Optimization of Ethanol Detection by Automatic Headspace Method for Cellulose Insulation Aging of Oil-immersed Transformers
Abstract
:1. Introduction
2. Materials and Instruments
2.1. Materials
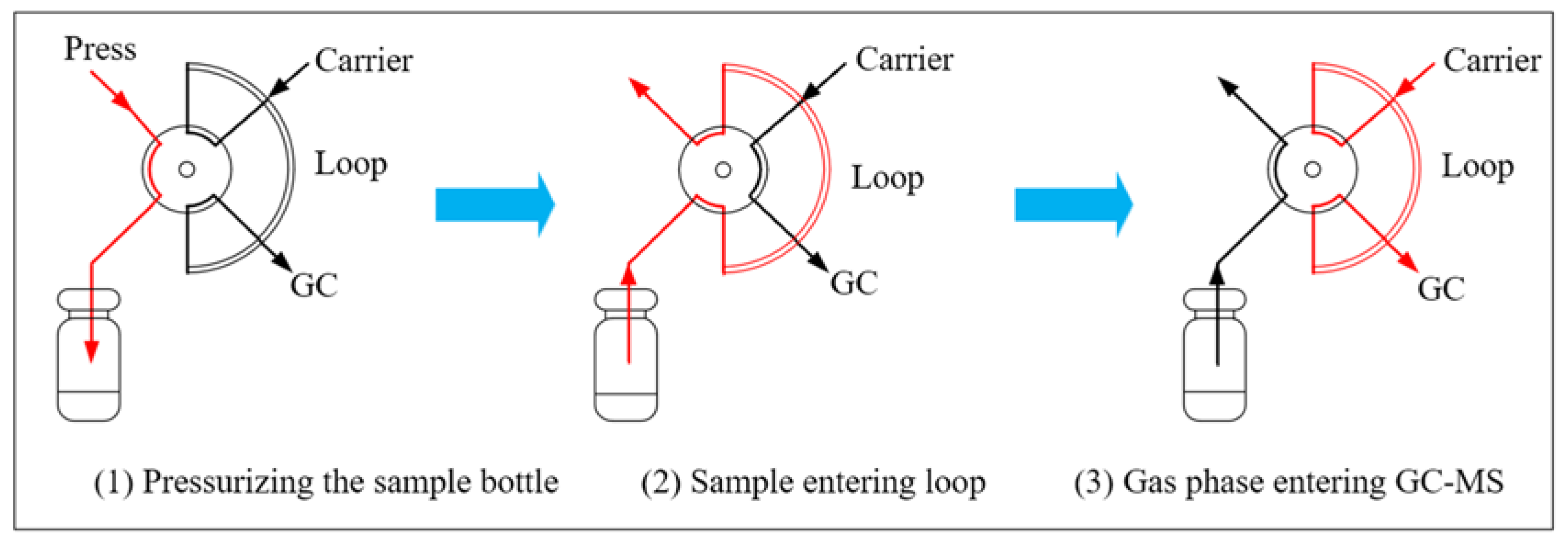
2.2. Instruments
3. Experimental Principle and Process Description
3.1. Selection of GC-MS Parameters
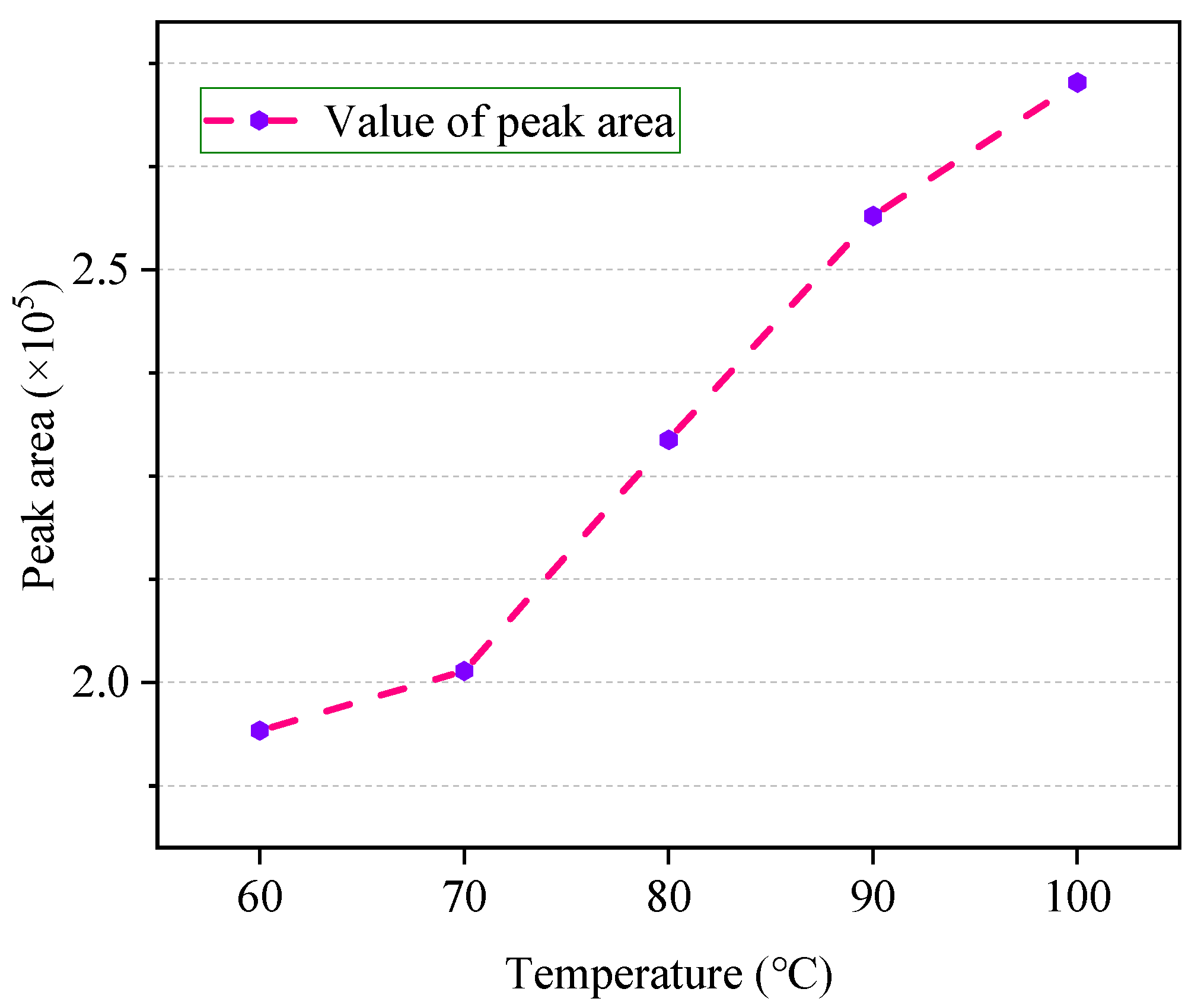
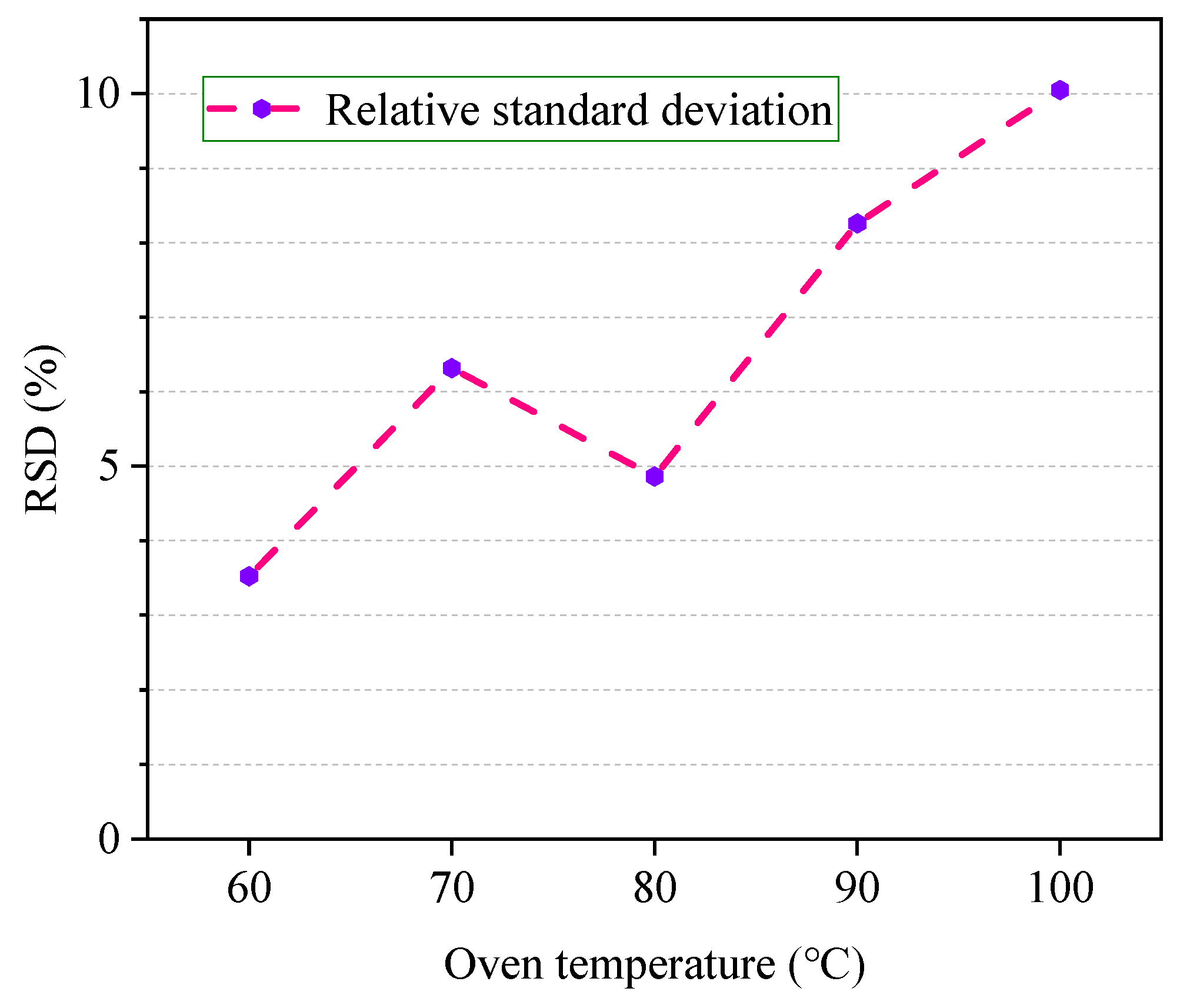
3.2. Selection of Equilibrium Temperature
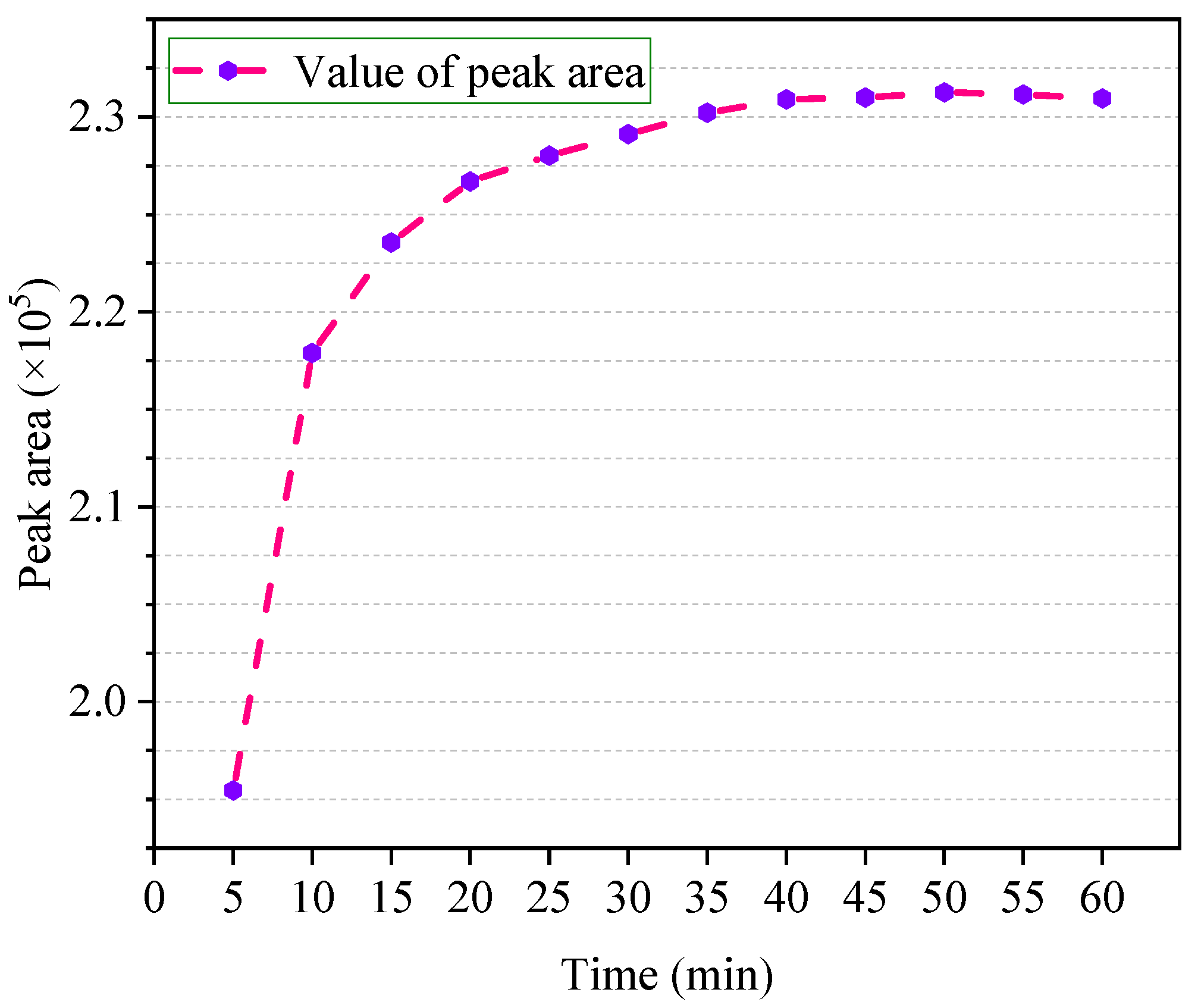
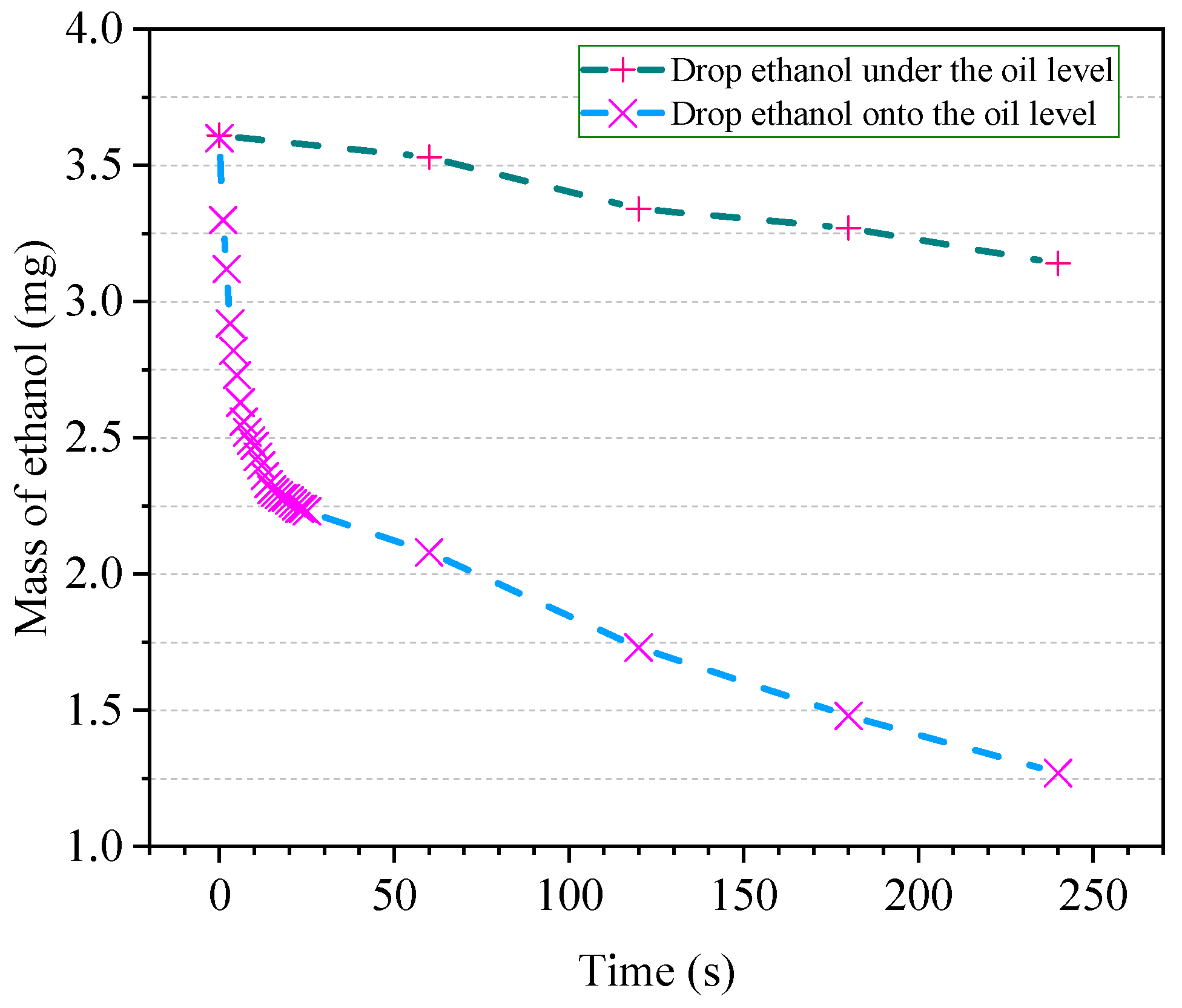
3.3. Selection of Equilibrium Time
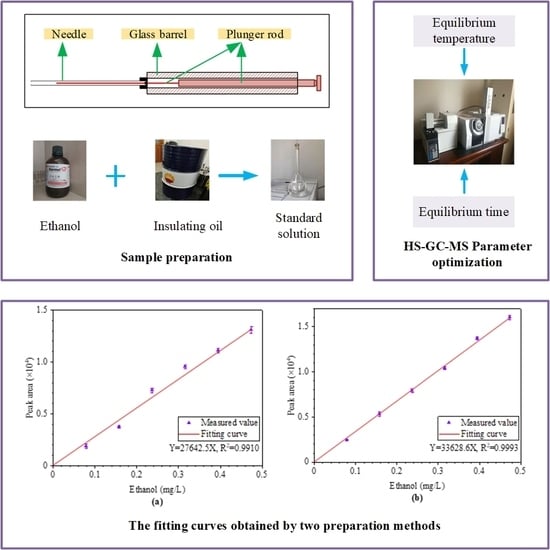
3.4. Standard Solution Preparation Method
3.4.1. Method by Stepwise Dilution
3.4.2. Method by Controlling Ethanol Volume
3.5. Methodology Validation
3.5.1. Standard Addition Recovery Test
3.5.2. Determination of Samples under Thermal Ageing Experiment
4. Results and Discussion
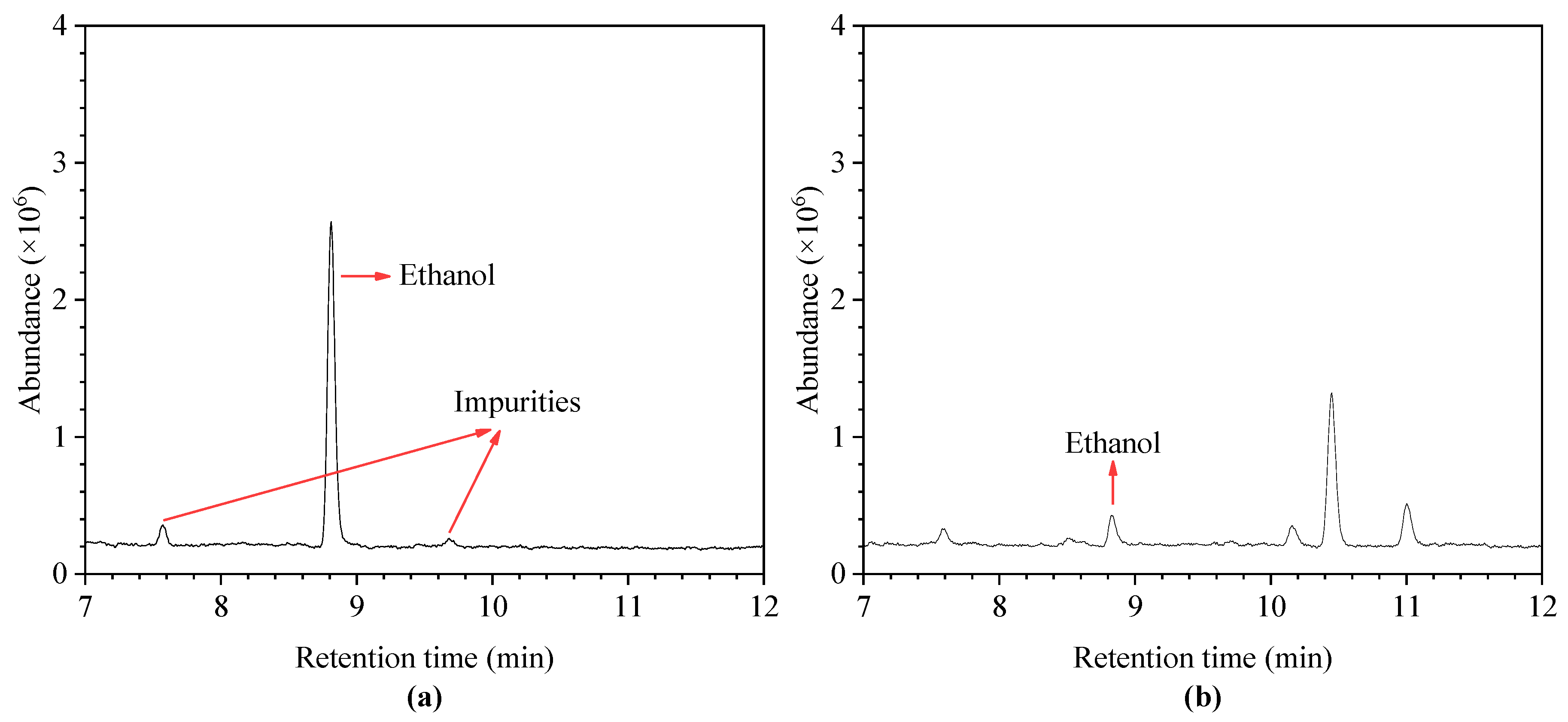
4.1. Optimal GC-MS Parameters
4.2. Optimal Equilibrium Temperature
4.3. Optimal Equilibrium Time
4.4. Comparison of Standard Solution Preparation Methods
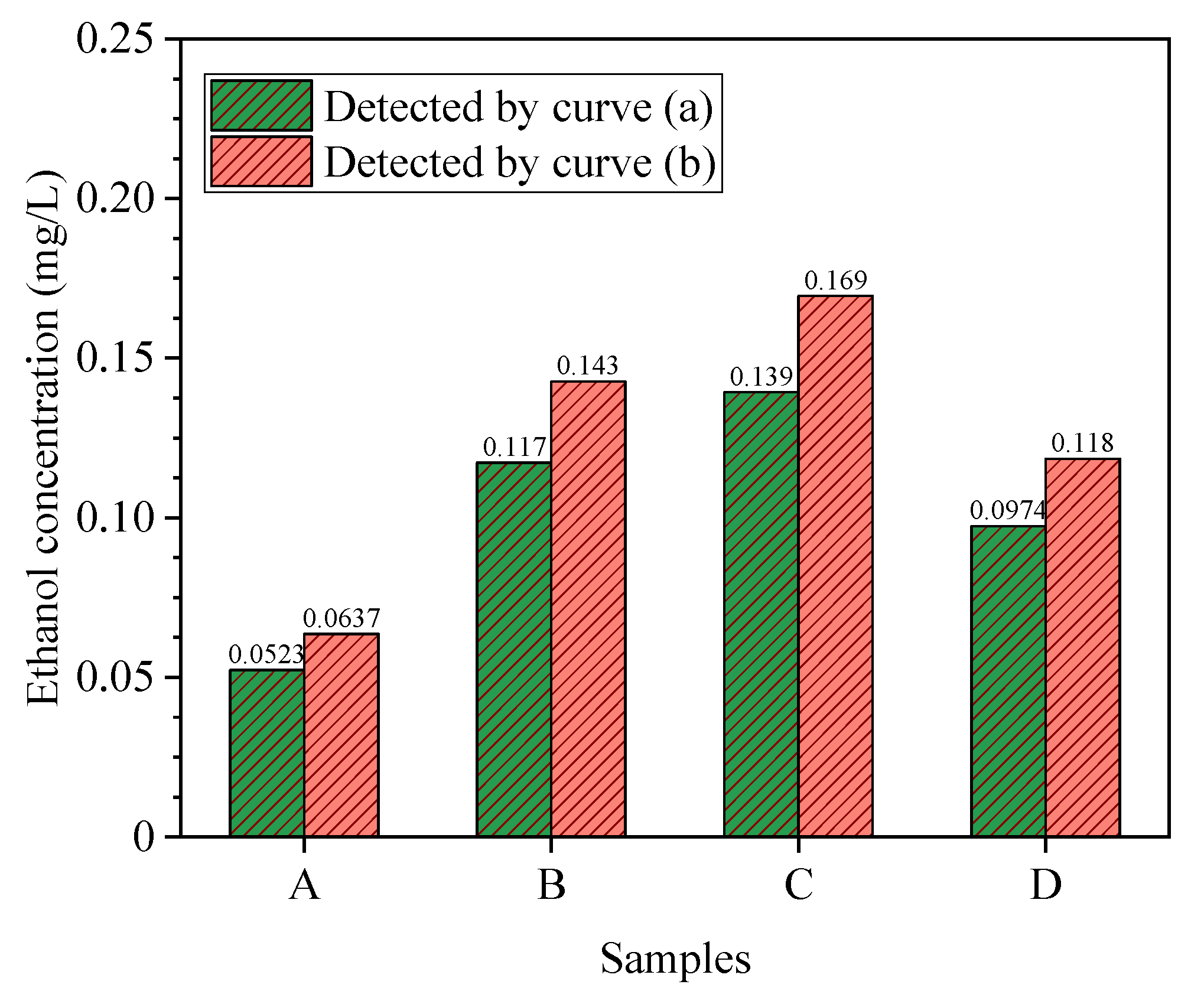
4.5. Results of Methodology Validation
5. Conclusions
- (1)
- The chromatographic peak area of ethanol in the sample was significantly affected by the headspace temperature and equilibrium time. In a certain range, the peak area increases gradually with the extension of the above two factors;
- (2)
- The reproducibility of the experiment results is best at about 80 °C and gas–liquid two phases equilibration will be obtained at about 40 min. This means that the ethanol detection results obtained under this condition are more scientific;
- (3)
- The standard curve obtained by controlling the sample volume of anhydrous ethanol when preparing standard solution can have a better linear relationship compared with the dilution method. Under the premise of satisfying the concentration range needed in detection, it is better to avoid by stepwise dilution when manufacturing the standard solution. If the standard curve cannot meet the detection requirements, a stock solution should be prepared and diluted stepwise.
Author Contributions
Funding
Conflicts of Interest
References
- Jalbert, J.; Duchesne, S.; Rodriguez-Celis, E.; Tétreault, P.; Collin, P. Robust and sensitive analysis of methanol and ethanol from cellulose degradation in mineral oils. J. Chromatogr. A 2012, 1256, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, J.; Gilbert, R.; Tétreault, P.; Morin, B.; Lessard-Déziel, D. Identification of a chemical indicator of the rupture of 1, 4-b-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 2007, 14, 295–309. [Google Scholar] [CrossRef]
- Christina, A.J.; Salam, M.A.; Rahman, Q.M.; Wen, F.; Ang, S.P.; Voon, W. Causes of transformer failures and diagnostic methods—A review. Renew. Sustain. Energy Rev. 2018, 82, 1442–1456. [Google Scholar]
- Gilbert, R.; Jalbert, J.; Tétreault, P.; Morin, B.; Denos, Y. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 1: Standard wood kraft insulation. Cellulose 2009, 16, 327–338. [Google Scholar] [CrossRef]
- Feng, D.; Hao, J.; Liao, R.; Chen, X.; Cheng, L.; Liu, M. Comparative study on the thermal-aging characteristics of cellulose insulation polymer immersed in new three-element mixed oil and mineral oil. Polymers 2019, 11, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IEC60450. Measurement of the Average Viscometric Degree of Polymerization of New and Aged Cellulosic Electrically Insulating Materials, 2nd ed.; NORMA, IEC: Geneva, Switzerland, 2004; Volume 4. [Google Scholar]
- ASTM D4243-99. Standard Test Method for Measurement of Average Viscometric Degree of Polymerization of New and Aged Electrical Papers and Boards; ASTM International: West Conshohocken, PA, USA, 2009; p. 6. [Google Scholar]
- Saha, T.K.; Purkait, P. Investigations of Temperature Effects on the Dielectric Response Measurements of Transformer Oil-Paper Insulation System. IEEE Trans. Power Del. 2007, 23, 252–260. [Google Scholar] [CrossRef]
- Bertrand, Y.; Tran-Duy, C.; Murin, V.; Schaut, A.; Autru, S.; Eeckhoudt, S. MV/LV distribution transformers: Research on paper ageing markers. Water Energy Int. 2013, 70, 50–51. [Google Scholar]
- Jalbert, J.; Lessard, M.C.; Ryadi, M. Cellulose chemical markers in transformer oil insulation part 1: Temperature correction factors. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2287–2291. [Google Scholar] [CrossRef]
- Bhumiwat, S.A. Identification of overheating in transformer solid insulation by polarization depolarization current analysis. In Proceedings of the Electrical Insulation Conference (EIC), Ottawa, ON, Canada, 2–5 June 2013. [Google Scholar]
- Rodriguez-Celis, E.; Jalbert, J.; Duchesne, S.; Noirhomme, B.; Lessard, M.C.; Ryadi, M. Chemical markers use for the diagnosis and life estimation of power transformers: A preliminary study of their origins. In Proceedings of the CIGRE Canada Conference, Montreal, QC, Canada, 24–26 September 2012. [Google Scholar]
- Rodriguez-Celis, E.M.; Duchesne, S.; Jalbert, J.; Ryadi, M. Understanding ethanol versus methanol formation from insulating paper in power transformers. Cellulose 2015, 22, 3225–3236. [Google Scholar] [CrossRef] [Green Version]
- Bruzzoniti, M.C.; Maina, R.; De Carlo, R.M.; Sarzanini, C.; Tumiatti, V. GC methods for the determination of methanol and ethanol in insulating mineral oils as markers of cellulose degradation in power transformers. Chromatographia 2014, 77, 1081–1089. [Google Scholar] [CrossRef]
- Matharage, S.Y.; Liu, Q.; Wang, Z.D. Ageing assessment of kraft paper insulation through methanol in oil measurement. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1589–1596. [Google Scholar] [CrossRef]
- Molavi, H.; Yousefpour, A.; Mirmostafa, A.; Sabzi, A.; Hamedi, S.; Narimani, M.; Abdi, N. Static headspace GC/MS method for determination of methanol and ethanol contents, as the degradation markers of solid insulation systems of power transformers. Chromatographia 2017, 80, 1129–1135. [Google Scholar] [CrossRef]
- Matharage, S.Y.; Liu, Q.; Davenport, E.; Wilson, G.; Walker, D.; Wang, Z.D. Methanol detection in transformer oils using gas chromatography and ion trap mass spectrometer. In Proceedings of the 2014 IEEE 18th International Conference on Dielectric Liquids (ICDL), Bled, Slovenia, 29 June–3 July 2014; pp. 1–4. [Google Scholar]
- Fu, Q.; Peng, L.; Li, L.; Lin, M.; Zhao, Y.; Li, S.; Chen, C. Detection of Methanol in Power Transformer Oil Using Spectroscopy. J. Electr. Eng. Technol. 2019, 14, 861. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Stahl, W.H.; Voelker, W.A.; Sullivan, J.H. A gas chromatographic method for determining gases in the headspace of cans and flexible packages. Food. Technol. 1960, 14, 14–16. [Google Scholar]
- Restek, A. Technical Guide for Static Headspace Analysis Using GC; Restek Corp.: Bellefonte, PA, USA, 2000; pp. 11–12. [Google Scholar]
- Snow, N.H.; Slack, G.C. Head-space analysis in modern gas chromatography. Trac-Trend. Anal. Chem. 2002, 21, 608–617. [Google Scholar] [CrossRef]
- Soria, A.C.; García-Sarrió, M.J.; Sanz, M.L. Volatile sampling by headspace techniques. Trac-Trend. Anal. Chem. 2015, 71, 85–99. [Google Scholar] [CrossRef]
- Agilent G1888 Technical Instruction. Agilent: California. Available online: http://www.agilent.com/cs/library/brochures/5989-6491_CHCN.pdf (accessed on 7 June 2020).
- DANI HSS 86.50 Plus Technical Instruction. DANI: Italy. Available online: https://www.dani-instruments.com/wordpress/wp-content/uploads/2019/01/8650_DANI_ANALITICA.pdf (accessed on 7 June 2020).
- Girard, B.; Nakai, S. Static headspace gas chromato-graphic method for volatiles in canned salmon. J. Food. Sci 1991, 56, 1271–1274. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.M.; Santiago, K.S. A novel application of electroactive polyimide doped with gold nanoparticles: As a chemiresistor sensor for hydrogen sulfide gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, P.; Nowruzi, H. Effects of Heavy Fuel Oil Blend with Ethanol, n-Butanol or Methanol Bioalcohols on the Spray Characteristics. J. Appl. Fluid. Mech. 2016, 9, 2413–2425. [Google Scholar] [CrossRef]

| Properties | Insulating Oil | Ethanol |
|---|---|---|
| Density (20 °C), kg/m3 | 895.7 | - |
| H2O, ppm | 29 | - |
| Density (20 °C), g/mL | - | 0.790 |
| H2O, % | - | 0.01 |
| Number | Aging Time (Days) | Temperature (°C) | Oil-Paper Ratio |
|---|---|---|---|
| A | 10 | 100 | 10:1 |
| B | 5 | 110 | 10:1 |
| C | 5 | 130 | 15:1 |
| D | 3 | 150 | 10:1 |
| Instrument Parameters | GC | MS |
|---|---|---|
| Split ratio | 30:01:00 | - |
| Helium carrier gas flow rate | 1.04 mL/min | - |
| Interface temperature | 250 °C | - |
| Pressure | 52.6 kPa | - |
| Temperature program | 40 °C for 10 min, then 10 °C/min to 210 °C, hold on 20 min. | - |
| Ion source | - | Electron impact |
| Ionization energy | - | 70 eV |
| Acquisition mode | - | Scan |
| mass-to-charge ratios | - | 20–100 amu |
| Ion source temperature | - | 200 °C |
| Quadrupole temperature | - | 150 °C |
| Instrument Parameters | Headspace Sample |
|---|---|
| Oven temperature | 80 °C |
| Equilibrium time | 40 min |
| Loop temperature | 110 °C |
| Transfer line temperature | 120 °C |
| Vial pressure | 15 psi |
| Pressurization time | 0.15 min |
| Injection time | 0.50 min |
| Loop fill time | 0.15 min |
| Initial Sample Concentration (mg/L) | Standard Addition Concentration (mg/L) | Ethanol Content after Standard Addition (μg) | Average Recovery (%) | ||
|---|---|---|---|---|---|
| 0 | 10 | 9.5 | 8.7 | 8.9 | 90.3 |
| 0 | 15 | 14.4 | 12.8 | 13.7 | 90.9 |
| 0 | 20 | 18.6 | 18.5 | 19.4 | 94.1 |
| 0.1 | 10 | 19.6 | 18.9 | 19.4 | 93.0 |
| 0.1 | 15 | 24.3 | 24.3 | 24.5 | 95.8 |
| 0.1 | 20 | 27.8 | 28.3 | 28.6 | 91.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Zhang, C.; Zhang, Y.; Liu, J.; Zhang, E.; Shi, Z.; Shao, G.; Shi, K.; Guo, J.; Zhang, C. Optimization of Ethanol Detection by Automatic Headspace Method for Cellulose Insulation Aging of Oil-immersed Transformers. Polymers 2020, 12, 1567. https://doi.org/10.3390/polym12071567
Zheng H, Zhang C, Zhang Y, Liu J, Zhang E, Shi Z, Shao G, Shi K, Guo J, Zhang C. Optimization of Ethanol Detection by Automatic Headspace Method for Cellulose Insulation Aging of Oil-immersed Transformers. Polymers. 2020; 12(7):1567. https://doi.org/10.3390/polym12071567
Chicago/Turabian StyleZheng, Hanbo, Chuansheng Zhang, Yiyi Zhang, Jiefeng Liu, Enze Zhang, Zhen Shi, Guangqi Shao, Kuikui Shi, Jing Guo, and Chaohai Zhang. 2020. "Optimization of Ethanol Detection by Automatic Headspace Method for Cellulose Insulation Aging of Oil-immersed Transformers" Polymers 12, no. 7: 1567. https://doi.org/10.3390/polym12071567
APA StyleZheng, H., Zhang, C., Zhang, Y., Liu, J., Zhang, E., Shi, Z., Shao, G., Shi, K., Guo, J., & Zhang, C. (2020). Optimization of Ethanol Detection by Automatic Headspace Method for Cellulose Insulation Aging of Oil-immersed Transformers. Polymers, 12(7), 1567. https://doi.org/10.3390/polym12071567