PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
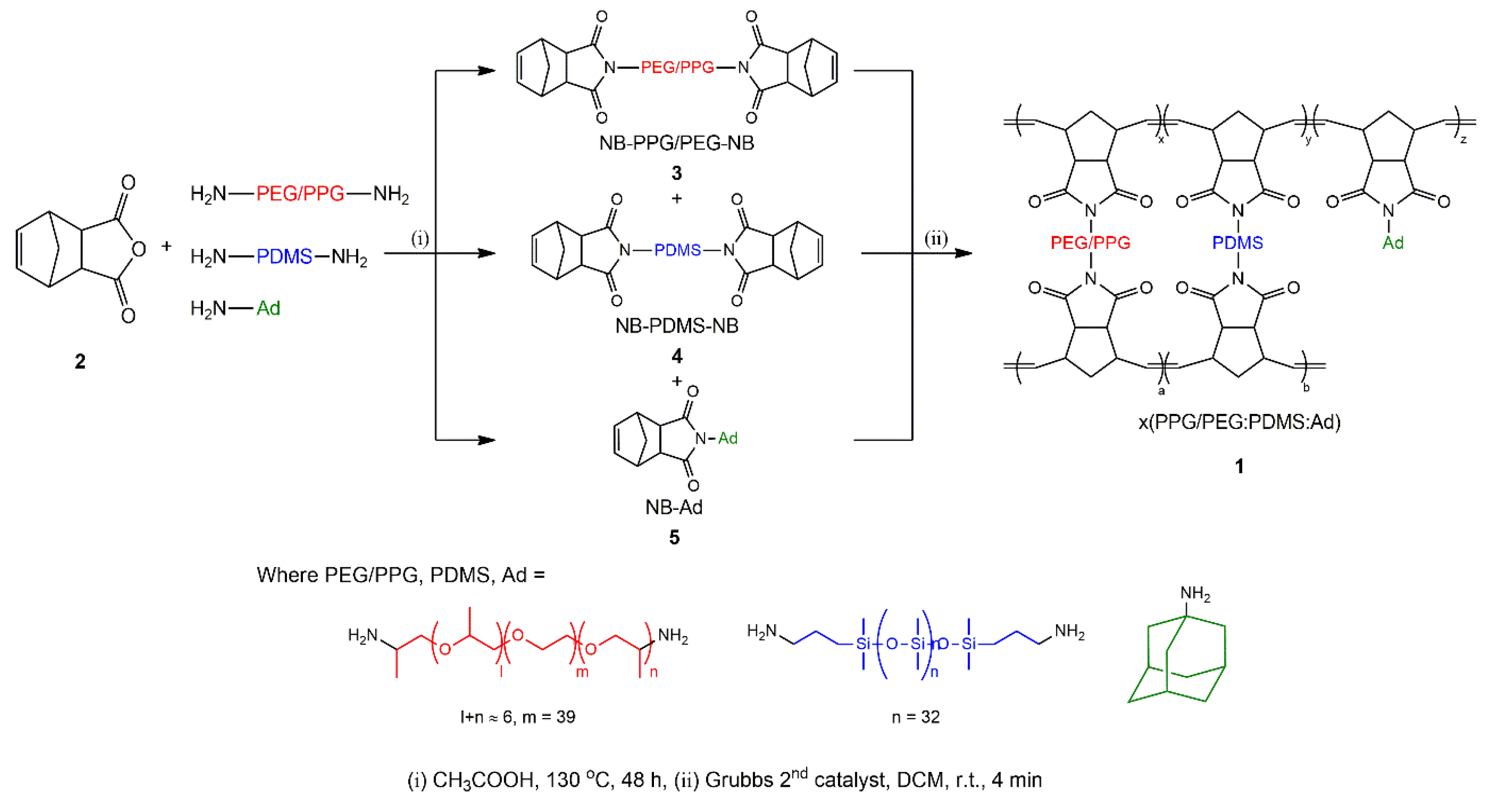
Synthesis of Norbornene-Functionalized Precursor Mmolecules 3, 4, 5
2.2. Membrane Fabrication
2.3. Characterization and Gas Separation Measurements
3. Results
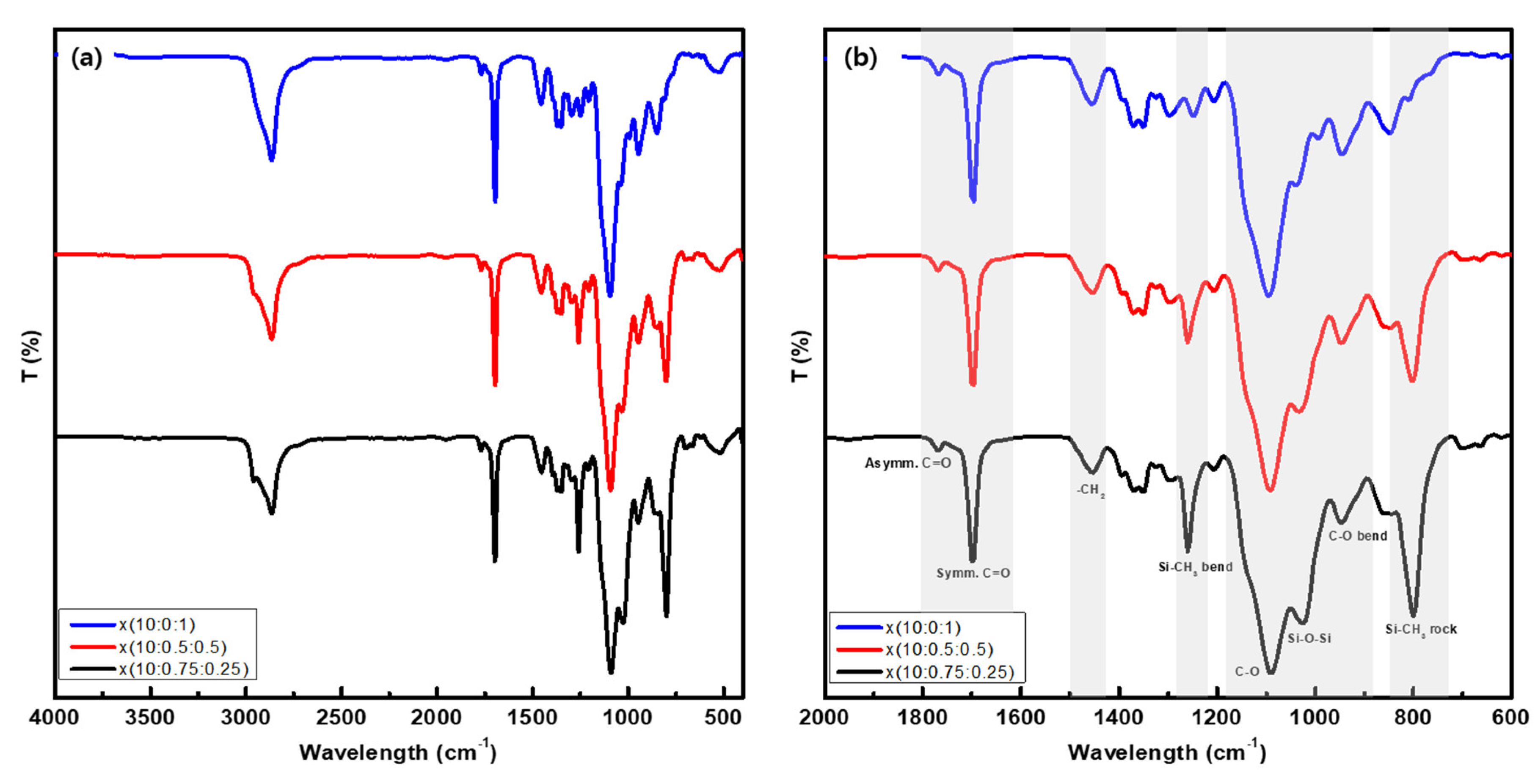
3.1. Synthesis of Precursor Molecules and Membrane Characterization
3.2. Thermal Properties of the x(PEG/PPG:PDMS:Ad) Membranes
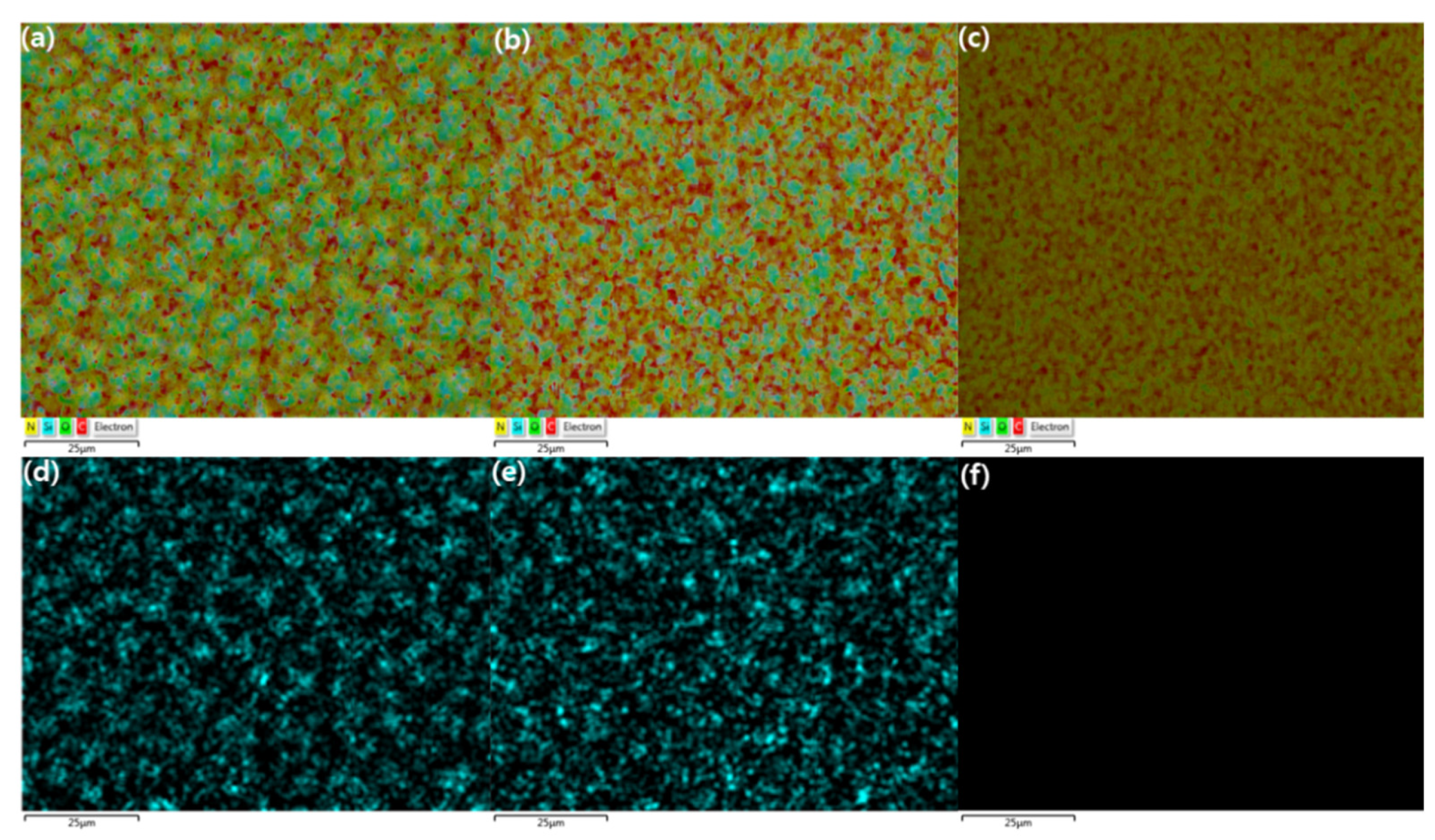
3.3. Morphological Analysis of the x(PEG/PPG:PDMS:Ad) Membranes
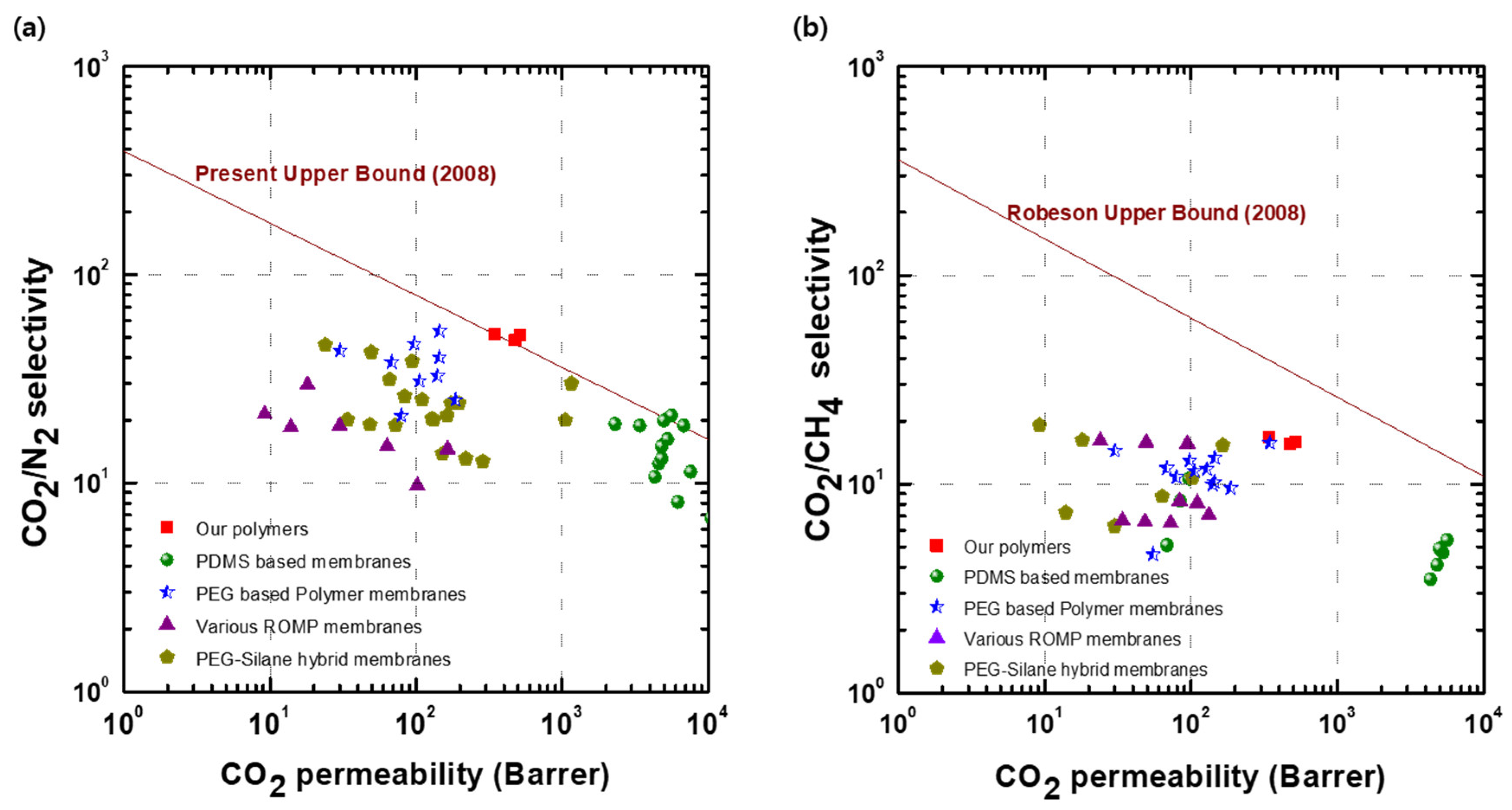
3.4. Gas Separation Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.H.; Foo, D.C.Y.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Smit, B.; Park, A.H.A.; Gadikota, G. The grand challenges in carbon capture, utilization, and storage. Front. Energy Res. 2014, 2, 2013–2015. [Google Scholar] [CrossRef] [Green Version]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Bandehali, S.; Moghadassi, A.; Parvizian, F.; Hosseini, S.M.; Matsuura, T.; Joudaki, E. Advances in high carbon dioxide separation performance of poly (ethylene oxide)-based membranes. J. Energy Chem. 2020, 46, 30–52. [Google Scholar] [CrossRef]
- Jujie, L.; He, X.; Si, Z. Polysulfone membranes containing ethylene glycol monomers: Synthesis, characterization, and CO2/CH4 separation. J. Polym. Res. 2017, 24, 1–14. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Kargari, A.; Rezaeinia, S. State-of-the-art modification of polymeric membranes by PEO and PEG for carbon dioxide separation: A review of the current status and future perspectives. J. Ind. Eng. Chem. 2020, 84, 1–22. [Google Scholar] [CrossRef]
- Khosravi, T.; Omidkhah, M. Preparation of CO2-philic polymeric membranes by blending poly(ether-b-amide-6) and PEG/PPG-containing copolymer. RSC Adv. 2015, 5, 12849–12859. [Google Scholar] [CrossRef]
- Isfahani, A.P.; Sadeghi, M.; Wakimoto, K.; Gibbons, A.H.; Bagheri, R.; Sivaniah, E.; Ghalei, B. Enhancement of CO2 capture by polyethylene glycol-based polyurethane membranes. J. Membr. Sci. 2017, 542, 143–149. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, X.; Zhang, Y.; Zheng, Y.; He, X.; Luo, J. Random and block copolymer membranes based on flexible etheric-aliphatic soft segments designed for CO2/CH4 separation. J. Nat. Gas Sci. Eng. 2018, 54, 92–101. [Google Scholar] [CrossRef]
- Cong, H.; Yu, B. Aminosilane cross-linked PEG/PEPEG/PPEPG membranes for CO2/N2 and CO2/H2 separation. Ind. Eng. Chem. Res. 2010, 49, 9363–9369. [Google Scholar] [CrossRef]
- Schauer, J.; Sysel, P.; Maroušek, V.; Pientka, Z.; Pokorný, J.; Bleha, M. Pervaporation and gas separation membranes made from polyimide/polydimethylsiloxane block copolymer. J. Appl. Polym. Sci. 1996, 61, 1333–1337. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes 2018, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Ansaloni, L.; Gin, D.L.; Noble, R.D.; Deng, L. Facile fabrication of CO2 separation membranes by cross-linking of poly(ethylene glycol) diglycidyl ether with a diamine and a polyamine-based ionic liquid. J. Membr. Sci. 2017, 523, 551–560. [Google Scholar] [CrossRef]
- Khan, M.M.; Halder, K.; Shishatskiy, S.; Filiz, V. Synthesis and crosslinking of polyether-based main chain benzoxazine polymers and their gas separation performance. Polymers 2018, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Liu, J. Synthesis and gas transport properties of polyamide membranes containing PDMS groups. RSC Adv. 2019, 9, 9737–9744. [Google Scholar] [CrossRef] [Green Version]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Lim, C.; Hong, S.I.; Kim, H. Effect of polyether diamine on gas permeation properties of organic-inorganic hybrid membranes. J. Sol. Gel. Sci. Technol. 2007, 43, 35–40. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, X.; Kumar, S.R.; Mark, J.E. Improved hydrophilicity from poly(ethylene glycol) in amphiphilic conetworks with poly(dimethylsiloxane). Silicon 2009, 1, 173–181. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; Al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.-H. PEG/PPG–PDMS-Based Cross-Linked Copolymer Membranes Prepared by ROMP and In Situ Membrane Casting for CO2 Separation: An Approach to Endow Rubbery Materials with Properties of Rigid Polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef] [PubMed]
- Sutthasupa, S.; Shiotsuki, M.; Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 2010, 42, 905–915. [Google Scholar] [CrossRef]
- Song, K.; Kim, K.; Hong, D.; Kim, J.; Heo, C.E.; Kim, H.I.; Hong, S.H. Highly active ruthenium metathesis catalysts enabling ring-opening metathesis polymerization of cyclopentadiene at low temperatures. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapala, P.P.; Borisov, I.L.; Bermeshev, M.V.; Volkov, V.V.; Finkelshtein, E.S. Synthesis and gas separation properties of metathesis poly(5-ethylidene-2-norbornene). Pet. Chem. 2016, 56, 1056–1060. [Google Scholar] [CrossRef]
- Spring, A.M.; Qiu, F.; Hong, J.; Bannaron, A.; Cheng, X.; Yokoyama, S. Adamantyl and carbazole containing trans-poly(norbornene-dicarboximide)s as electro-optic chromophore hosts. Polymer 2019, 172, 382–390. [Google Scholar] [CrossRef]
- Vargas, J.; Martínez, A.; Santiago, A.A.; Tlenkopatchev, M.A.; Aguilar-Vega, M. Synthesis and gas permeability of new polynorbornene dicarboximide with fluorine pendant groups. Polymer 2007, 48, 6546–6553. [Google Scholar] [CrossRef]
- Liu, F.; Long, Y.; Zhao, Q.; Liu, X.; Qiu, G.; Zhang, L.; Ling, Q.; Gu, H. Gallol-containing homopolymers and block copolymers: ROMP synthesis and gelation properties by metal-coordination and oxidation. Polymer 2018, 143, 212–227. [Google Scholar] [CrossRef]
- Hong, T.; Lai, S.; Mahurin, S.M.; Cao, P.-F.; Voylov, D.N.; Meyer, H.M.; Jacobs, C.B.; Carrillo, J.-M.Y.; Kisliuk, A.; Ivanov, I.N.; et al. Highly Permeable Oligo(ethylene oxide)-co-poly(dimethylsiloxane) Membranes for Carbon Dioxide Separation. Adv. Sustain. Syst. 2018, 2, 1700113. [Google Scholar] [CrossRef]
- Hong, T.; Chatterjee, S.; Mahurin, S.M.; Fan, F.; Tian, Z.; Jiang, D.E.; Long, B.K.; Mays, J.W.; Sokolov, A.P.; Saito, T. Impact of tuning CO2-philicity in polydimethylsiloxane-based membranes for carbon dioxide separation. J. Membr. Sci. 2017, 530, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Hossain, I.; Al Munsur, A.Z.; Kim, T.H. A facile synthesis of (PIM-polyimide)-(6FDA-durene-polyimide) copolymer as novel polymer membranes for CO2 separation. Membranes 2019, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Chung, T. In situ fabrication of cross-linked PEO/silica reverse-selective membranes for hydrogen purification. Int. J. Hydrogen Energy 2009, 34, 6492–6504. [Google Scholar] [CrossRef]
- Chen, C.; Bu, X.; Feng, Q.; Li, D. Cellulose nanofiber/carbon nanotube conductive nano-network as a reinforcement template for polydimethylsiloxane nanocomposite. Polymers 2018, 10, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Lozano, D.; Comesaña-Gándara, B.; de la Viuda, M.; Seong, J.G.; Palacio, L.; Prádanos, P.; de la Campa, J.G.; Cuadrado, P.; Lee, Y.M.; Hernández, A.; et al. New aromatic polyamides and polyimides having an adamantane bulky group. Mater. Today Commun. 2015, 5, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.P.; Cerda, A.M.; Tlenkopatchev, M.A. Synthesis of high-Tg polymers by ring-opening metathesis polymerization of N-cycloalkylnorbornene dicarboximide. Macromol. Chem. Phys. 2002, 203, 1811–1818. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.H.; Cho, J.H.; Hwang, M.; Jang, Y.; Cho, K. Effect of the phase states of self-assembled monolayers on pentacene growth and thin-film transistor characteristics. J. Am. Chem. Soc. 2008, 130, 10556–10564. [Google Scholar] [CrossRef]
- Kang, D.A.; Kim, K.; Lim, J.Y.; Park, J.T.; Kim, J.H. Mixed matrix membranes consisting of ZIF-8 in rubbery amphiphilic copolymer: Simultaneous improvement in permeability and selectivity. Chem. Eng. Res. Des. 2020, 153, 175–186. [Google Scholar] [CrossRef]
- Roberts, C.; Graham, A.; Nemer, M.; Phinney, L.; Garcia, R.; Stirrup, E. Physical Properties of Low-Molecular Weight Polydimethylsiloxane Fluids; SAND-2017-1242; Sandia National Laboratories: Albuquerque, NM, USA, 2017. [CrossRef] [Green Version]
- Fang, H.; Zhou, S.; Wu, L. Microphase separation behavior on the surfaces of PEG-MDI-PDMS multiblock copolymer coatings. Appl. Surf. Sci. 2006, 253, 2978–2983. [Google Scholar] [CrossRef]
- Scofield, J.M.P.; Gurr, P.A.; Kim, J.; Fu, Q.; Halim, A.; Kentish, S.E.; Qiao, G.G. High-performance thin film composite membranes with well-defined poly(dimethylsiloxane)-b-poly(ethylene glycol) copolymer additives for CO2 separation. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1500–1511. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Ghalei, B.; Fardi, M.; Esmaeeli, M.; Vakili, E. Structural and transport properties of polydimethylsiloxane based polyurethane/silica particles mixed matrix membranes for gas separation. Korean J. Chem. Eng. 2014, 31, 841–848. [Google Scholar] [CrossRef]
- Kim, H.; Lim, C.; Hong, S.I. Gas permeation properties of organic-inorganic hybrid membranes prepared from hydroxyl-terminated polyether and 3-isocyanatopropyltriethoxysilane. J. Sol. Gel. Sci. Technol. 2005, 36, 213–221. [Google Scholar] [CrossRef]
- Adrees, M.; Iqbal, S.S.; Ahmad, A.; Jamshaid, F.; Haider, B.; Khan, M.H.; Khan, R.; Zahid Butt, M.T.; Bahadar, A. Characterization of novel polydimethylsiloxane (PDMS) and copolymer polyvinyl chloride-co-vinyl acetate (PVCA) enhanced polymer blend membranes for CO2 separation. Polym. Test. 2019, 80, 106163. [Google Scholar] [CrossRef]
- Sadeghi, M.; Semsarzadeh, M.A.; Barikani, M.; Ghalei, B. Study on the morphology and gas permeation property of polyurethane membranes. J. Membr. Sci. 2011, 385–386, 76–85. [Google Scholar] [CrossRef]






| Membrane Code | Density (g/cm3) | Gel-Fraction (%) | PDMS | Td (°C) a | Tg | |
|---|---|---|---|---|---|---|
| d-Spacing(Å) | 2θ (°) | |||||
| x(10:0.75:0.25) | 1.12 | 98.3 | 7.59 | 11.64 | 312 | −53.6 |
| x(10:0.5:0.5) | 1.13 | 94.6 | 7.31 | 12.08 | 325 | −53.2 |
| x(10:0:1) | 1.15 | 87.8 | - | - | 335 | −52.9 |
| poly(NB-Ad) | 1.19 | - | ||||
| Membrane Code | Atomic % (K series) | Si/O | Si/C | |||
|---|---|---|---|---|---|---|
| C | N | O | Si | |||
| x(10:0.75:0.25) | 69.90 | 0.00 | 27.91 | 2.19 | 0.078 | 0.031 |
| x(10:0.5:0.5) | 70.50 | 0.00 | 27.76 | 1.74 | 0.063 | 0.025 |
| x(10:0:1) | 75.06 | 0.00 | 24.94 | 0.00 | - | - |
| Membrane Code | Permeability (Barrer) | Selectivity (α) | |||
|---|---|---|---|---|---|
| CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | |
| x(10:0.75:0.25) | 475.0 ± 2.0 | 9.8 ± 0.1 | 30.7 ± 0.1 | 48.5 | 15.5 |
| x(10:0.5:0.5) | 514.5 ± 2.5 | 10.1 ± 0.0 | 32.4 ± 0.1 | 50.9 | 15.9 |
| x(10:0:1) | 342.6 ± 3.5 | 6.6 ± 0.1 | 20.5 ± 0.1 | 51.9 | 16.7 |
| Membrane Code | Diffusivity (D) | Solubility (S) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | |
| x(10:0.75:0.25) | 101.8 ± 0.1 | 61.1 ± 0.4 | 56.6 ± 3.7 | 1.67 | 1.80 | 4.67 ± 0.03 | 0.16 ± 0.00 | 0.54 ± 0.04 | 29.2 | 8.6 |
| x(10:0.5:0.5) | 95.2 ± 0.1 | 57.5 ± 0.7 | 54.1 ± 3.0 | 1.66 | 1.76 | 5.40 ± 0.03 | 0.17 ± 0.00 | 0.60 ± 0.03 | 31.8 | 9.0 |
| x(10:0:1) | 68.5 ± 0.8 | 44.0 ± 1.5 | 51.9 ± 0.1 | 1.56 | 1.32 | 5.00 ± 0.01 | 0.15 ± 0.00 | 0.39 ± 0.00 | 33.3 | 12.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.-H. PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers 2020, 12, 1674. https://doi.org/10.3390/polym12081674
Kim D, Hossain I, Kim Y, Choi O, Kim T-H. PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers. 2020; 12(8):1674. https://doi.org/10.3390/polym12081674
Chicago/Turabian StyleKim, Dongyoung, Iqubal Hossain, Yeonho Kim, Ook Choi, and Tae-Hyun Kim. 2020. "PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane" Polymers 12, no. 8: 1674. https://doi.org/10.3390/polym12081674
APA StyleKim, D., Hossain, I., Kim, Y., Choi, O., & Kim, T.-H. (2020). PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers, 12(8), 1674. https://doi.org/10.3390/polym12081674









