Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells
Abstract
1. Introduction
2. Materials
3. Experimental Procedure
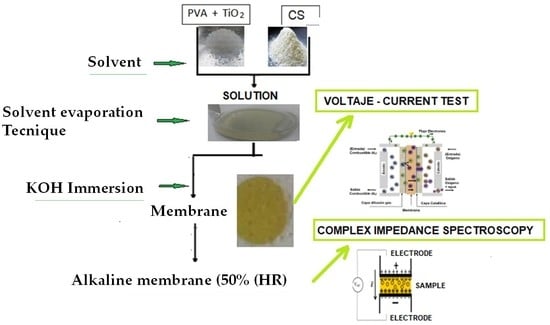
3.1. Preparation of PVA/CS-TiO2 Polymeric Membranes
3.2. Morphological Characterization
3.3. Thermal Characterization (DSC and TGA)
3.4. Moisture Absorption
3.5. Complex Impedance Spectroscopy
3.6. Mechanical Characterization (Tensile Tests)
3.7. X-ray Diffraction (XRD)
3.8. Current–Voltage Tests
4. Results and Discussion
4.1. SEM Morphology
4.2. Moisture Adsorption
4.3. Thermogravimetric Analysis (TGA)
4.4. Differential Scanning Calorimetry (DSC)
4.5. X-ray Diffraction
4.6. Tensile Tests
4.7. Complex Impedance Spectroscopy
4.8. Current–Voltage Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripathi, B.P.; Shahi, V.K. Organic-Inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2020, 36, 945–979. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Preparation and properties of organic–inorganic alkaline hybrid membranes for direct methanol fuel cell application. Solid State Ion. 2013, 255, 96–103. [Google Scholar] [CrossRef]
- Ayse, A.; Bozkurt, A. Nanocomposite polymer electrolyte membranes based on poly (vinyl phosphonic acid)/sulfated nano-titania. J. Power Sources 2012, 21, 158–163. [Google Scholar] [CrossRef]
- González, Y.F.; Vargas, R.A. Estudio de las Propiedades Termodinámicas y Eléctricas de Materiales Compuestos Poliméricos Basados En El Poli (Vinil Alcohol) (PVA) + H3PO2 + TiO2. Rev. Iberoam. Polím. 2011, 12, 64–75. Available online: http://www.reviberpol.iibcaudo.com.ve/pdf/MAR11/gonzalez.pdf (accessed on 28 August 2018).
- Gonçalves, R.P.; Ferreira, W.H.; Gouvêa, R.F.; Andrade, C.T. Effect of chitosan on the properties of electrospun fibers from mixed poly(vinyl alcohol)/chitosan solutions. Mater. Res. 2017, 20, 984–993. [Google Scholar] [CrossRef]
- Ali, M.; Gherissi, A. Synthesis and characterization of the composite material PVA/chitosan/5% sorbitol with different ratio of chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA blending on structural and ion transport properties of CS:AgNt-Based polymer electrolyte membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef]
- Benítez, M.; Diosa, J.E.; Vargas, R.A. Effect of H3PO2 on the mechanical, thermal, and electrical properties of polymers based on poly (vinyl alcohol) (PVA) and chitosan (CS). Ionics 2018, 24, 2029–2034. [Google Scholar] [CrossRef]
- Quintana, D.A.; Baca, E.; Mosquera, E.; Vargas, R.A.; Diosa, J.E. Improving the ionic conductivity in nanostructured membranes based on poly(vinyl alcohol) (PVA), chitosan (CS), phosphoric acid (H3PO4), and niobium oxide (Nb2O5). Ionics 2019, 25, 1131–1136. [Google Scholar] [CrossRef]
- Habiba, U.; Islam, M.S.; Siddique, T.A.; Afifi, A.M.; Ang, B.C. Adsorption and photocatalytic degradation of anionic dyes on Chitosan/PVA/Na–Titanate/TiO2 composites synthesized by solution casting method. Carbohydr. Polym. 2016, 149, 317–331. [Google Scholar] [CrossRef]
- Paipitak, K.; Pornpra, T.; Mongkontalang, P.; Techitdheera, W.; Pecharapa, W. Characterization of PVA-chitosan nanofibers prepared by electrospinning. Procedia Eng. 2010, 8, 101–105. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Zhang, Y.; Shi, Z.; Tan, J.; Wang, F.; Wang, Y. Aramid nanofibers and poly (vinyl alcohol) nanocomposites for ideal combination of strength and toughness via hydrogen bonding interactions. Compos. Sci. Technol. 2017, 144, 193–201. [Google Scholar] [CrossRef]
- ASTM D882-09. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM E104-02. Standard Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Hoogers, G. Fuel Cell Technology Handbook; Hoogers, G., Ed.; CRC PRESS: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2003; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjugJONgb_qAhWwnuAKHSu4CiYQFjAAegQIBRAB&url=http%3A%2F%2Fbizkhan.tistory.com%2Fattachment%2Fcfile25.uf%401130D0134A3F5E853726D9.pdf&usg=AOvVaw2sdJPTP4aXrDvsRRlZPw8S (accessed on 15 June 2020).
- EG&G TechnicalServices Inc. Fuel Cell Handbook; U.S. Deparment of Energy: Morgantown, WV, USA, November 2004. Available online: https://netl.doe.gov/sites/default/files/netl-file/FCHandbook7.pdf (accessed on 23 June 2020).
- Cruz, J.A.; Savage, L.J.; Zegarac, R.; Kovac, W.K.; Chen, J.; Kramer, D.M. Dynamic environmental photosynthetic imaging reveals emergent phenotypes. Cell Syst. 2016, 22, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Li, Y.J.; Liou, T.H. Preparation of novel poly (vinyl alcohol)/SiO2 Nanocomposite membranes by a sol–gel process and their application on alkaline DMFCs. Desalination 2011, 276, 366–372. [Google Scholar] [CrossRef]
- Mollá, S.; Campañ, V. Performance of composite Nafion/PVA membranes for direct methanol fuel cells. J. Power Sources 2010, 196, 2699–2708. [Google Scholar] [CrossRef]
- Vargas, M.A.; Vargas, R.A.; Mellander, B.E. More studies on the PVA + H3PO2 + H2O proton conductor gels. Electrochim. Acta 2000, 45, 1399–1403. [Google Scholar] [CrossRef]
- Yang, J.M.; Chiu, H.C. Preparation and characterization of polyvinyl alcohol/chitosan blended membrane for alkaline direct methanol fuel cells. J. Membr. Sci. 2012, 419, 65–71. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, S.A. Preparation of graphene-based poly(vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- García, L.; Cruz, C.; Casado-Coterillo, J.; Iniesta, V.; Montiel, A. Preparation and characterization of novel chitosan-based mixed matrix membranes resistant in alkaline media. J. Appl. Polym. Sci. 2015, 132, 42240. [Google Scholar] [CrossRef]
- Mohamad, A.A.; Mohamed, N.S.; Yahya, M.Z.; Othman, R.; Ramesh, S.; Aliasf, Y.; Arof, A.K. Ionic conductivity studies of poly (vinyl alcohol) alkaline solid polymer electrolyte and its use in nickel–zinc cells. Solid State Ion. 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.; Peppley, B.; Tam Bui, V. Chitosan-based solid electrolyte composite membranes I. Preparation and characterization. J. Membr. Sci. 2006, 280, 666–674. [Google Scholar] [CrossRef]
- González-Campos, J.; Betzabe del Río Rosa, E. Compuestos de quitosano/nanopartículas de Ag: Conductividad y mecanismos de relajación y su relación con sus propiedades macroscópicas. Sociedad Mexicana de Ciencia y Tecnología de Superficies y Materiales. Superf. Vacío 2012, 25, 43–48. [Google Scholar]
- Permana, D.; Ilimu, E.; Faariu, N.M.; Setyawati, A.; Kadidae, L.O.; Ramadhan, L.O.A.N. Synthesis and characterization of chitosan-polyvinyl alcohol-Fe2O3 composite membrane for DMFC application. Makara J. Sci. 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, W.T.; Mahesh, K.P.; Yang, C.C. Enhanced performance of a direct methanol alkaline fuel cell (DMAFC) using a polyvinyl alcohol/fumed silica/KOH electrolyte. J. Power Sources 2010, 195, 7991–7999. [Google Scholar] [CrossRef]
- Li, P.C.; Liao, G.M.; Kumar, S.R.; Shih, C.M.; Yang, C.C.; Wang, D.M.; Lue, S.J. Fabrication and characterization of chitosan nanoparticle-incorporated quaternized poly (vinyl alcohol) composite membranes as solid electrolytes for direct methanol alkaline fuel cells. Electrochim. Acta 2015, 187, 616–628. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Gómez, E.E.; Mina Hernández, J.H.; Diosa Astaiza, J.E. Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells. Polymers 2020, 12, 1691. https://doi.org/10.3390/polym12081691
Ruiz Gómez EE, Mina Hernández JH, Diosa Astaiza JE. Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells. Polymers. 2020; 12(8):1691. https://doi.org/10.3390/polym12081691
Chicago/Turabian StyleRuiz Gómez, Elio Enrique, José Herminsul Mina Hernández, and Jesús Evelio Diosa Astaiza. 2020. "Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells" Polymers 12, no. 8: 1691. https://doi.org/10.3390/polym12081691
APA StyleRuiz Gómez, E. E., Mina Hernández, J. H., & Diosa Astaiza, J. E. (2020). Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells. Polymers, 12(8), 1691. https://doi.org/10.3390/polym12081691