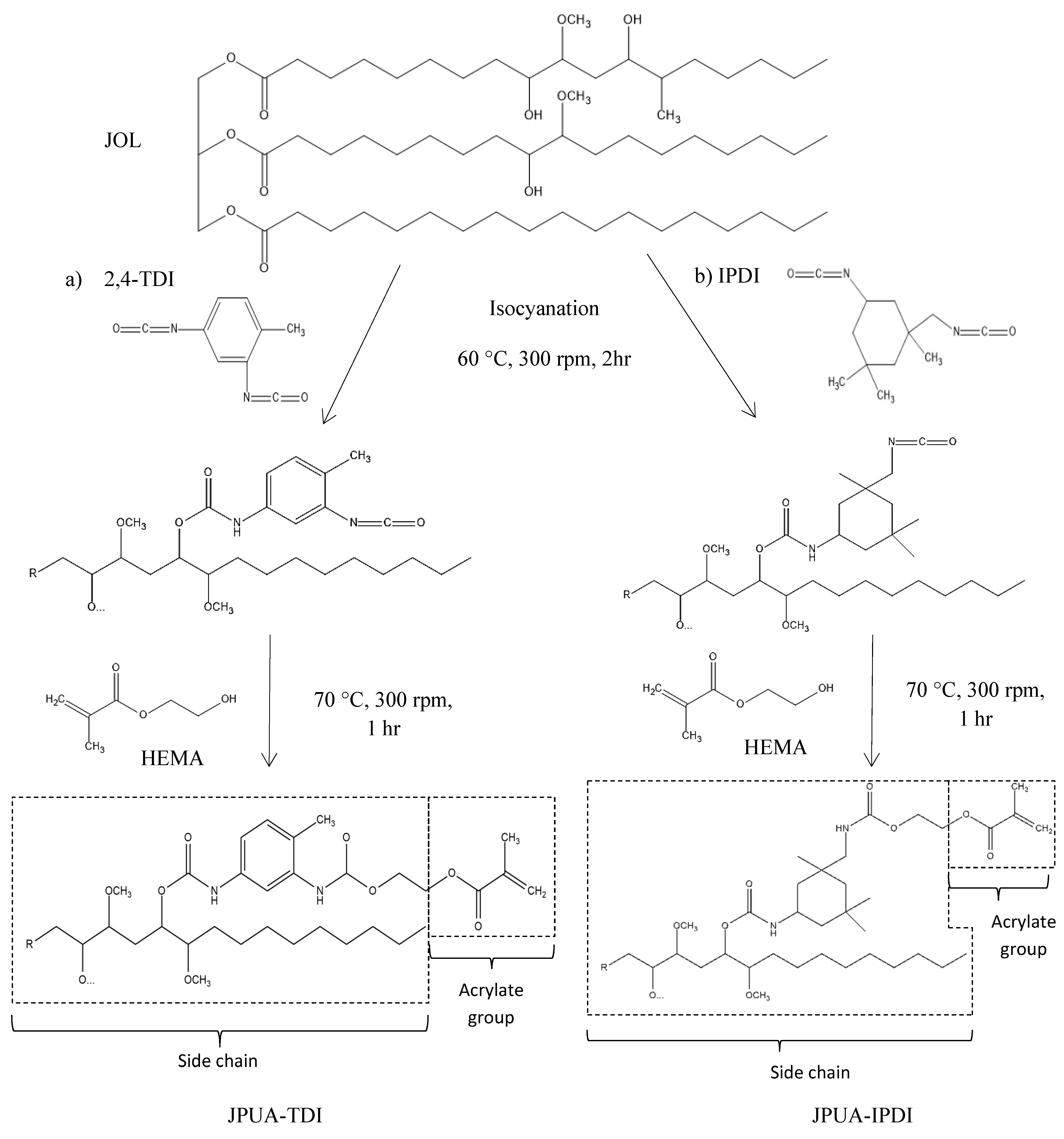
2.3. Synthesis of JPUA
2.4. Formulation and Curing of JPUA
2.5. Preparation of UV-Cured Film
2.6. Characterisation
2.6.1. Viscosity
2.6.2. Tintometer
2.6.3. Fourier Transform Infra-Red (FTIR) Spectroscopy
2.6.4. Gel Permeation Chromatography (GPC)
2.6.5. Thermal Analysis
2.6.6. Pendulum Hardness
2.6.7. Adhesion Test
2.6.8. Contact Angle
2.6.9. Transmittance and Haze Tests
3. Results and Discussion
3.1. Synthesis of JPUA
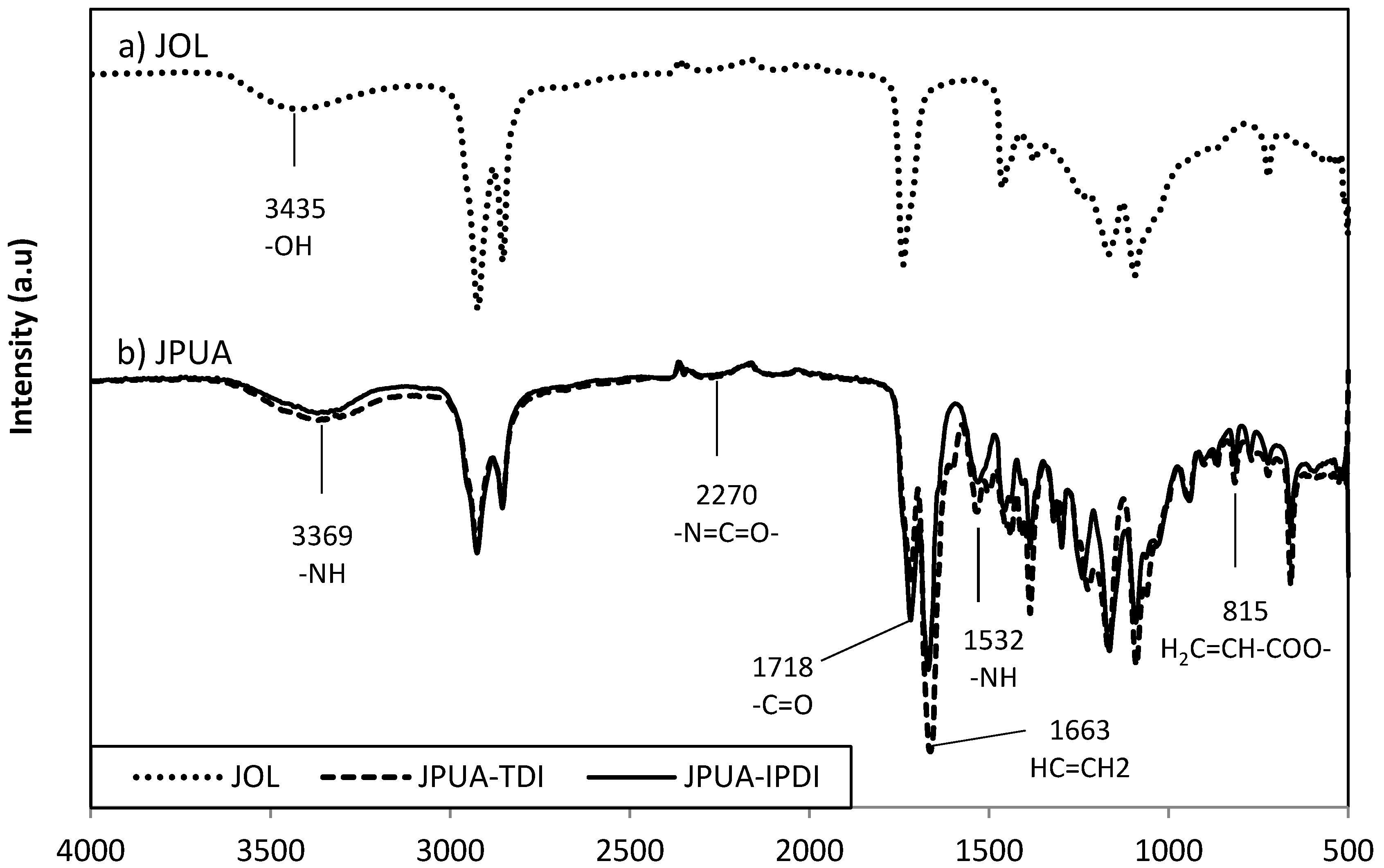
3.2. FTIR Analysis
3.3. Viscoelasticity Property
3.4. Thermal Analysis
3.5. UV Curing of the Coating
3.6. Mechanical Properties of Cured Film
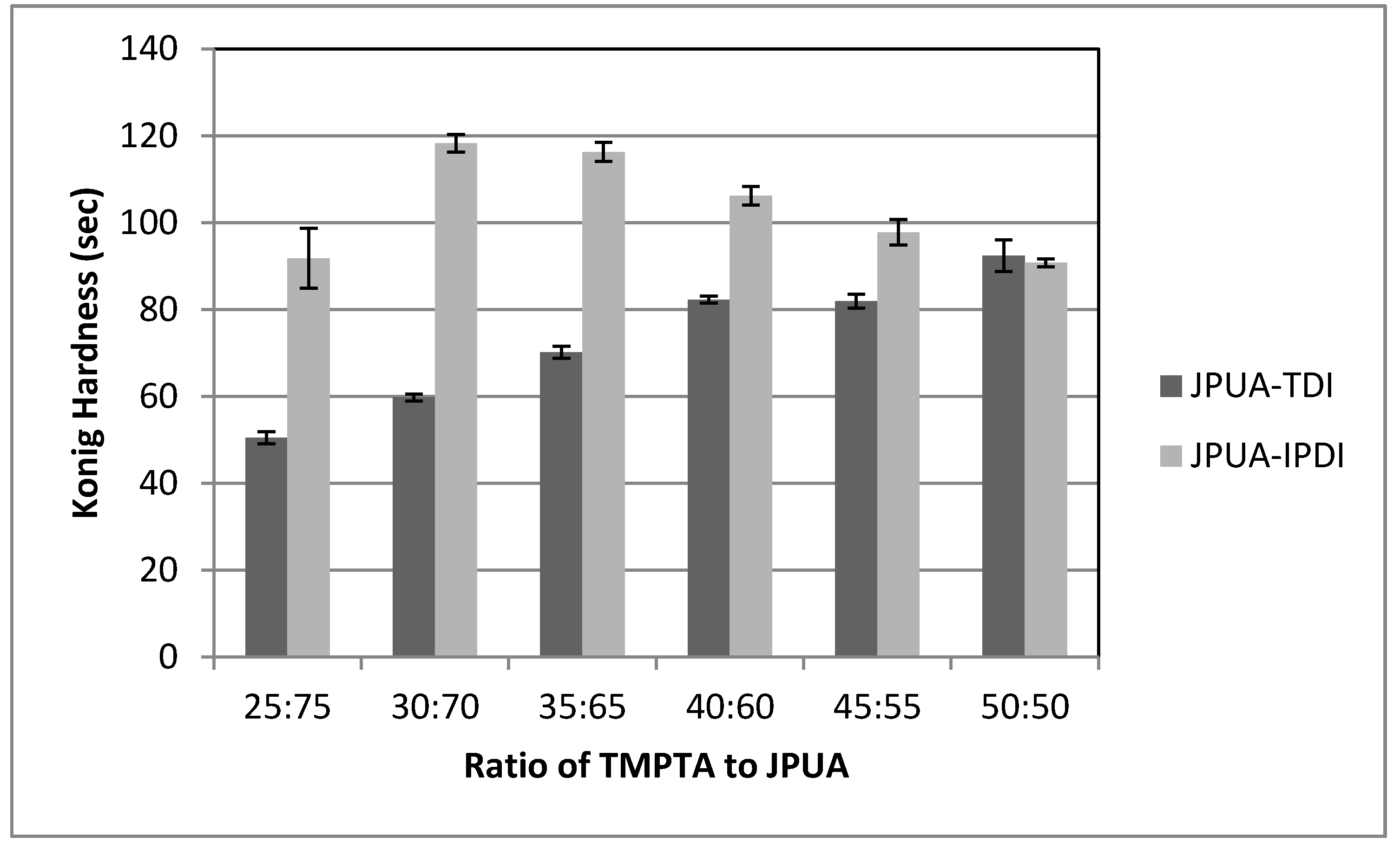
3.6.1. Hardness Test
3.6.2. Adhesion Test
3.6.3. Water Contact Angle
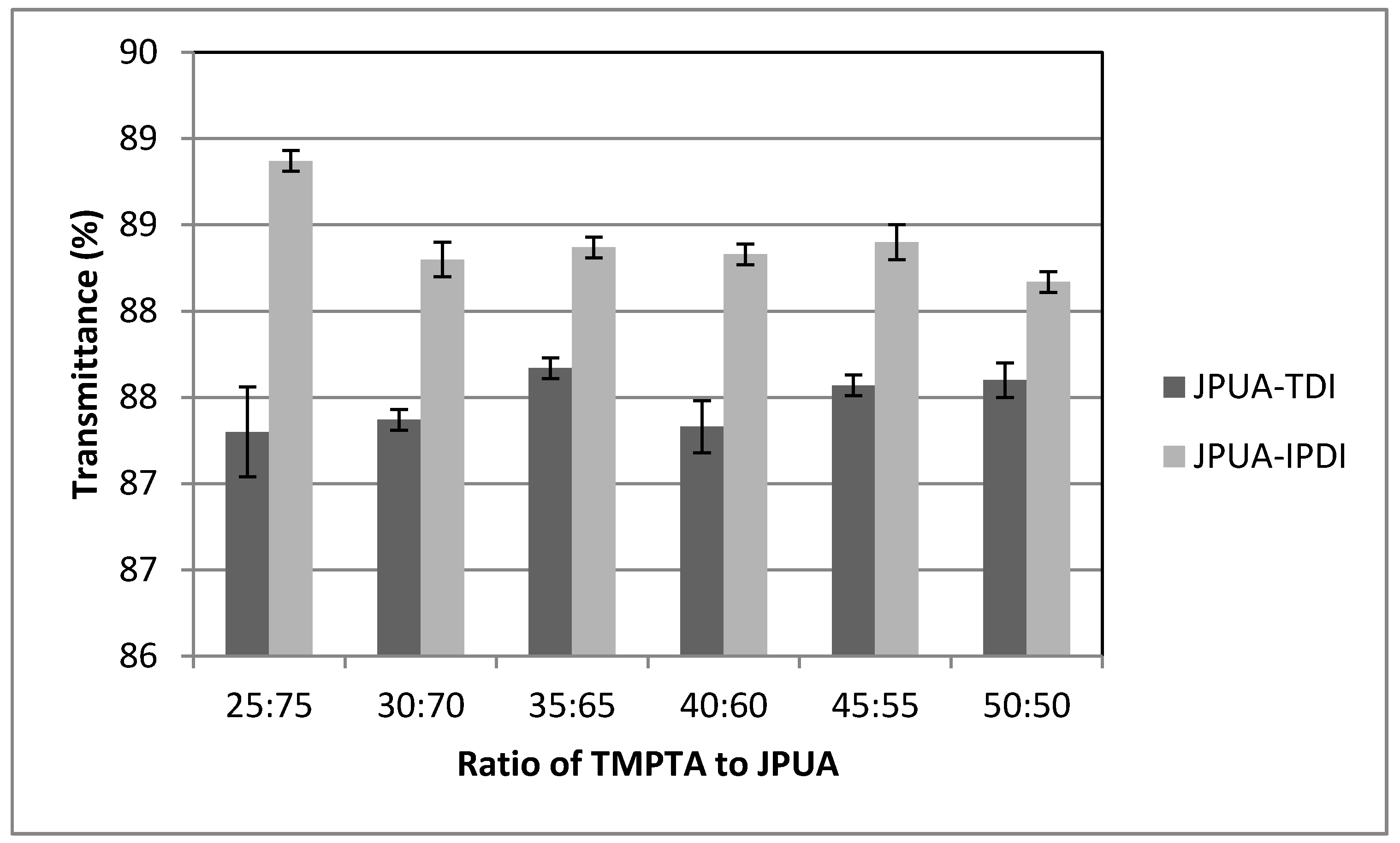
3.6.4. Transmittance Test
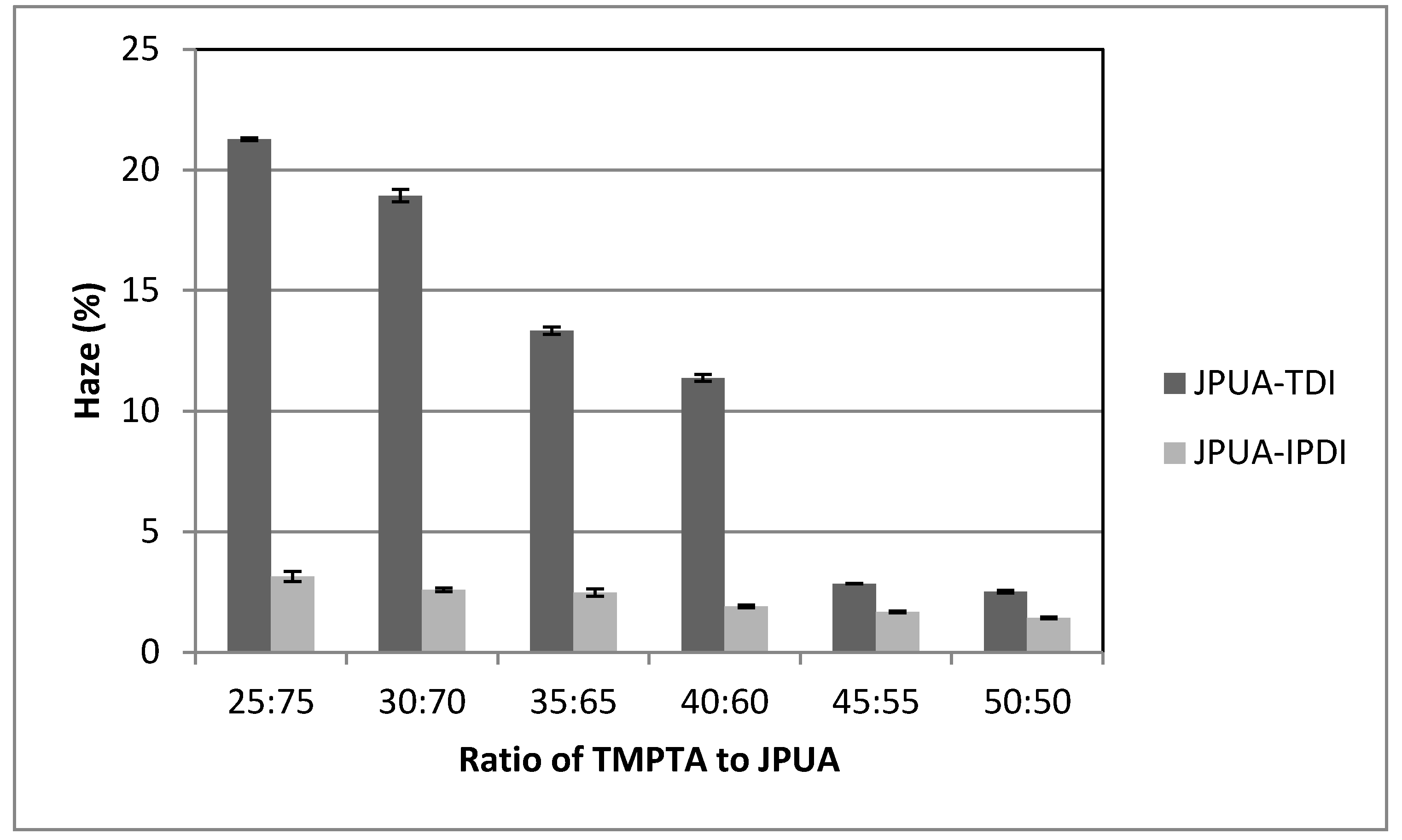
3.6.5. Haze Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent advances in vegetable oil-based polyurethanes. ChemSusChem 2011, 4, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Ling, J.S.; Ahmed Mohammed, I.; Ghazali, A.; Khairuddean, M. Novel poly(alkyd-urethane)s from vegetable oils: Synthesis and properties. Ind. Crops Prod. 2014, 52, 74–84. [Google Scholar] [CrossRef]
- Marathe, R.; Tatiya, P.; Chaudhari, A.; Lee, J.; Mahulikar, P.; Sohn, D.; Gite, V. Neem acetylated polyester polyol-Renewable source based smart PU coatings containing quinoline (corrosion inhibitor) encapsulated polyurea microcapsules for enhance anticorrosive property. Ind. Crops Prod. 2015, 77, 239–250. [Google Scholar] [CrossRef]
- Kalam, M.A.; Ahamed, J.U.; Masjuki, H.H. Land availability of Jatropha production in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 3999–4007. [Google Scholar] [CrossRef]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Ahmed, W.A.; Salimon, J. Phorbol ester as toxic constituents of tropical Jatropha curcas seed oil. Eur. J. Sci. Res. 2009, 31, 429–436. [Google Scholar]
- Goud, V.V.; Dinda, S.; Patwardhan, A.V.; Pradhan, N.C. Epoxidation of Jatropha (Jatropha curcas) oil by peroxyacids. Asia-Pac. J. Chem. Eng. 2010. [Google Scholar] [CrossRef]
- Hazmi, A.S.A.; Aung, M.M.; Abdullah, L.C.; Salleh, M.Z.; Mahmood, M.H. Producing Jatropha oil-based polyol via epoxidation and ring opening. Ind. Crops Prod. 2013, 50, 563–567. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Jusoh, E.R.; Mamat, S. Physicochemical Properties of jatropha oil-based polyol produced by a two steps method. Molecules 2017, 22, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, M.M.; Yaakob, Z.; Chuah Abdullah, L.; Rayung, M.; Jia Li, W. A comparative study of acrylate oligomer on Jatropha and Palm oil-based UV-curable surface coating. Ind. Crop. Prod. 2015, 77, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Rayung, M.; Aung, M.M.; Ahmad, A.; Su’ait, M.S.; Abdullah, L.C.; Ain Md Jamil, S.N. Characteristics of ionically conducting jatropha oil-based polyurethane acrylate gel electrolyte doped with potassium iodide. Mater. Chem. Phys. 2019, 222, 110–117. [Google Scholar] [CrossRef]
- Kai Ling, C.; Aung, M.M.; Rayung, M.; Chuah Abdullah, L.; Lim, H.N.; Mohd Noor, I.S. Performance of Ionic Transport Properties in Vegetable Oil-Based Polyurethane Acrylate Gel Polymer Electrolyte. ACS Omega 2019, 4, 2554–2564. [Google Scholar] [CrossRef]
- Gaikwad, M.S.; Gite, V.V.; Mahulikar, P.P.; Hundiwale, D.G.; Yemul, O.S. Eco-friendly polyurethane coatings from cottonseed and karanja oil. Prog. Org. Coat. 2015, 86, 164–172. [Google Scholar] [CrossRef]
- Bolognesi, C.; Baur, X.; Marczynski, B.; Norppa, H.; Sepai, O.; Sabbioni, G. Carcinogenic risk of toluene diisocyanate and 4,4′-methylenediphenyl diisocyanate: Epidemiological and experimental evidence. Crit. Rev. Toxicol. 2001, 31, 737–772. [Google Scholar] [CrossRef] [PubMed]
- Shiotsuka, R.N.; Stuart, B.P.; Charles, J.M.; Simon, G.S.; Malichky, P.; Mostowy, J.M. Chronic inhalation exposures of Fischer 344 rats to 1,6-hexamethylene diisocyanate did not reveal a carcinogenic potential. Inhal. Toxicol. 2010, 22, 875–887. [Google Scholar] [CrossRef] [PubMed]
- NIOSH Skin Notation Profile: Isophorone Diisocyanate. DHHS 2014, CAS No. 40, 1689–1699. [CrossRef]
- Scrinzi, E.; Rossi, S.; Deflorian, F.; Zanella, C. Evaluation of aesthetic durability of waterborne polyurethane coatings applied on wood for interior applications. Prog. Org. Coat. 2011, 72, 81–87. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.; Ibrahim, N.A.; Mohd Dahlan, K.Z.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Synthesis of radiation curable palm oil-based epoxy acrylate: NMR and FTIR spectroscopic investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardini, O.; Amalvy, J. FTIR, 1H-NMR Spectra, and Thermal Characterization of Water-Based Polyurethane/Acrylic Hybrids. J. Appl. Polym. Sci. 2008, 107, 1207–1214. [Google Scholar] [CrossRef]
- Taib, E.R.J.; Abdullah, L.C.; Aung, M.M.; Basri, M.; Salleh, M.Z.; Saalah, S.; Mamat, S.; Chee, C.Y.; Wong, J.L. Physico-chemical characterisation of epoxy acrylate resin from jatropha seed oil. Pigment Resin Technol. 2017, 46, 485–495. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Rajput, S.D.; Mahulikar, P.P.; Gite, V.V. Biobased dimer fatty acid containing two pack polyurethane for wood finished coatings. Prog. Org. Coat. 2014, 77, 38–46. [Google Scholar] [CrossRef]
- Du, P.; Liu, X.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Synthesis and characterization of linear self-healing polyurethane based on thermally reversible Diels-Alder reaction. RSC Adv. 2013. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-curable waterborne polyurethane-acrylate: Preparation, characterization and properties. Prog. Org. Coat. 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Habib, F.; Bajpai, M. Synthesis and Characterization of Acrylated Epoxidized Soybean Oil for Uv Cured Coatings. Chem. Chem. Technol. 2011, 5, 317–326. [Google Scholar] [CrossRef]
- Rahman, N.A.; Badri, K.H.; Salleh, N.G.N. UV-curable acrylated coating from epoxidized palm oil. AIP Conf. Proc. 2014, 1614, 439–445. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Rheology of lignin-based chemical oleogels prepared using diisocyanate crosslinkers: Effect of the diisocyanate and curing kinetics. Eur. Polym. J. 2017, 89, 311–323. [Google Scholar] [CrossRef]
- Raychura, A.J.; Jauhari, S.; Prajapati, V.S.; Dholakiya, B.Z. Synthesis and performance evaluation of vegetable oil based wood finish polyurethane coating. Bioresour. Technol. Rep. 2018, 3, 88–94. [Google Scholar] [CrossRef]
- Yildiz, Z.; Onen, H.A. Dual-curable PVB based adhesive formulations for cord/rubber composites: The influence of reactive diluents. Int. J. Adhes. Adhes. 2017, 78, 38–44. [Google Scholar] [CrossRef]
- Oprea, S. Properties of crosslinked polyurethanes obtained by acrylic side-group polymerization and of their blends with various plant oils. J. Appl. Polym. Sci. 2013, 129, 3640–3649. [Google Scholar] [CrossRef]
- Miller, D.C.; Bengoechea, J.; Bokria, J.G.; Köhl, M.; Powell, N.E.; Smith, M.E.; White, M.D.; Wilson, H.R.; Wohlgemuth, J.H. Examination of an optical transmittance test for photovoltaic encapsulation materials. In Proceedings of the Reliability of Photovoltaic Cells, Modules, Components, and Systems VI, San Diego, CA, USA, 27–30 August 2013. [Google Scholar]
- Chen, Y.H.; Liu, L.X.; Zhan, M.S. The preparation and characterization of abrasion-resistant coatings on polycarbonate. J. Coat. Technol. Res. 2013. [Google Scholar] [CrossRef]



| Code | TMPTA (%) | JPUA-TDI (%) | Benzophenone (%) |
|---|---|---|---|
| TDI 25 | 25 | 75 | 4 |
| TDI 30 | 30 | 70 | 4 |
| TDI 35 | 35 | 65 | 4 |
| TDI 40 | 40 | 60 | 4 |
| TDI 45 | 45 | 45 | 4 |
| TDI 50 | 50 | 50 | 4 |
| Code | TMPTA (%) | JPUA-IPDI (%) | Benzophenone (%) |
|---|---|---|---|
| IPDI 25 | 25 | 75 | 4 |
| IPDI 30 | 30 | 70 | 4 |
| IPDI 35 | 35 | 65 | 4 |
| IPDI 40 | 40 | 60 | 4 |
| IPDI 45 | 45 | 45 | 4 |
| IPDI 50 | 50 | 50 | 4 |
| Grade | Removed Area (%) | Appearance |
|---|---|---|
| 5B | None |  |
| 4B | Less than 5% |  |
| 3B | 5–15% | 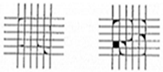 |
| 2B | 15–35% | 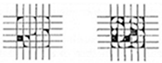 |
| 1B | 35–65% | 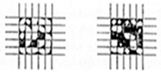 |
| 0B | Greater than 65% | 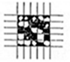 |
| Sample | Chemical Reaction | Appearance | Tintometer | State at Room Temp | OOC (% per mole) | OHV (mg KOH/g) |
|---|---|---|---|---|---|---|
| JO | No reaction |  | Red: 3.9 Yellow: 1.0 Blue: 0 Neutral: 0.2 | Liquid | - | - |
| EJO | Epoxidation |  | Red: 1.2 Yellow: 4.4 Blue: 0 Neutral: 0 | Liquid | 4.25 ± 0.08 | - |
| JOL | Hydroxylation |  | Red: 2.2 Yellow: 9.0 Blue: 0 Neutral: 0 | Liquid | 0.2 ± 0.03 | 149.44 ± 0.23 |
| JPUA-TDI | Isocyanation (aromatic) |  | Red: 2.9 Yellow: 0 Blue: 0 Neutral: 2.0 | Semi liquid | - | - |
| JPUA-IPDI | Isocyanation (cycloaliphatic) |  | Red: 1.6 Yellow: 0 Blue: 0 Neutral: 3.0 | Liquid | - | - |
| Sample | Mw | Mn | PDI (Mw/Mn) | Viscosity (Pas) |
|---|---|---|---|---|
| JO | 1278 | 948 | 1.35 | 0.056 |
| EJO | 1452 | 1041 | 1.39 | 0.342 |
| JOL | 1768 | 1426 | 1.23 | 2.336 |
| JPUA-TDI | 6871 | 3192 | 2.15 | 10.820 |
| JPUA-IPDI | 3151 | 2457 | 1.28 | 0.096 |
| JPUA Resin | Sample | Adhesion Score |
|---|---|---|
| JPUA-TDI | TDI 25 | 4B |
| TDI 30 | 4B | |
| TDI 35 | 4B | |
| TDI 40 | 1B | |
| TDI 45 | 1B | |
| TDI 50 | 0B | |
| JPUA-IPDI | IPDI 25 | 1B |
| IPDI 30 | 1B | |
| IPDI 35 | 1B | |
| IPDI 40 | 0B | |
| IPDI 45 | 0B | |
| IPDI 50 | 0B |
 |  |  |  |  |  |
| TDI 25 | TDI 30 | TDI 35 | TDI 40 | TDI 45 | TDI 50 |
 |  |  |  |  |  |
| IPDI 25 | IPDI 30 | IPDI 35 | IPDI 40 | IPDI 45 | IPDI 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Rayung, M. Comparative Study of Aromatic and Cycloaliphatic Isocyanate Effects on Physico-Chemical Properties of Bio-Based Polyurethane Acrylate Coatings. Polymers 2020, 12, 1494. https://doi.org/10.3390/polym12071494
Mudri NH, Abdullah LC, Aung MM, Salleh MZ, Awang Biak DR, Rayung M. Comparative Study of Aromatic and Cycloaliphatic Isocyanate Effects on Physico-Chemical Properties of Bio-Based Polyurethane Acrylate Coatings. Polymers. 2020; 12(7):1494. https://doi.org/10.3390/polym12071494
Chicago/Turabian StyleMudri, Nurul Huda, Luqman Chuah Abdullah, Min Min Aung, Mek Zah Salleh, Dayang Radiah Awang Biak, and Marwah Rayung. 2020. "Comparative Study of Aromatic and Cycloaliphatic Isocyanate Effects on Physico-Chemical Properties of Bio-Based Polyurethane Acrylate Coatings" Polymers 12, no. 7: 1494. https://doi.org/10.3390/polym12071494





