Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines
Abstract
:1. Introduction
2. Experimental
2.1. Materials
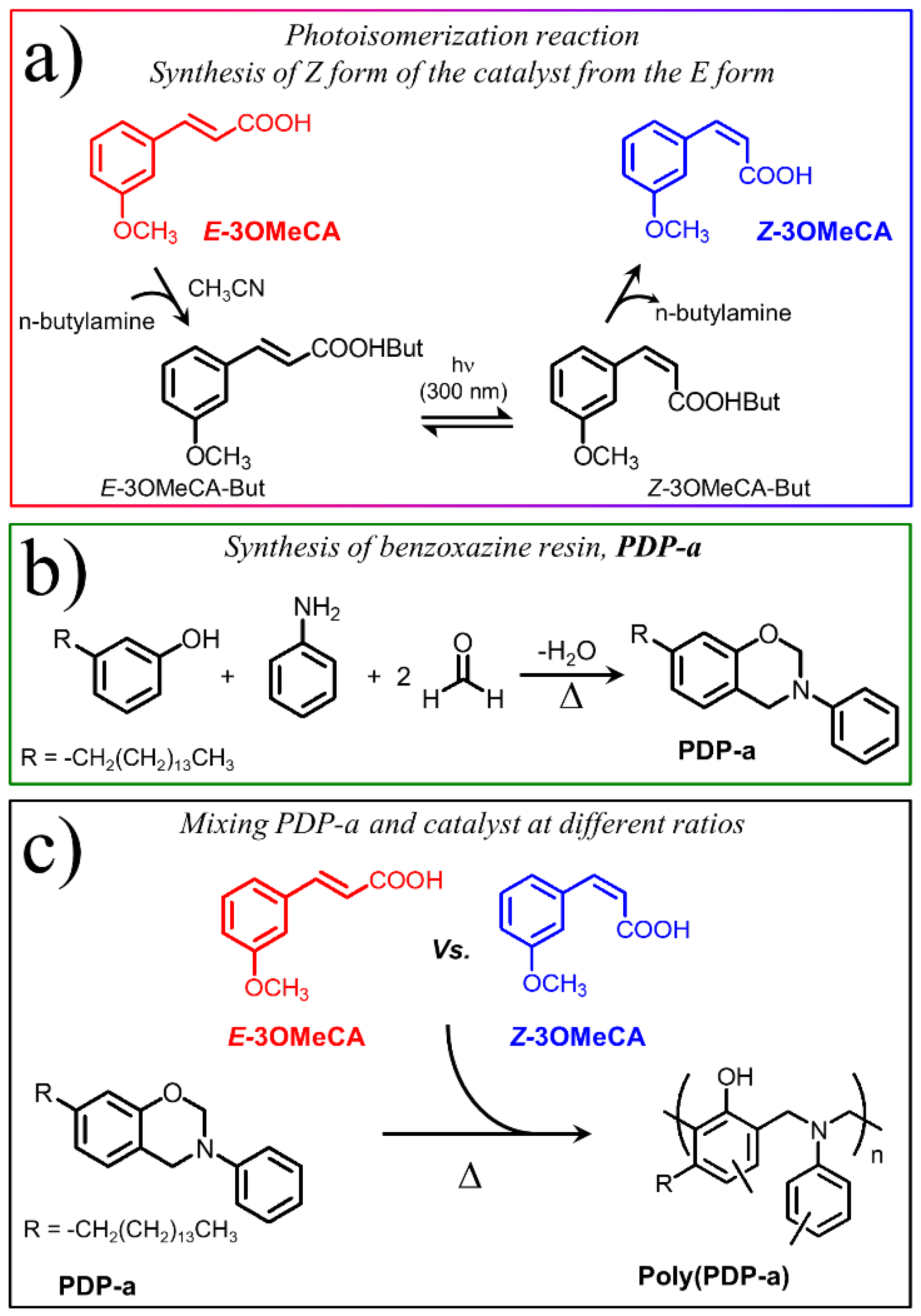
2.2. Synthesis of Z-3-Methoxycinnamic Acid (Z-3OMeCA)
2.3. Characterization of E-3-Methoxycinnamic Acid (E-3OMeCA)
2.4. Synthesis of Benzoxazine Monomer (PDP-a)
2.5. Preparation of Cinnamic Catalyst/Benzoxazine Resin Mixtures
2.6. Instrumentation
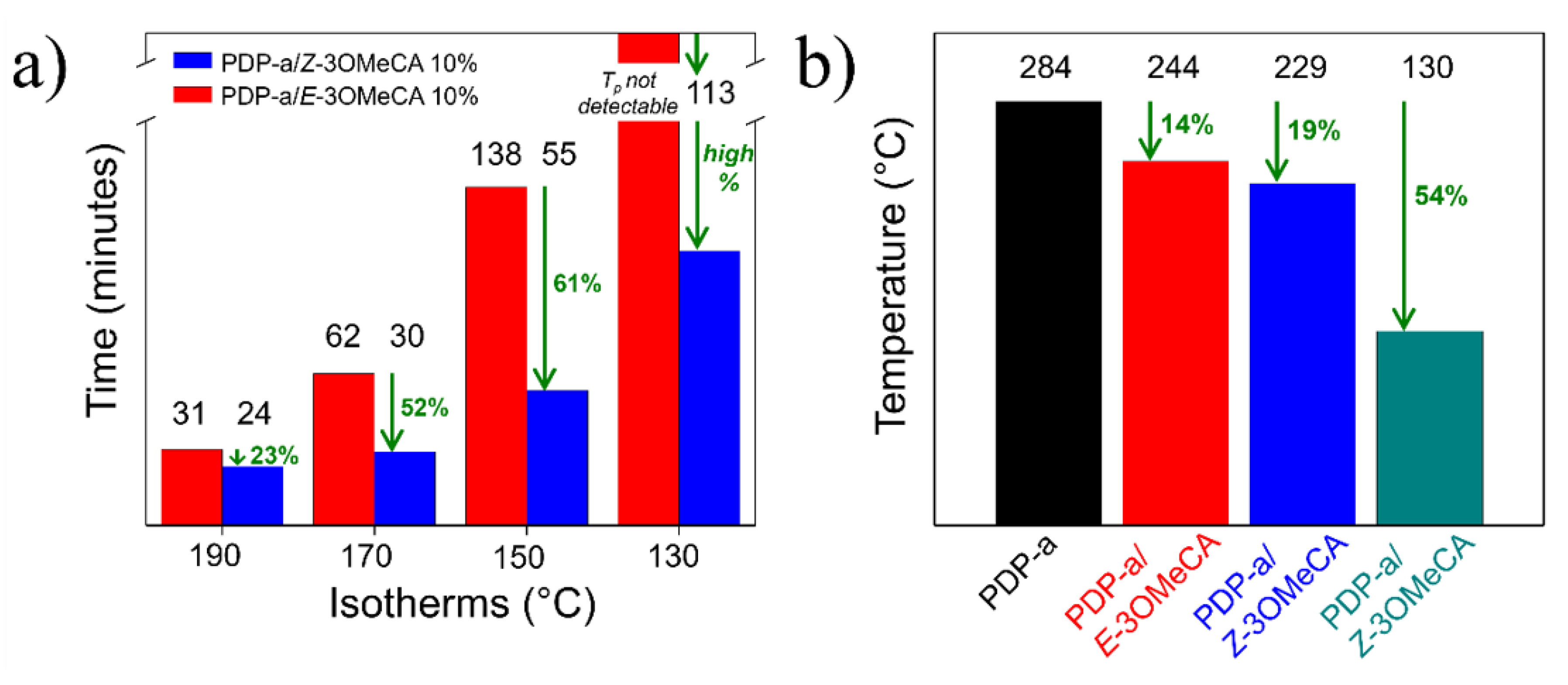
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ishida, H.; Froimowicz, P. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier Science BV: Amsterdam, The Netherlands, 2017; pp. 1–1097. [Google Scholar]
- Zhou, C.L.; Tao, M.; Liu, J.; Liu, T.; Lu, X.; Xin, Z. Effects of interfacial interaction on corrosion resistance of polybenzoxazine/sio2 nanocomposite coatings. ACS Appl. Polym. Mater. 2019, 1, 381–391. [Google Scholar] [CrossRef]
- Froimowicz, P.; Arza, C.R.; Han, L.; Ishida, H. Smart, sustainable, and ecofriendly chemical design of fully bio-based thermally stable thermosets based on benzoxazine chemistry. ChemSusChem 2016, 9, 1921–1928. [Google Scholar] [CrossRef]
- Salum, M.L.; Iguchi, D.; Arza, C.R.; Han, L.; Ishida, H.; Froimowicz, P. Making benzoxazines greener: Design, synthesis, and polymerization of a biobased benzoxazine fulfilling two principles of green chemistry. ACS Sustain. Chem. Eng. 2018, 6, 13096–13106. [Google Scholar] [CrossRef]
- Jubsilp, C.; Panyawanitchakun, C.; Rimdusit, S. Flammability and thermomechanical properties of dianhydride-modified polybenzoxazine composites reinforced with carbon fiber. Polym. Compos. 2013, 34, 2067–2075. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.Q.; Han, L.; Wang, J.Y.; Ishida, H. Synthesis and thermally induced structural transformation of phthalimide and nitrile-functionalized benzoxazine: Toward smart ortho-benzoxazine chemistry for low flammability thermosets. RSC Adv. 2019, 9, 1526–1535. [Google Scholar] [CrossRef] [Green Version]
- Ishida, H.; Low, H.Y. A study on the volumetric expansion of benzoxazine-based phenolic resin. Macromolecules 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Amarnath, N.; Shukla, S.; Lochab, B. Isomannide-derived chiral rigid fully biobased polybenzoxazines. ACS Sustain. Chem. Eng. 2019, 7, 18700–18710. [Google Scholar] [CrossRef]
- Malakooti, S.; Qin, G.Q.; Mandal, C.; Soni, R.; Taghvaee, T.; Ren, Y.; Chen, H.L.; Tsao, N.; Shiao, J.; Kulkarni, S.S.; et al. Low-cost, ambient-dried, superhydrophobic, high strength, thermally insulating, and thermally resilient polybenzoxazine aerogels. ACS Appl. Polym. Mater. 2019, 1, 2322–2333. [Google Scholar] [CrossRef]
- Zhang, W.F.; Froimowicz, P.; Arza, C.R.; Ohashi, S.; Xin, Z.; Ishida, H. Latent catalyst-containing naphthoxazine: Synthesis and effects on ring-opening polymerization. Macromolecules 2016, 49, 7129–7140. [Google Scholar] [CrossRef]
- Kaya, G.; Kiskan, B.; Yagci, Y. Phenolic naphthoxazines as curing promoters for benzoxazines. Macromolecules 2018, 51, 1688–1695. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.Q.; Han, M.C.; Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Deliballi, Z.; Kiskan, B.; Yagci, Y. Advanced polymers from simple benzoxazines and phenols by ring-opening addition reactions. Macromolecules 2020, 53, 2354–2361. [Google Scholar] [CrossRef]
- Akkus, B.; Kiskan, B.; Yagci, Y. Cyanuric chloride as a potent catalyst for the reduction of curing temperature of benzoxazines. Polym. Chem. 2020, 11, 1025–1032. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. The journey of phenolics from the first spark to advanced materials. Isr. J. Chem. 2020, 60, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Salum, M.L.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic self-initiating thermal ring-opening polymerization of 1,3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci. Pol. Chem. 2017, 55, 3434–3445. [Google Scholar] [CrossRef]
- Ishida, H.; Rodriguez, Y. Catalyzing the curing reaction of a new benzoxazine-based phenolic resin. J. Appl. Polym. Sci. 1995, 58, 1751–1760. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J. Polym. Sci. Pol. Chem. 1999, 37, 1913–1921. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 56. [Google Scholar] [CrossRef]
- Lligadas, G.; Tuzun, A.; Ronda, J.C.; Galia, M.; Cadiz, V. Polybenzoxazines: New players in the bio-based polymer arena. Polym. Chem. 2014, 5, 6636–6644. [Google Scholar] [CrossRef]
- Monisha, M.; Amarnath, N.; Mukherjee, S.; Lochab, B. Cardanol benzoxazines: A versatile monomer with advancing applications. Macromol. Chem. Phys. 2019, 220, 14. [Google Scholar] [CrossRef]
- Minigher, A.; Benedetti, E.; De Giacomo, O.; Campaner, P.; Aroulmoji, V. Synthesis and characterization of novel cardanol based benzoxazines. Nat. Prod. Commun. 2009, 4, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.F.; Sun, J.Q.; Liu, X.D.; Sudo, A.; Endo, T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012, 14, 2799–2806. [Google Scholar] [CrossRef]
- Comi, M.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Renewable benzoxazine monomers from “lignin-like” naturally occurring phenolic derivatives. J. Polym. Sci. Pol. Chem. 2013, 51, 4894–4903. [Google Scholar] [CrossRef]
- Steenackers, W.; El Houari, I.; Baekelandt, A.; Witvrouw, K.; Dhondt, S.; Leroux, O.; Gonzalez, N.; Corneillie, S.; Cesarino, I.; Inze, D.; et al. cis-Cinnamic acid is a natural plant growth-promoting compound. J. Exp. Bot. 2019, 70, 6293–6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, R.B.; Zapata, R.L.; Salum, M.L.; Erra-Balsells, R. Understanding the role played by protic ionic liquids (PILs) and the substituent effect for enhancing the generation of Z-cinnamic acid derivatives. Photochem. Photobiol. Sci. 2020, 19, 819–830. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Thermal behaviour of cardanol-based benzoxazines. J. Therm. Anal. Calorim. 2010, 102, 769–774. [Google Scholar] [CrossRef]
- Hanai, K.; Kuwae, A.; Takai, T.; Senda, H.; Kunimoto, K.K. A comparative vibrational and NMR study of cis-cinnamic acid polymorphs and trans-cinnamic acid. Spectroc. Acta Part A-Mol. Biomol. Spectrosc. 2001, 57, 513–519. [Google Scholar] [CrossRef]
- Salum, M.L.; Robles, C.J.; Erra-Balsells, R. Photoisomerization of ionic liquid ammonium cinnamates: One-pot synthesis-isolation of z-cinnamic acids. Org. Lett. 2010, 12, 4808–4811. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. An anomalous trade-off effect on the properties of smart ortho-functional benzoxazines. Polym. Chem. 2015, 6, 2541–2550. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Ishida, H.; Qutubuddin, S. Polymerization behavior of methylol-functional benzoxazine monomer. React. Funct. Polym. 2013, 73, 360–368. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, M.; Lochab, B.; Kumar, D.; Ramanan, A.; Roy, P.K. Metal-organic frameworks as curing accelerators for benzoxazines. ChemistrySelect 2016, 1, 3924–3932. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.W.; Yu, D.S. Multiwalled carbon nanotube/polybenzoxazine nanocomposites: Preparation, characterization and properties. Polymer 2006, 47, 7711–7719. [Google Scholar] [CrossRef]
- Wang, L.; Du, W.J.; Wu, Y.X.; Xu, R.W.; Yu, D.S. Synthesis and characterizations of a latent polyhedral oligomeric silsequioxane-containing catalyst and its application in polybenzoxazine resin. J. Appl. Polym. Sci. 2012, 126, 150–155. [Google Scholar] [CrossRef]
- He, J.; Li, X.Y.; Fu, Y.Z.; Zhu, H.B.; Zhao, G.Z.; Wang, Z. Curing reaction mechanism and heat resistance properties of hexa-(4-carboxyl-phenoxy)-cyclotriphosphazene/bisphenol A aniline benzoxazine blends. J. Appl. Polym. Sci. 2018, 135, 7. [Google Scholar] [CrossRef]





| \ | CHCl3 | MeOH | DMSO | H2O |
|---|---|---|---|---|
| E-3OMeCA | −/± | ✓ | ✓ | ✘ |
| Z-3OMeCA | ✓ | ✓ | ✓ | −/± |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, R.B.; Iguchi, D.; Erra-Balsells, R.; Salum, M.L.; Froimowicz, P. Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines. Polymers 2020, 12, 1527. https://doi.org/10.3390/polym12071527
Rodríguez RB, Iguchi D, Erra-Balsells R, Salum ML, Froimowicz P. Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines. Polymers. 2020; 12(7):1527. https://doi.org/10.3390/polym12071527
Chicago/Turabian StyleRodríguez, Rocío B., Daniela Iguchi, Rosa Erra-Balsells, M. Laura Salum, and Pablo Froimowicz. 2020. "Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines" Polymers 12, no. 7: 1527. https://doi.org/10.3390/polym12071527
APA StyleRodríguez, R. B., Iguchi, D., Erra-Balsells, R., Salum, M. L., & Froimowicz, P. (2020). Design and Effects of the Cinnamic Acids Chemical Structures as Organocatalyst on the Polymerization of Benzoxazines. Polymers, 12(7), 1527. https://doi.org/10.3390/polym12071527







