Effects of Electrospun Fibrous Membranes of PolyCaprolactone and Chitosan/Poly(Ethylene Oxide) on Mouse Acute Skin Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Electrospun Scaffolds Preparation
2.2. Methods
2.2.1. In Vivo Studies
2.2.2. Surgical Procedures
2.2.3. Experimental Design and Lesion Treatment
2.2.4. Sample Collection
2.2.5. Histological Analysis
2.2.6. Histomorphometric Analysis with Light Microscopy
2.2.7. Collagen Fiber Analysis
2.2.8. Immunohistochemical Analysis
2.2.9. Immunoblotting
2.2.10. Statistical Analysis
3. Results
3.1. Morphological Aspect and Other Relevant Characteristics of the Membranes
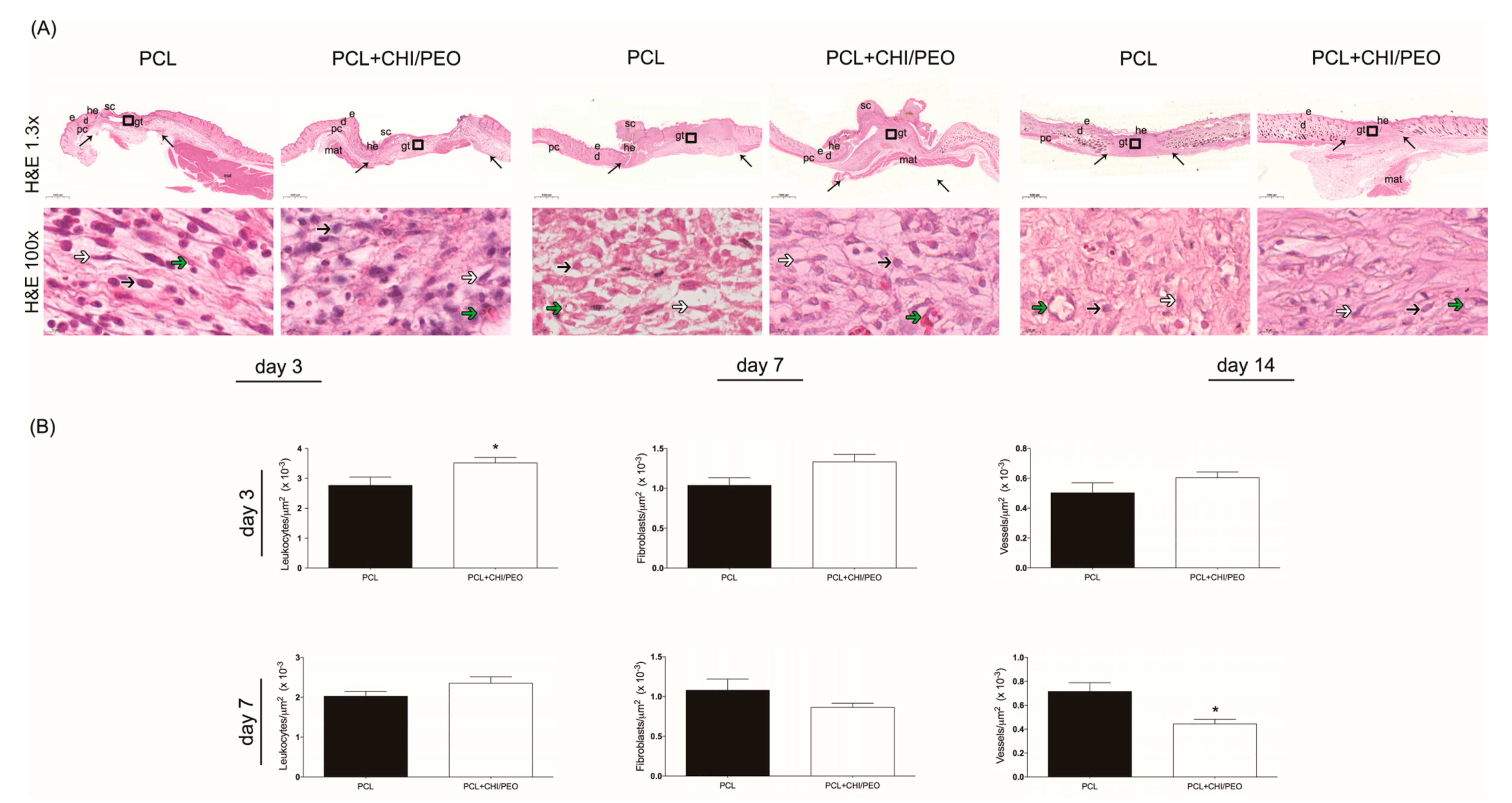
3.2. Morphological Analysis of the Lesions
3.3. Histological Analysis
3.4. Morphometric Analysis
3.5. Immunohistochemical Analysis
3.6. Collagen Fiber Analysis
3.7. Immunoblotting
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Bierhalz, A.C.K.; Pires, A.L.R.; Moraes, A.M. Biopolyer-based Films and Membranes as Wound Dressings In Biopolymer Membranes and Films: Health, Food, Environment and Energy Applications; Silva, M.d.M.C., Vieira, R., Eds.; Elsevier: Oxford, UK, 2020; pp. 165–194. [Google Scholar]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide Based Scaffolds for Soft Tissue Engineering Applications. Polymers (Basel) 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Laird, C.; Porter, K.; Bloch, M. Haemostatic dressings in prehospital care. Emerg. Med. J. 2013, 30, 784–789. [Google Scholar] [CrossRef]
- Bombaldi de Souza, F.C.; Bombaldi de Souza, R.F.; Moraes, Â.M. Incorporation and release kinetics of alpha-bisabolol from PCL and Chitosan/Guar Gum membranes. Braz. J. Chem. Eng. 2016, 33, 453–467. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Schoukens, G. Bioactive dressing to promote wound healing. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 114–152. [Google Scholar]
- Miles, K.B.; Ball, R.L.; Matthew, H.W. Chitosan films with improved tensile strength and toughness from N-acetyl-cysteine mediated disulfide bonds. Carbohydr. Polym. 2016, 139, 1–9. [Google Scholar] [CrossRef]
- Khan, Z.; Jamil, S.; Akhtar, A.; Bashir, M.; Yar, M. Chitosan based hybrid materials used for wound healing applications- A short review. Int. J. Polym. Mater. 2019, 1–18. [Google Scholar]
- Cai, E.Z.; Teo, E.Y.; Jing, L.; Koh, Y.P.; Qian, T.S.; Wen, F.; Lee, J.W.; Hing, E.C.; Yap, Y.L.; Lee, H.; et al. Bio-conjugated polycaprolactone membranes: A novel wound dressing. Arch. Plast Surg 2014, 41, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.; Sanchez, E.; Mano, J.F.; Moraes, A.M. Characterization of chitosan and polycaprolactone membranes designed for wound repair application. J. Mater. Sci. 2012, 47, 659–667. [Google Scholar] [CrossRef]
- Croisier, F.; Atanasova, G.; Poumay, Y.; Jérôme, C. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv. Healthc Mater. 2014, 3, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 10, 1217–1256. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun poly(ε-caprolactone)-based skin substitutes: In vivo evaluation of wound healing and the mechanism of cell proliferation. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1445–1454. [Google Scholar] [CrossRef]
- Yoon, C.; Ji, D. Effects of in vitro degradation on the weight loss and tensile properties of PLA/LPCL/HPCL blend fibers. Fibers Polym. 2005, 6, 13–18. [Google Scholar] [CrossRef]
- Rajam, M.; Pulavendran, S.; Rose, C.; Mandal, A.B. Chitosan nanoparticles as a dual growth factor delivery system for tissue engineering applications. Int. J. Pharm. 2011, 410, 145–152. [Google Scholar] [CrossRef]
- Silver, F.H. Wound dressings and skin replacement. In Biomaterials, Medical Devices and Tissue Engineering: An Integrated Approach, 1st ed.; Springer Science+Business Media Dordrecht: London, UK, 1994; pp. 46–91. [Google Scholar]
- Hwang, P.T.; Murdock, K.; Alexander, G.C.; Salaam, A.D.; Ng, J.I.; Lim, D.J.; Dean, D.; Jun, H.W. Poly(ɛ-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 1017–1029. [Google Scholar] [CrossRef]
- Xiang, P.; Wu, K.C.; Zhu, Y.; Xiang, L.; Li, C.; Chen, D.L.; Chen, F.; Xu, G.; Wang, A.; Li, M.; et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014, 35, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chung, M.Y.; Unnithan, A.R.; Kim, C.S. Creation of a functional graded nanobiomembrane using a new electrospinning system for drug release control and an in vitro validation of drug release behavior of the coating membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Hackensack, NJ, USA; London, UK, 2005. [Google Scholar]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine (Lond.) 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int. Wound J. 2014, 11, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Drumheller, P.D.; Hubbell, J.A. Densely crosslinked polymer networks of poly(ethylene glycol) in trimethylolpropane triacrylate for cell-adhesion-resistant surfaces. J. Biomed. Mater. Res. 1995, 29, 207–215. [Google Scholar] [CrossRef]
- Trinca, R.; Felisberti, M. Segmented polyurethanes based on poly(L-lactide), poly(ethylene glycol) and poly(trimethylene carbonate): Physico-chemical properties and morphology. Eur. Polym. J. 2015, 62, 77–86. [Google Scholar] [CrossRef]
- Trinca, R.; Westin, C.; da Silva, J.; Moraes, A. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Pessoa, A.F.; Florim, J.C.; Rodrigues, H.G.; Andrade-Oliveira, V.; Teixeira, S.A.; Vitzel, K.F.; Curi, R.; Saraiva Câmara, N.O.; Muscará, M.N.; Lamers, M.L.; et al. Oral administration of antioxidants improves skin wound healing in diabetic mice. Wound Repair Regen. 2016, 24, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.M.; Hankenson, F.C.; Heng, K.; McKinstry-Wu, A.; Kelz, M.B.; Marx, J.O. Dose regimens, variability, and complications associated with using repeat-bolus dosing to extend a surgical plane of anesthesia in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 684–691. [Google Scholar]
- Hish, G.A.; Diaz, J.A.; Hawley, A.E.; Myers, D.D.; Lester, P.A. Effects of analgesic use on inflammation and hematology in a murine model of venous thrombosis. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 485–493. [Google Scholar]
- Consonni, S.R.; Rosa, R.G.; Nascimento, M.A.; Vinagre, C.M.; Toledo, O.M.; Joazeiro, P.P. Recovery of the pubic symphysis on primiparous young and multiparous senescent mice at postpartum. Histol. Histopathol. 2012, 27, 885–896. [Google Scholar]
- Cantaruti, T.A.; Costa, R.A.; de Souza, K.S.; Vaz, N.M.; Carvalho, C.R. Indirect effects of immunological tolerance to a regular dietary protein reduce cutaneous scar formation. Immunology 2017, 151, 314–323. [Google Scholar] [CrossRef]
- Montes, G.S.; Junqueira, L.C. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem. Inst. Oswaldo. Cruz. 1991, 86 (Suppl. 3), 1–11. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef]
- Clasen, C.; Wilhelms, T.; Kulicke, W.M. Formation and characterization of chitosan membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [CrossRef]
- Weller, C.; Sussman, G. Wound Dressings Update. J. Pharm. Pract. Res. 2006, 36, 318–324. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar]
- Stricker-Krongrad, A.H.; Alikhassy, Z.; Matsangos, N.; Sebastian, R.; Marti, G.; Lay, F.; Harmon, J.W. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. Eplasty 2018, 18, e14. [Google Scholar]
- Chen, S.L.; Fu, R.H.; Liao, S.F.; Liu, S.P.; Lin, S.Z.; Wang, Y.C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transplant. 2018, 27, 275–284. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Suh, W.; Kim, K.L.; Kim, J.M.K.; Shin, I.S.; Lee, Y.S.; Lee, J.Y.; Jang, H.S.; Byun, J.; Choi, J.H.; Jeon, E.S.; et al. Transplantation of Endothelial Progenitor Cells Accelerates Dermal Wound Healing with Increased Recruitment of Monocytes/Macrophages and Neovascularization. Stem Cells J. 2005, 23, 1571–1578. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. (Maywood) 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Low, Q.E.; Drugea, I.A.; Duffner, L.A.; Quinn, D.G.; Cook, D.N.; Rollins, B.J.; Kovacs, E.J.; DiPietro, L.A. Wound healing in MIP-1alpha(-/-) and MCP-1(-/-) mice. Am. J. Pathol. 2001, 159, 457–463. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116. [Google Scholar]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.L.R.; Moraes, Â.M. Improvement of the mechanical properties of chitosan-alginate wound dressings containing silver through the addition of a biocompatible silicone rubber. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Caetano, G.F.; Frade, M.A.; Andrade, T.A.; Leite, M.N.; Bueno, C.Z.; Moraes, Â.; Ribeiro-Paes, J.T. Chitosan-alginate membranes accelerate wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchetta, F.C.; Trinca, R.B.; Gomes Silva, J.L.; Breder, J.d.S.C.; Cantarutti, T.A.; Consonni, S.R.; Moraes, Â.M.; Pereira de Araújo, E.; Saad, M.J.A.; Adams, G.G.; et al. Effects of Electrospun Fibrous Membranes of PolyCaprolactone and Chitosan/Poly(Ethylene Oxide) on Mouse Acute Skin Lesions. Polymers 2020, 12, 1580. https://doi.org/10.3390/polym12071580
Zanchetta FC, Trinca RB, Gomes Silva JL, Breder JdSC, Cantarutti TA, Consonni SR, Moraes ÂM, Pereira de Araújo E, Saad MJA, Adams GG, et al. Effects of Electrospun Fibrous Membranes of PolyCaprolactone and Chitosan/Poly(Ethylene Oxide) on Mouse Acute Skin Lesions. Polymers. 2020; 12(7):1580. https://doi.org/10.3390/polym12071580
Chicago/Turabian StyleZanchetta, Flávia Cristina, Rafael Bergamo Trinca, Juliany Lino Gomes Silva, Jéssica da Silva Cunha Breder, Thiago Anselmo Cantarutti, Sílvio Roberto Consonni, Ângela Maria Moraes, Eliana Pereira de Araújo, Mario José Abdalla Saad, Gary G. Adams, and et al. 2020. "Effects of Electrospun Fibrous Membranes of PolyCaprolactone and Chitosan/Poly(Ethylene Oxide) on Mouse Acute Skin Lesions" Polymers 12, no. 7: 1580. https://doi.org/10.3390/polym12071580






