Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration
Abstract
1. Introduction
2. Experimental Details
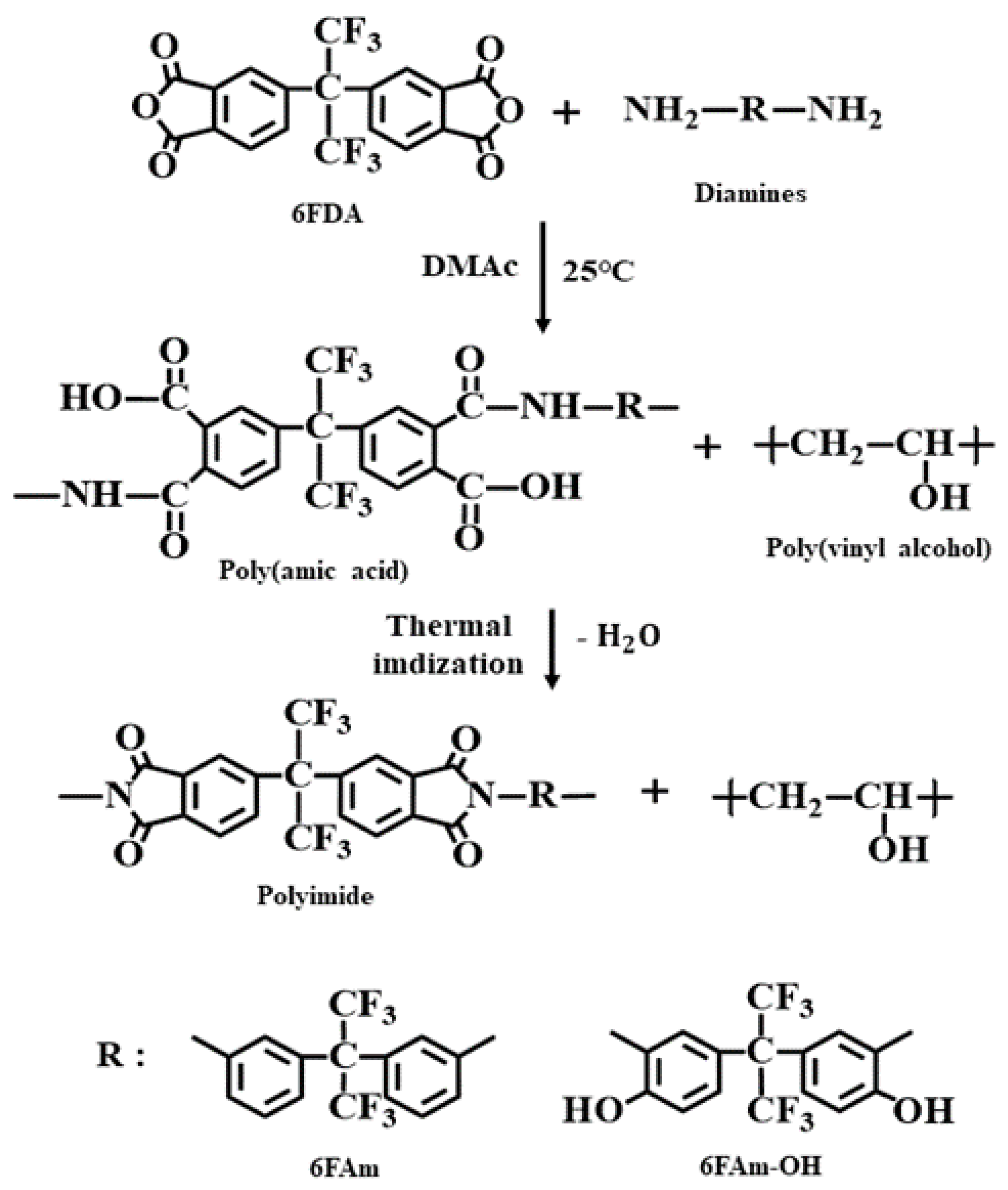
2.1. Materials
2.2. Preparation of the CPI/PVA Blend Film
2.3. Preparation of a Porous CPI Membrane Film
2.4. Characterization
3. Results and Discussion
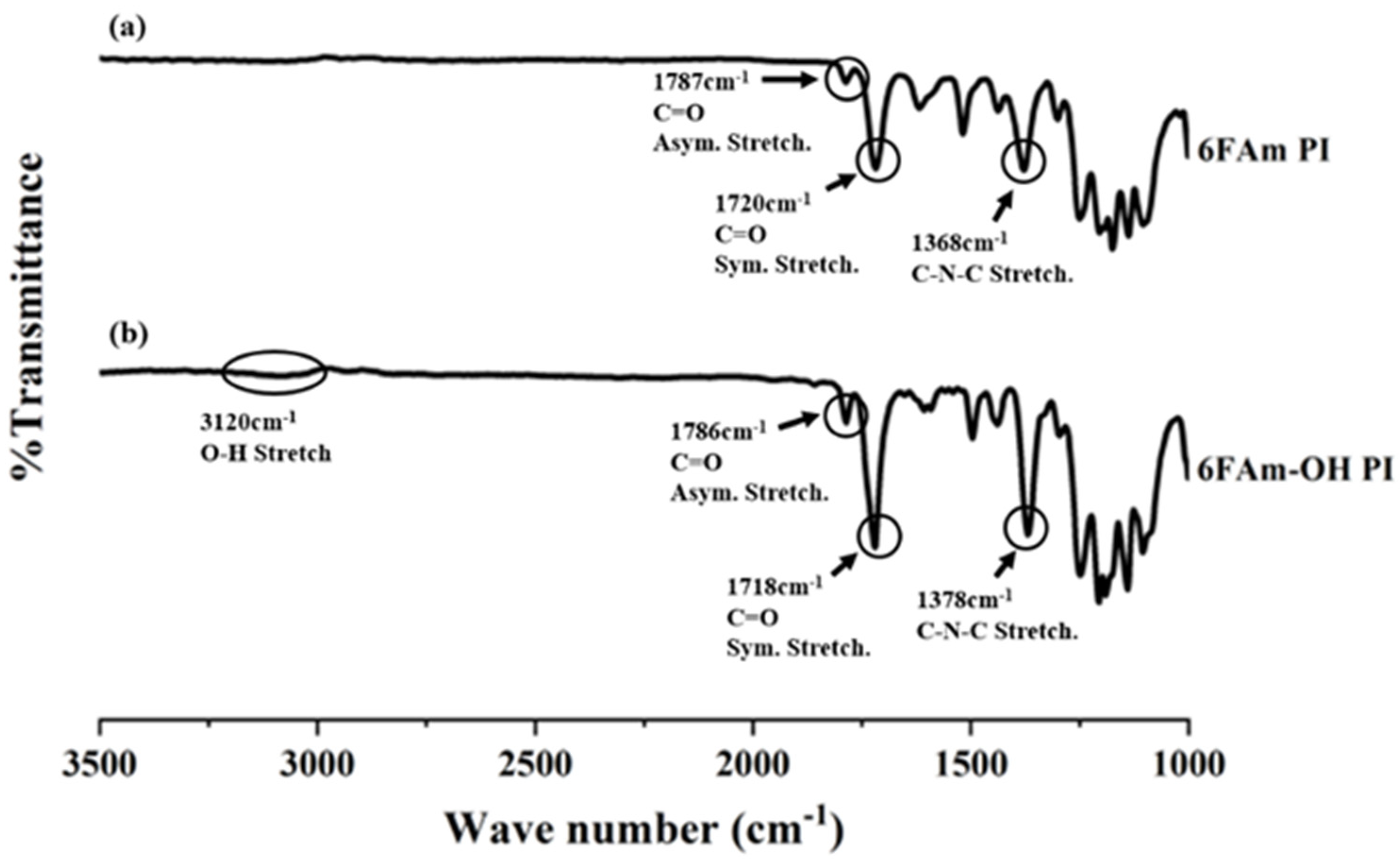
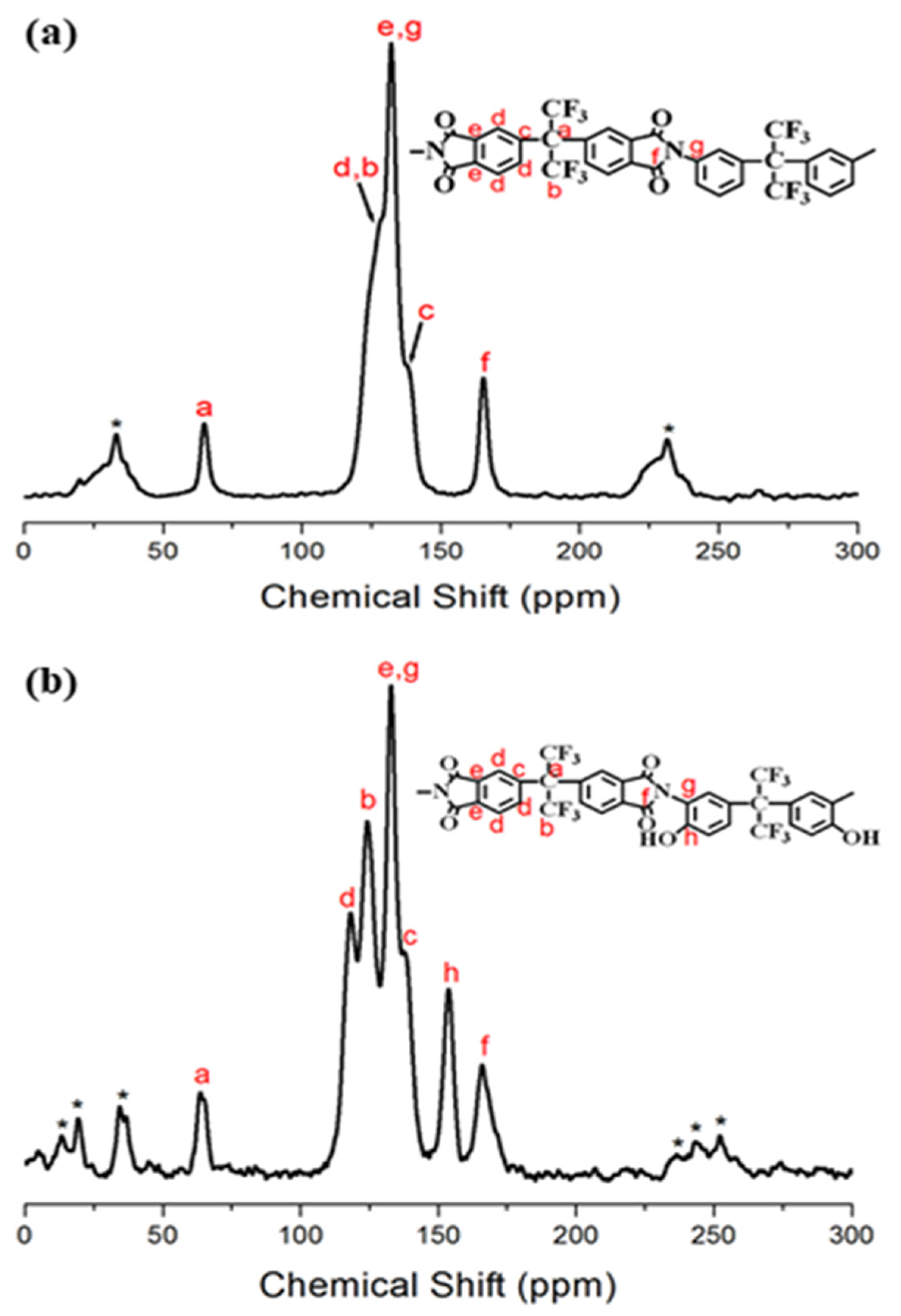
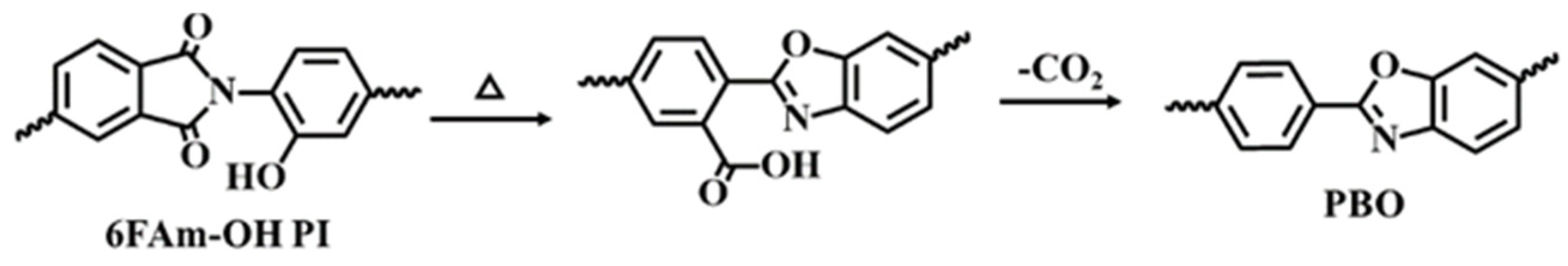
3.1. FTIR and NMR
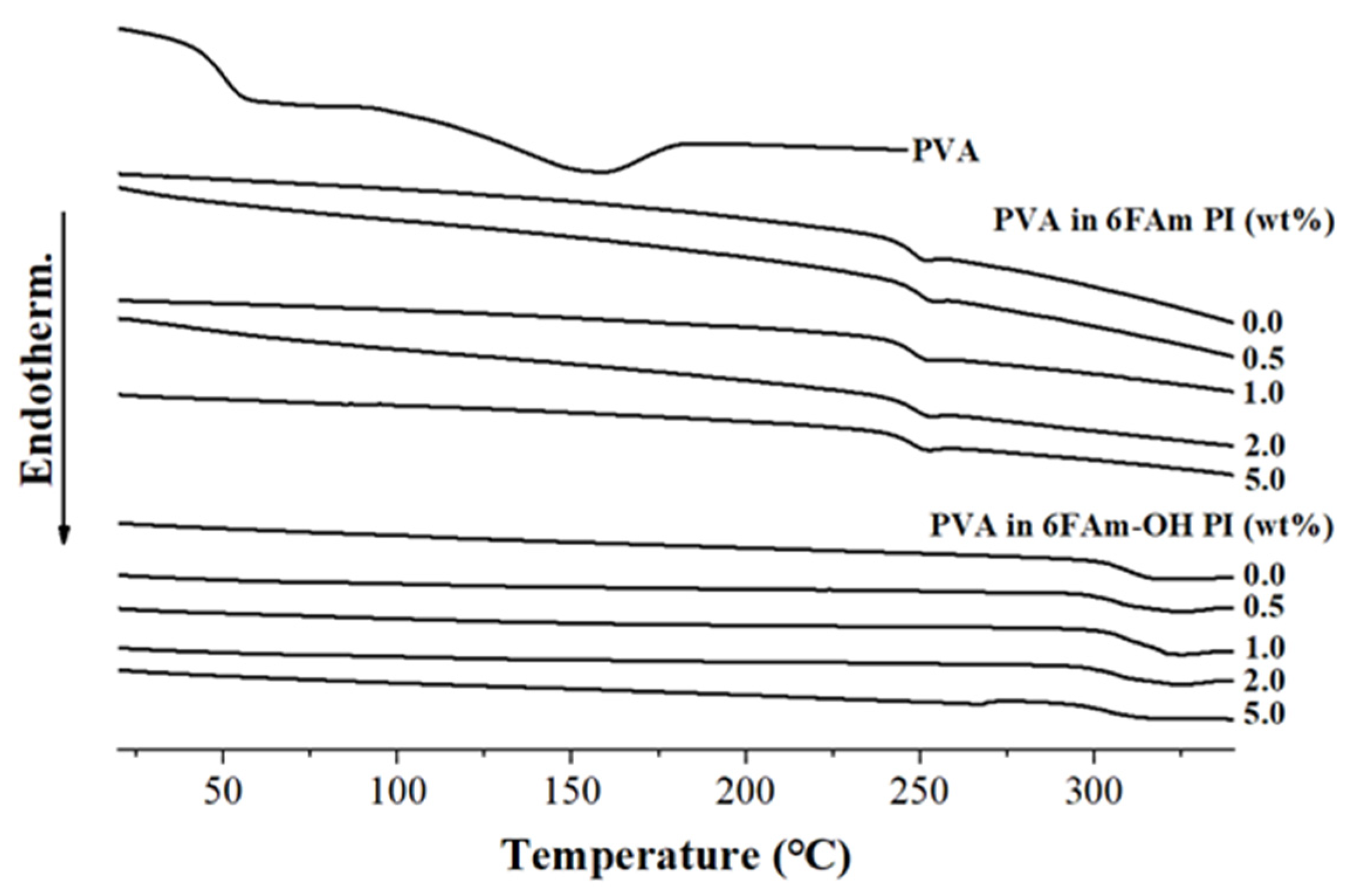
3.2. Thermal Properties
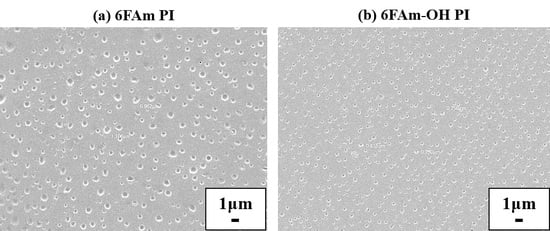
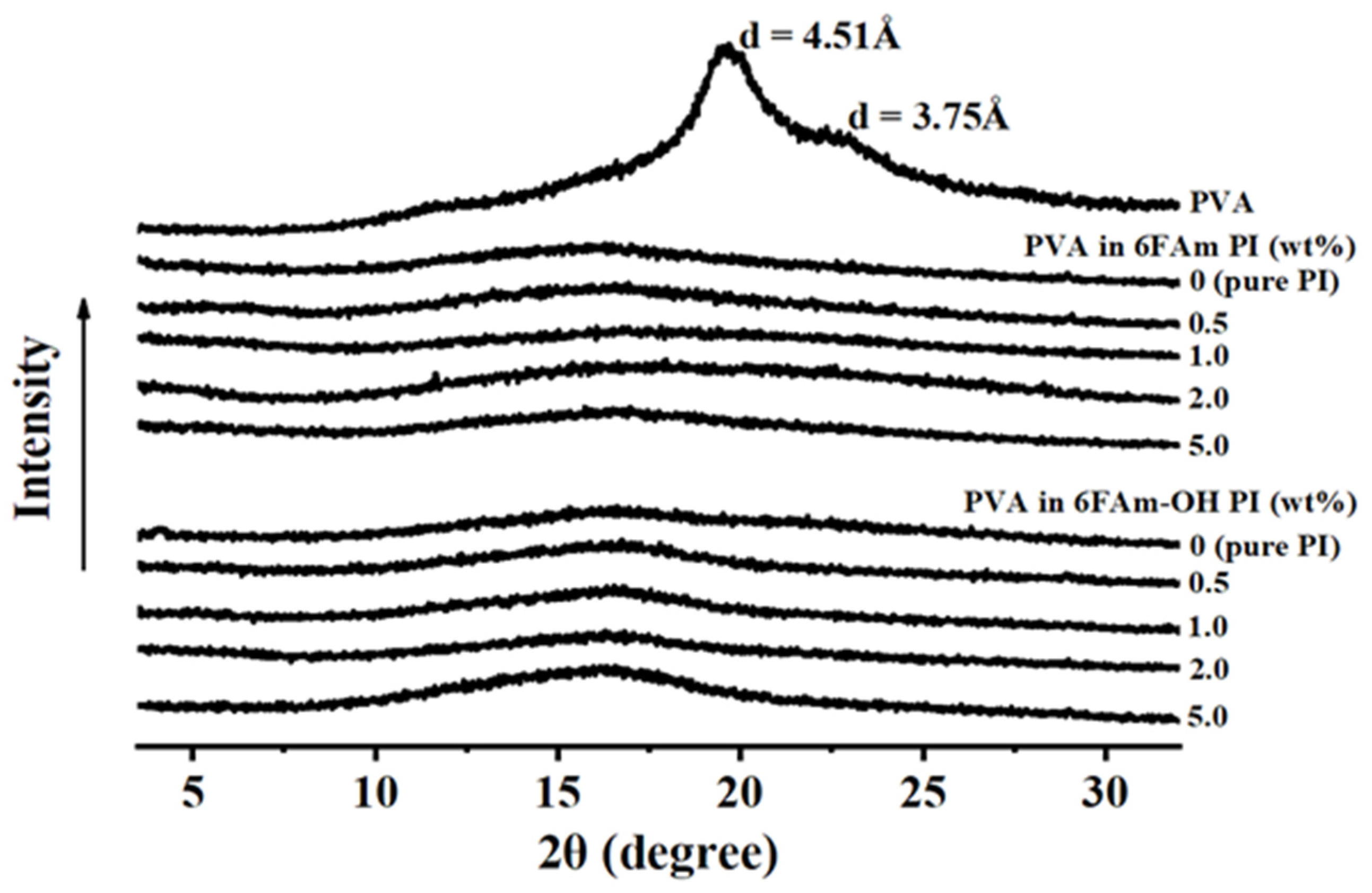
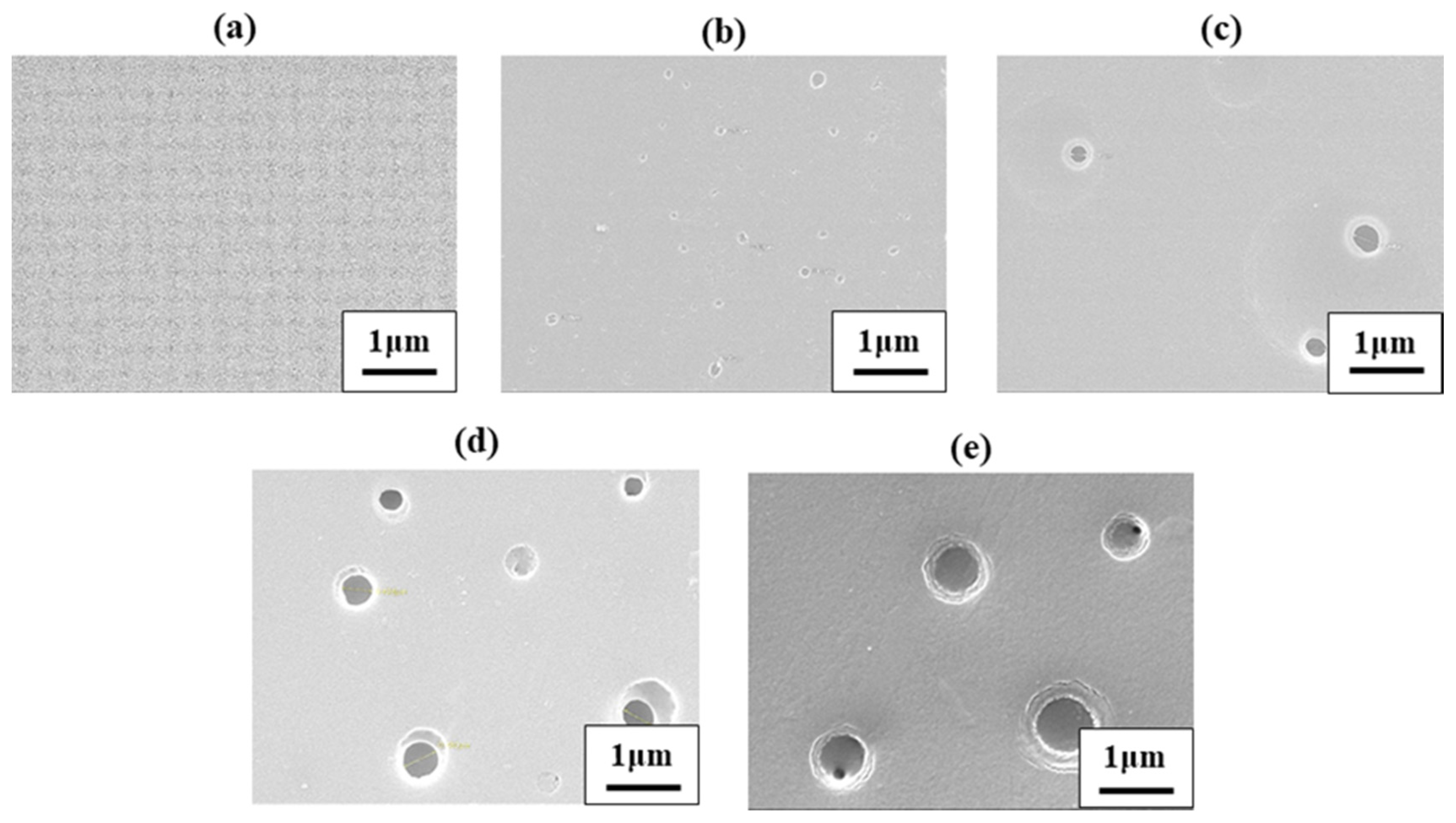
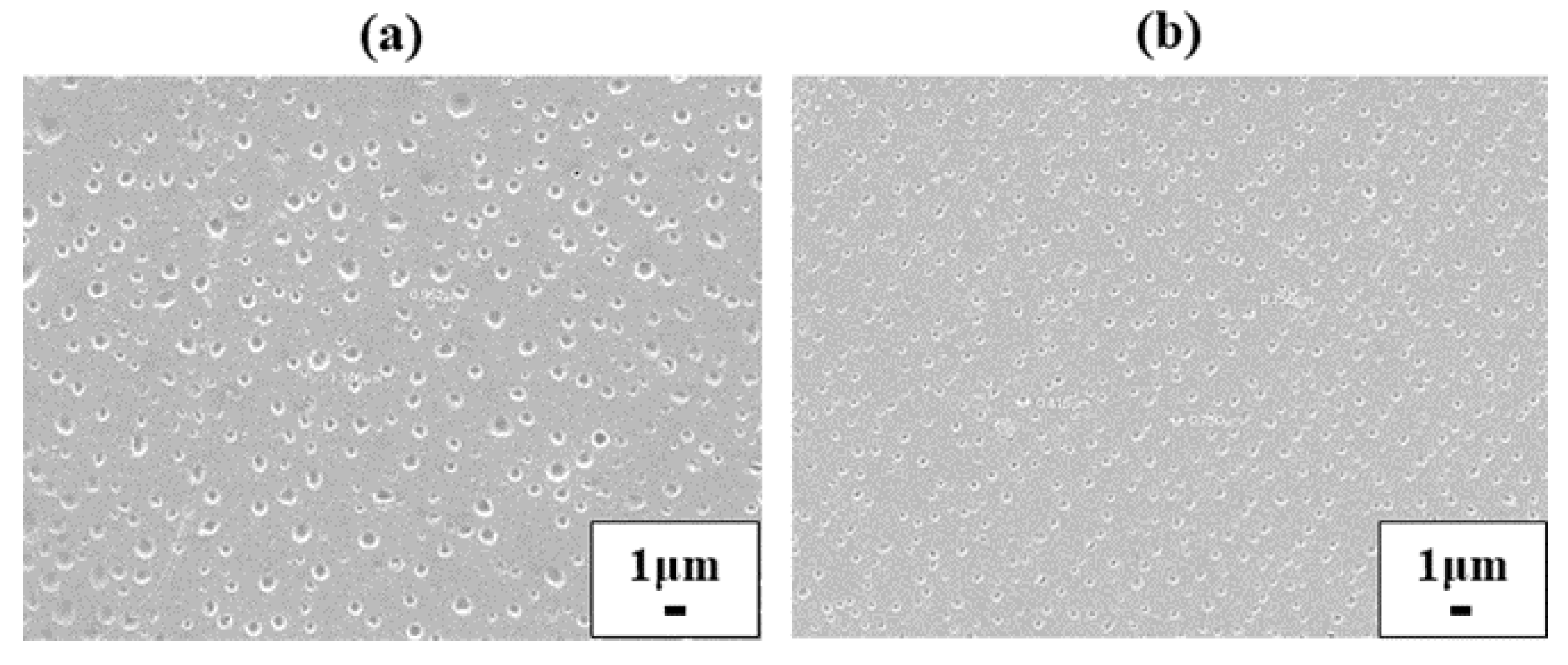
3.3. Morphology
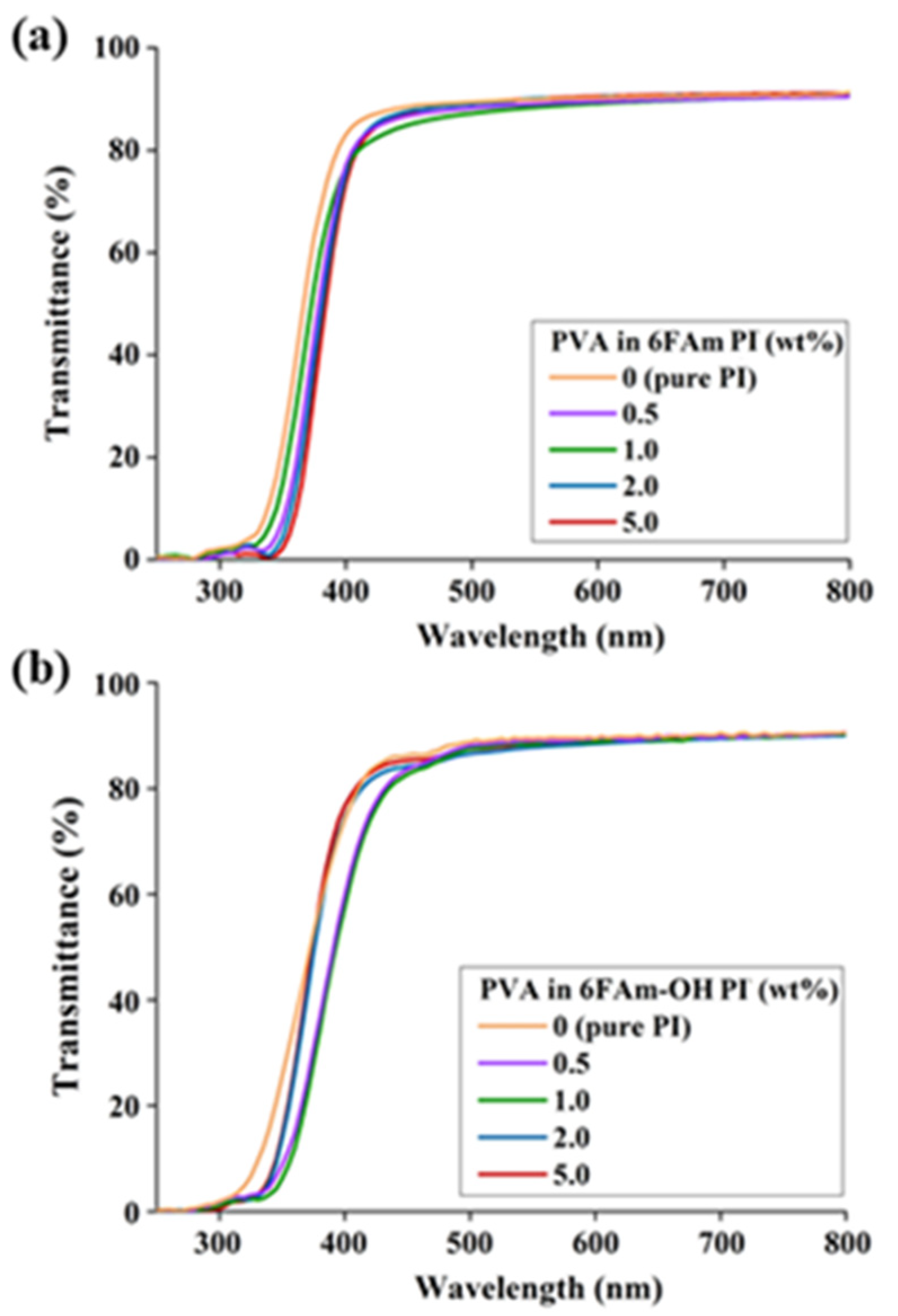
3.4. Optical Transparency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosso, V.; Vuono, D.; Bahattab, M.A.; Di Profio, G.; Curcio, E.; Al-Jilil, S.A.; Alsubaie, F.; Alfife, M.; Nagy, J.B.; Drioli, E.; et al. Polymeric and mixed matrix polyimide membranes. Sep. Purif. Technol. 2014, 132, 684–696. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K. Removal of Pharmaceuticals by Ultrafiltration (UF), Nanofiltration (NF), and Reverse Osmosis (RO). In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 319–344. [Google Scholar]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Charcosset, C. Ultrafiltration, Microfiltration, Nanofiltration and Reverse Osmosis in Integrated Membrane Processes. In Integrated Membrane Systems and Processes; Basile, A., Charcosset, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar]
- Mueller, J.; Cen, Y.; Davis, R.H. Crossflow microfiltration of oily water. J. Membr. Sci. 1997, 129, 221–235. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trands in membrane technology implementation for produced water treatment: A review. J. Water. Proc. Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z. Mineralization-inspired preparation of composite membranes with polyethyleneimine-nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of photocatalytic membrane reactors in organic synthesis, energy storage and environmental applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.; Wang, Y. Network cross-linking of polyimide membranes for pervaporation dehydration. Separ. Purif. Technol. 2017, 185, 215–226. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Cheng, C.-H. Mass Transfer Mechanisms and Transport Resistances in Membrane Separation Process. In Mass Transfer—Advancement in Process Modelling; Solecki, M., Ed.; InTech: London, UK, 2015; Chapter 2; pp. 15–40. [Google Scholar]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Constantin, C.-P.; Bruma, M.; Belomoina, N.M. Highly fluorinated polyimide blends—insights into physico-chemical characterization. Polymer 2014, 55, 4488–4497. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and Properties of Novel Asymmetric Biphenyl Type Polyimides. Homo- and Copolymers and Blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of Aromatic Polyimides and Electronic Properties of Their Source Materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Min, U.K.; Chang, J.-H. Colorless and Transparent Polyimide Films from Poly(amic acid)s with Cross-Linkable Anhydride End. Polymer 2010, 34, 495–500. [Google Scholar]
- Ke, F.; Song, N.; Liang, D.; Xu, H. A method to break charge transfer complex of polyimide: A study on solution behavior. J. Appl. Polym. Sci. 2013, 127, 797–803. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Akhtar, M.; Kadhum, A.; Mohamad, A.; Al-Amiery, A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, S.; Gu, K.; Song, X.; Zhou, Y.; Gao, C. Gradient cross-linked structure: Towards superior PVA nanofiltration membrane performance. J. Membr. Sci. 2019, 569, 83–90. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Chang, J.H. Permeation Properties of Water-Soluble Polymer Nanocomposite Systems. In Barrier Properties of Polymer Clay Nanocomposites; Vikas, M., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; Chapter 6; pp. 117–137. [Google Scholar]
- Shin, H.I.; Chang, J.-H. Transparent Polyimide/Organoclay Nanocomposite Films Containing Different Diamine Monomers. Polymers 2020, 12, 135. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008; Chapter 2; pp. 15–104. [Google Scholar]
- Lim, A.R.; Novak, B.M. Helix conformation in helical polycarbodiimides studied by solid state 13C NMR. Chem. Phys. 2001, 272, 199–212. [Google Scholar] [CrossRef]
- Pouchert, C.; Behnke, J. The Aldrich Library of 13C and 1H FT NMR Spectra; Aldrich Chemical Co. Inc.: Milwaukee, WI, USA, 1993. [Google Scholar]
- Tang, C.; Li, X.; Li, Z.; Hao, J. Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres. Polymers 2017, 9, 504. [Google Scholar] [CrossRef]
- Jiang, Y.; Willmore, F.T.; Sanders, D.; Smith, Z.P.; Ribeiro, C.P.; Doherty, C.M.; Thornton, A.; Hill, A.J.; Freeman, B.D.; Sanchez, I.C. Cavity size, sorption and transport characteristics of thermally rearranged (TR) polymers. Polymer 2011, 52, 2244–2254. [Google Scholar] [CrossRef]
- Kostina, J.; Rusakova, O.; Bondarenko, G.; Alentiev, A.; Meleshko, T.; Kukarkina, N.; Yakimanskii, A.; Yampolskii, Y. Thermal Rearrangement of Functionalized Polyimides: IR-Spectral, Quantum Chemical Studies, and Gas Permeability of TR Polymers. Ind. Eng. Chem. Res. 2013, 52, 10476–10483. [Google Scholar] [CrossRef]
- Scholes, C.A.; Freeman, B.D. Thermal rearranged poly(imide-co-ethylene glycol) membranes for gas separation. J. Membr. Sci. 2018, 563, 676–683. [Google Scholar] [CrossRef]
- Meis, D.; Tena, A.; Neumann, S.; Georgopanos, P.; Emmler, T.; Shishatskiy, S.; Rangou, S.; Filiz, V.; Abetz, V. Thermal rearrangement of ortho-allyloxypolyimide membranes and the effect of the degree of functionalization. Polym. Chem. 2018, 9, 3987–3999. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, J.-H.; Kim, J.-C. Optically transparent and colorless polyimide hybrid films with various clay contents. Macromol. Res. 2012, 20, 1257–1263. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]


| PVA in PI (wt%) | 6FAm PI | 6FAm-OH PI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness (μm) | Tg (°C) | TDi a (°C) | wtR600 b (%) | D.C c (%) | Dia d (μm) | Thickness (μm) | Tg (°C) | TDi (°C) | wtR600 (%) | D.C (%) | Dia. (μm) | |
| 0 (pure PI) | 19 | 237 | 350 | 77 | 0 | - | 18 | 302 | 328 | 55 | 0 | - |
| 0.5 | 21 | 238 | 349 | 76 | 0 | 0.14 | 20 | 302 | 329 | 55 | 0 | 0.12 |
| 1.0 | 20 | 235 | 348 | 74 | 0 | 0.29 | 21 | 303 | 328 | 57 | 0 | 0.13 |
| 2.0 | 18 | 233 | 351 | 76 | 0 | 0.41 | 21 | 302 | 327 | 58 | 0 | 0.37 |
| 5.0 | 20 | 234 | 349 | 73 | 0 | 0.69 | 19 | 303 | 329 | 54 | 0 | 0.61 |
| 100 (pure PVA) | - | 44 | 201 | 30 | 18 | - | - | 44 | 201 | 30 | 18 | - |
|
PVA in PI (wt%) | 6FA6FAm PI m PI | 6FAm-OH PI | ||||
|---|---|---|---|---|---|---|
| λ0 (nm) | 500 nmtrans (%) | YI a | λ0 (nm) | 500 nmtrans (%) | YI | |
| 0 (pure PI) | 280 | 89 | 3 | 350 | 87 | 2 |
| 0.5 | 285 | 89 | 3 | 353 | 87 | 3 |
| 1.0 | 280 | 87 | 2 | 360 | 88 | 3 |
| 2.0 | 290 | 88 | 3 | 355 | 88 | 2 |
| 5.0 | 280 | 89 | 3 | 345 | 89 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Chang, J.-H. Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration. Polymers 2020, 12, 1610. https://doi.org/10.3390/polym12071610
Kim JW, Chang J-H. Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration. Polymers. 2020; 12(7):1610. https://doi.org/10.3390/polym12071610
Chicago/Turabian StyleKim, Jong Won, and Jin-Hae Chang. 2020. "Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration" Polymers 12, no. 7: 1610. https://doi.org/10.3390/polym12071610
APA StyleKim, J. W., & Chang, J.-H. (2020). Syntheses of Colorless and Transparent Polyimide Membranes for Microfiltration. Polymers, 12(7), 1610. https://doi.org/10.3390/polym12071610