A Review on Citric Acid as Green Modifying Agent and Binder for Wood
Abstract
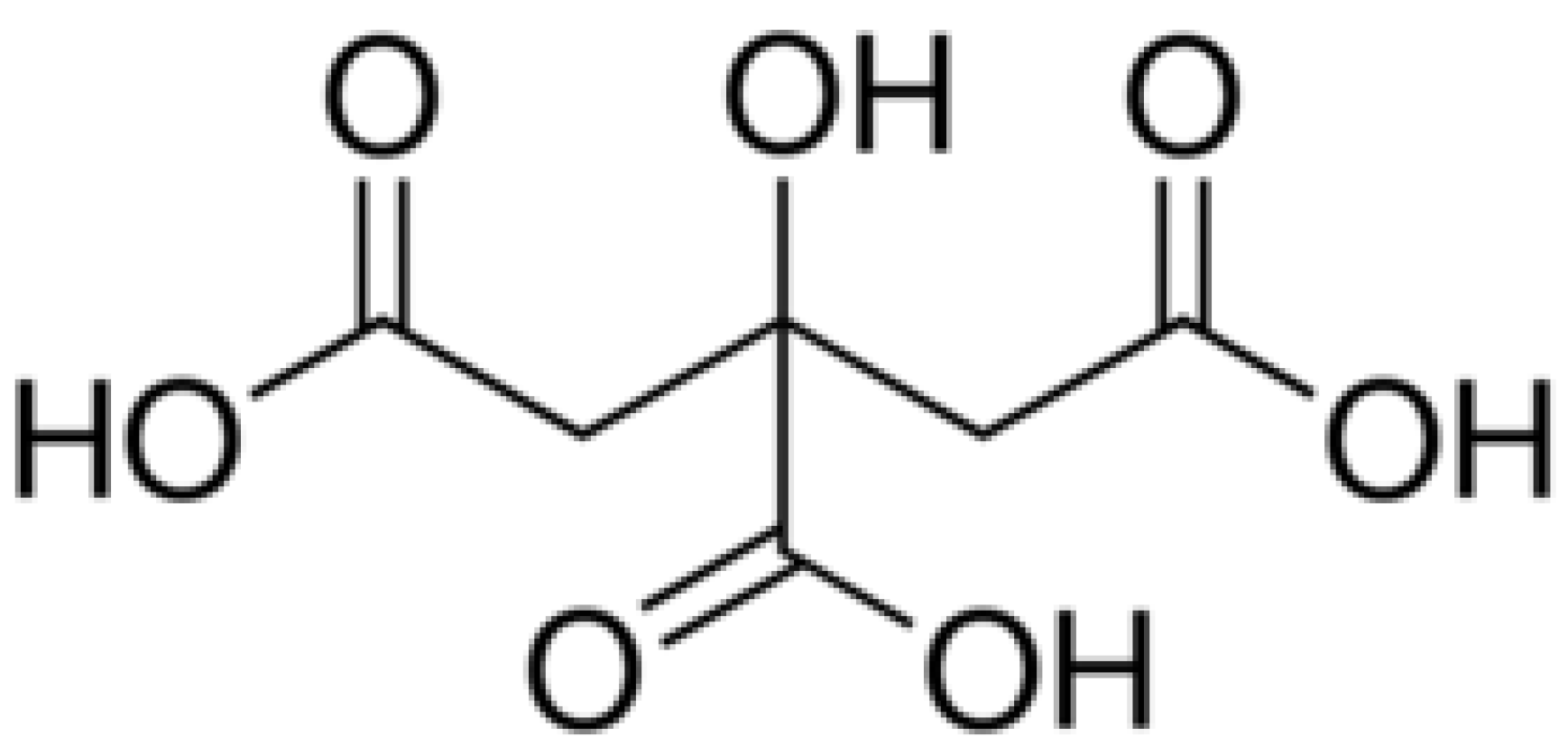
1. Introduction
2. Esterification for Wood Modification
2.1. Reaction Mechanism of Interaction between Wood and Citric Acid
2.2. Citric Acid Compared to Other Polycarboxylic Acids
2.3. Citric Acid for Wood Modification
2.3.1. Citric Acid Alone
2.3.2. Citric Acid with Glycerol
2.3.3. Citric Acid with Glucose
2.3.4. Citric Acid with Sorbitol
2.4. Factor That Affect the Effectiveness of Wood Modification by Citric Acid
2.4.1. Catalyst Type
2.4.2. Wood Species
2.4.3. Curing Temperature
3. Citric Acid as Main Bonding Component for Wood Composites
3.1. Bonding Mechanism
3.2. Wood-Based Composites
3.2.1. Wood Based Molding
3.2.2. Particleboard
- Pressing temperature of more than 180 °C for 10 min
- Board density of 800 kg/m3
- CA content of 20 wt % or above
- Pre-drying treatment of 12 h at 80 °C is preferential
3.2.3. Fibreboard
3.2.4. Veneer-Based Panels
4. Comparison between CA-Treated Particleboard with Conventional Particleboard
5. Environmental Impact and Future Outlook
5.1. Environmental Impact of CA-Treated Wood
5.2. Future Outlook
5.3. Economic Perspective
6. Conclusions
- Wood modified with CA displayed an improvement in MOE, compression strength and dimensional stability and biological resistance as well as reduced WA.
- Glycerol, glucose, and sorbitol can be combined with CA to resulted in a better treatment.
- Several factors have been identified as able to affect the effectiveness of CA treatment on wood, namely catalyst type, wood species, and curing temperature. Apart from SHP, chlorhydric acid (HCl) and para-toluenesulphonic acid (p-TSA) are also among the most effective catalyst. A temperature of at least 140 °C is required to ensure the polyesterification occurs to a greater extent.
- In the fabrication of wood-based composites, CA alone could attain sufficient bonding properties for the resultant boards. However, sucrose and starch could be added to improve some selected properties of the boards.
- For the manufacturing of particleboard, particleboard with the optimum properties could be attained when 20 wt % of CA/sucrose adhesive with ratio of 25/75 (CA/sucrose) was pressed at a temperature >180 °C for 10 min, with the condition that pre-drying treatment of 12 h at 80 °C was adopted.
- Contrarily, bonding the flat surfaces involves a completely different application compared to particleboard and fiberboard. Therefore, to fabricate CA/sucrose-bonded plywood with acceptable performance, one has to: 1) synthesize the CA/sucrose adhesive with ratio of 75/25 (CA/sucrose) at 100 °C for 2 h, and 2) hot-press the plywood at 190 °C for 7 min using a glue spread rate of 140 g/m2.
- One of the challenges faced by CA is that it requires a higher pressing temperature and longer pressing time. Further study should be conducted to match the industrial practice.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apelblat, A. (Ed.) Citric acid chemistry. In Citric Acid; Springer: Cham, Switzerland, 2014; pp. 213–266. [Google Scholar]
- He, Z.; Umemura, K. Utilization of citric acid in wood bonding. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 221–238. [Google Scholar]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef]
- Zahoor, A. Jabir Ibn Haiyan (Geber). Available online: http://www.unhas.ac.id/rhiza/arsip/saintis/haiyan.html (accessed on 19 April 2020).
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Ghani, A.; Ashaari, Z.; Bawon, P.; Lee, S.H. Reducing formaldehyde emission of urea formaldehyde-bonded particleboard by addition of amines as formaldehyde scavenger. Build. Environ. 2018, 142, 1881–1894. [Google Scholar] [CrossRef]
- Kanazawa, A.; Saito, I.; Araki, A.; Takeda, M.; Ma, M.; Saijo, Y.; Kishi, R. Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air 2010, 20, 72–84. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Di, M. Green modification of corn stalk lignin and preparation of environmentally friendly lignin-based wood adhesive. Polymers 2018, 10, 631. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, S.; Wu, D.; Zhang, M.; Huang, C.; Umemura, K.; Yong, Q. Synthesis and characterization of sucrose and ammonium dihydrogen phosphate (SADP) adhesive for plywood. Polymers 2019, 11, 1909. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hayashi, S.; Xu, W.; Wu, Z.; Tanaka, S.; Sun, S.; Zhang, M.; Kanayama, K.; Umemura, K. A novel eco-friendly wood adhesive composed by sucrose and ammonium dihydrogen phosphate. Polymers 2018, 10, 1251. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Kan, Y.; Gao, J.; Zhang, Y.; Gao, Z. The effect of thermo-chemical treatment on the water resistance of defatted soybean flour-based wood adhesive. Polymers 2018, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Miao, Y.; Yang, Z.; Wang, H.; Sang, R.; Fu, Y.; Huang, C.; Wu, Z.; Zhang, M.; Sun, S.; et al. Effects of sulfuric acid on the curing behavior and bonding performance of tannin–sucrose adhesive. Polymers 2018, 10, 651. [Google Scholar] [CrossRef]
- Cahyono, T.D. Syahidah. Citric acid, an environmentally friendly adhesive and wood impregnation material-review of research. IOP Conf. Ser. Mater. Sci. Eng. 2019, 593, 012009. [Google Scholar] [CrossRef]
- Militz, H.; Lande, S. Challenges in wood modification technology on the way to practical applications. Wood Mater. Sci. Eng. 2009, 4, 23–29. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Bikiaris, D.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials 2019, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.S. Wood Modification—Chemical, Thermal and other Processes; John Wiley and Sons Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Berube, M.A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- Hill, C.A. Wood modification: An update. BioResources 2011, 6, 918–919. [Google Scholar]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef]
- Treu, A.; Nunes, L.; Larnøy, E. Macrobiological Degradation of Esterified Wood with Sorbitol and Citric Acid. Forests 2020, 11, 776. [Google Scholar] [CrossRef]
- Welch, C.M.; Andrews, K.A. Tetracarboxylic acids as formaldehyde-free durable press finishing agents. Text. Res. J. 1988, 58, 480–486. [Google Scholar] [CrossRef]
- Vukusic, S.B.; Katovic, D.; Schramm, C.; Trajkovic, J.; Sefc, B. Polycarboxylic acids as non-formaldehyde anti-swelling agents for wood. Holzforschung 2006, 60, 439–444. [Google Scholar] [CrossRef]
- Yang, C.Q. FT-IR spectroscopy study of the ester crosslinking mechanism of cotton cellulose. Text. Res. J. 1991, 61, 433–440. [Google Scholar] [CrossRef]
- Peyer, S.M.; Wolcott, M.P.; Fenoglio, D.J. Reducing moisture swell of densified wood with polycarboxylic acid resin. Wood Fiber Sci. 2007, 32, 520–526. [Google Scholar]
- Katovic, D.; Trajkovic, J.; Bischof Vukusic, S.; Sefc, B. Alternative agents and methods for chemical modification of wood. Drv. Ind. 2004, 55, 175–180. [Google Scholar]
- Schramm, C.; Rinderer, B. Influence of additives on the formation of saturated PCA produced during durable-press curing with citric acid. Coloration Technol. 1999, 115, 306–311. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Al-Sammerrai, D.A. Thermal decomposition of citric acid. Thermochim. Acta 1986, 98, 119–126. [Google Scholar] [CrossRef]
- Fang, G.; Li, J.; Xu, X. The intermediate of crosslinking reaction between wood and polycarboxylic acid. Sci. Silvae Sin. 2000, 36, 51–54. [Google Scholar]
- McSweeny, J.; Rowell, R.M.; Min, S. Effect of citric acid modification of aspen wood on sorption of copper ion. J. Nat. Fibers 2006, 3, 43–58. [Google Scholar] [CrossRef]
- Caulfield, D.F. Ester crosslinking to improve wet performance of paper using multifunctional carboxylic acids, butanetetracarboxylic and citric acid. Tappi J. 1994, 77, 205–212. [Google Scholar]
- Zoldners, J.; Kiseleva, T. Modification of hemicelluloses with polycarboxylic acids. Holzforschung 2013, 67, 567–571. [Google Scholar] [CrossRef]
- Welch, C.M.; Kottes-Andrews, B.A. Ester crosslinks: A route to high performance nonformaldehyde finishing of cotton. Text. Chem. Colorist 1989, 21, 13–17. [Google Scholar]
- Morris, N.M.; Catalano, E.A.; Kottes-Andrews, B.A. FT-IR determination of degree of esterification in polycarboxylic acid cross-link finishing of cotton. Cellulose 1995, 2, 31–39. [Google Scholar]
- Yang, C.Q.; Wang, X.; Kang, I.S. Ester cross-linking of cotton fabric by polymeric carboxylic acids and citric acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Šefc, B.; Trajković, J.; Sinković, T.; Hasan, M.; Ištok, I. Compression strength of fir and beech wood modified by citric acid. Drv. Ind. 2012, 63, 45–50. [Google Scholar] [CrossRef]
- Miklečić, J.; Jirouš-Rajković, V. Accelerated weathering of coated and uncoated beech wood modified with citric acid. Drv. Ind. 2011, 62, 277–282. [Google Scholar] [CrossRef]
- Tarasin, M.; Rattanapun, W. Termite resistance of Melaleuca cajuputi wood treated with citric acid. Agric. Nat. Resour. 2019, 53, 662–666. [Google Scholar]
- Toyoshima, I.; Takahashi, M.; Tsunoda, K.; Yoshimura, T. Comparative toxicity, residual nature and effect on respiration of boron compounds in a lower termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Mater. Org. 1997, 31, 217–226. [Google Scholar]
- Xie, Y.; Krause, A.; Militz, H.; Turkulin, H.; Richter, K.; Mai, C. Effect of treatments with 1,3-dimethylol-4,5-dihydroxyethyleneurea (DMDHEU) on the tensile properties of wood. Holzforschung 2007, 61, 43–50. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, Z.; Sui, S.; Wang, Q.; Xie, Y. Esterification of wood with citric acid: The catalytic effects of sodium hypophosphite (SHP). Holzforschung 2014, 68, 427–433. [Google Scholar] [CrossRef]
- L’Hostis, C.; Thévenon, M.F.; Fredon, E.; Gérardin, P. Improvement of beech wood properties by in situ formation of polyesters of citric and tartaric acid in combination with glycerol. Holzforschung 2018, 72, 291–299. [Google Scholar] [CrossRef]
- Halpern, J.M.; Urbanski, R.; Weinstock, A.K.; Iwig, D.F.; Mathers, R.T.; von Recum, H.A. A biodegradable thermoset polymer made by esterification of citric acid and glycerol. J. Biomed. Mater. Res. Part 2014, 102, 1467–1477. [Google Scholar] [CrossRef]
- Goebel, W.F. On the oxidation of glucose in alkaline solutions of iodine. J. Biol. Chem. 1927, 72, 801–807. [Google Scholar]
- Yang, Q. Diensional Stability of Chemical Modified Aspen by Using Oxidized Glucose. Master’s Thesis, Lulea University of Technology, Lulea, Sweden, 2012. [Google Scholar]
- He, X.; Xiao, Z.; Feng, X.; Sui, S.; Wang, Q.; Xie, Y. Modification of poplar wood with glucose crosslinked with citric acid and 1, 3-dimethylol-4, 5-dihydroxy ethyleneurea. Holzforschung 2016, 70, 47–53. [Google Scholar] [CrossRef]
- Guo, W.; Xiao, Z.; Wentzel, M.; Emmerich, L.; Xie, Y.; Militz, H. Modification of Scots pine with activated glucose and citric acid: Physical and mechanical properties. BioResources 2019, 14, 3445–3458. [Google Scholar]
- Xiao, Z.; Xie, Y.; Militz, H.; Mai, C. Effects of modification with glutaraldehyde on the mechanical properties of wood. Holzforschung 2010, 64, 475–482. [Google Scholar] [CrossRef]
- Young, N.W.G.; O’Sullivan, G.R. The influence of ingredients on product stability and shelf life. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Cambridge, UK; pp. 132–183.
- Romero, A.; Esther, E.; Sastre, Á.; Nieto-Márquez, A. Conversion of biomass into sorbitol: Cellulose hydrolysis on MCM-48 and d-Glucose hydrogenation on Ru/MCM-48. Microporous Mesoporous Mater. 2016, 224, 1–8. [Google Scholar] [CrossRef]
- Doll, K.M.; Shogren, R.L.; Willett, J.L.; Swift, G. Solvent-free polymerization of citric acid and D-sorbitol. J. Polym. Sci. Part 2006, 44, 4259–4267. [Google Scholar] [CrossRef]
- Kiljunen, S.; Koski, A.; Kuntitu, M. Impregnation of chemicals into wood. World Patent WO2011/042609 A1, 14 April 2011. [Google Scholar]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of wood using sorbitol and citric acid under aqueous conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Beck, G. Leachability and decay resistance of wood polyesterified with sorbitol and citric acid. Forests 2020, 11, 650. [Google Scholar] [CrossRef]
- Šefc, B.; Trajković, J.; Hasan, M.; Katović, D.; Bischof Vukušić, S.; Frančić, M. Dimensional stability of wood modified by citric acid using different catalysts. Drv. Ind. 2009, 60, 23–26. [Google Scholar]
- Schramm, C.; Bischof Vukusic, S.; Katovic, D. Non-formaldehyde durable press finishing of dyed fabrics: Evaluation of cotton-bound polycarboxylic acids. Coloration Technol. 2002, 118, 244–249. [Google Scholar] [CrossRef]
- Lee, S.H.; Ashaari, Z.; Lum, W.C.; Halip, J.A.; Ang, A.F.; Tan, L.P.; Chin, K.L.; Tahir, P.M. Thermal treatment of wood using vegetable oils: A review. Constr. Build. Mater. 2018, 181, 408–419. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Munawar, S.S.; Kawai, S. Application of citric acid as natural adhesive for wood. J. Appl. Polym. Sci. 2012, 123, 1991–1996. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with wood carbohydrates and lignin of citric acid as a bond promoter of wood veneer panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Amirou, S.; Pizzi, A.; Delmotte, L. Citric acid as waterproofing additive in butt joints linear wood welding. Eur. J. Wood Wood Prod. 2017, 75, 651–654. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Kawai, S. Characterization of wood-based molding bonded with citric acid. J. Wood Sci. 2012, 58, 38–45. [Google Scholar] [CrossRef]
- Widyorini, R.; Nugraha, P.; Rahman, M.; Prayitno, T. Bonding ability of a new adhesive composed of citric acid-sucrose for particleboard. BioResources 2016, 11, 4526–4535. [Google Scholar] [CrossRef]
- Widyorini, R.; Umemura, K.; Isnan, R.; Putra, D.R.; Awaludin, A.; Prayitno, T.A. Manufacture and properties of citric acid-bonded particleboard made from bamboo materials. Eur. J. Wood Wood Prod. 2016, 74, 57–65. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2015, 61, 40–44. [Google Scholar] [CrossRef]
- Widyorini, R.; Umemura, K.; Kusumaningtyas, A.R.; Prayitno, T.A. Effect of starch addition on properties of citric acid-bonded particleboard made from bamboo. BioResources 2017, 12, 8068–8077. [Google Scholar]
- Kusumah, S.S.; Umemura, K.; Yoshioka, K.; Miyafuji, H.; Kanayama, K. Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard I: Effects of pre-drying treatment and citric acid content on the board properties. Ind. Crop. Prod. 2016, 84, 34–42. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Umemura, K.; Guswenrivo, I.; Yoshimura, T.; Kanayama, K. Utilization of sweet sorghum bagasse and citric acid for manufacturing of particleboard II: Influences of pressing temperature and time on particleboard properties. J. Wood Sci. 2017, 63, 161–172. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K.; Kanayama, K. Effects of the addition of citric acid on tannin-sucrose adhesive and physical properties of the particleboard. BioResources 2016, 11, 1319–1333. [Google Scholar] [CrossRef]
- Santoso, M.; Widyorini, R.; Prayitno, T.A.; Sulistyo, J. The effects of extractives substances for bonding performance of three natural binder on nipa fronds particleboard. KnE Life Sci. 2019, 2019, 227–238. [Google Scholar] [CrossRef]
- Santoso, M.; Widyorini, R.; Prayitno, T.A.; Sulistyo, J. Bonding performance of maltodextrin and citric acid for particleboard made from nipa fronds. J. Korean Wood Sci. Technol. 2017, 45, 432–443. [Google Scholar]
- Syamani, F.A.; Kusumah, S.S.; Astari, L.; Prasetiyo, K.W.; Wibowo, E.S.; Subyakto. Effect of pre-drying time and citric acid content on Imperata cylindrica particleboards properties. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 01203. [Google Scholar] [CrossRef]
- Ferrandez-Garcia, M.T.; Ferrandez-Garcia, C.E.; Garcia-Ortuño, T.; Ferrandez-Garcia, A.; Ferrandez-Villena, M. Experimental evaluation of a new giant reed (Arundo Donax L.) composite using citric acid as a natural binder. Agronomy 2019, 9, 882. [Google Scholar] [CrossRef]
- Liao, R.; Xu, J.; Umemura, K. Low density sugarcane bagasse particleboard bonded with citric acid and sucrose: Effect of board density and additive content. BioResources 2016, 11, 2174–2185. [Google Scholar] [CrossRef]
- Indrayani, Y.; Setyawati, D.; Munawar, S.S.; Umemura, K.; Yoshimura, T. Evaluation of termite resistance of medium density fiberboard (MDF) manufacture from agricultural fiber bonded with citric acid. Procedia Environ. Sci. 2015, 28, 778–782. [Google Scholar] [CrossRef][Green Version]
- Widyorini, R.; Dewi, G.K.; Nugroho, W.D.; Prayitno, T.A.; Jati, A.S.; Tejolaksono, M.N. Properties of citric acid-bonded composite board from elephant dung fibers. J. Korean Wood Sci. Technol. 2018, 46, 132–142. [Google Scholar]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further exploration of sucrose—Citric acid adhesive: Investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers 2019, 11, 1996. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further exploration of sucrose-citric acid adhesive: Synthesis and application on plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kelly, M.W. Critical literature review of relationships between processing parameters and physical properties of particleboard. In General Technical Report FPL-10; USDA Forest Service, Forest Product Laboratory: Madison, WI, USA, 1977. [Google Scholar]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Raina, A.; Bland, J.; Doolittle, A.; Boopathy, R.; Folkins, M. Effect of orange oil on Formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2007, 100, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Latibari, A.; Roohnia, M. Potential of utilization of the residues from poplar plantation for particleboard production in Iran. J. For. Res. 2010, 21, 503–508. [Google Scholar] [CrossRef]
- Lee, S.H.; Lum, W.C.; Zaidon, A.; Maminski, M. Microstructural, mechanical and physical properties of post heat-treated melamine-fortified urea formaldehyde-bonded particleboard. Eur. J. Wood Wood Prod. 2015, 73, 607–616. [Google Scholar] [CrossRef]
- Lee, S.H.; Ashaari, Z.; Ang, A.F.; Halip, J.A.; Lum, W.C.; Dahali, R.; Halis, R. Effects of two-step post heat-treatment in palm oil on the properties of oil palm trunk particleboard. Ind. Crop. Prod. 2018, 116, 249–258. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Y.; Zhang, R.; Jenkins, B.M. Physical properties of thin particleboard made from saline eucalyptus. Ind. Crop. Prod. 2007, 26, 185–194. [Google Scholar] [CrossRef]
- Amirou, S.; Zerizer, A.; Pizzi, A.; Haddadou, I.; Zhou, X. Particleboards production from date palm biomass. Eur. J. Wood Wood Prod. 2013, 71, 717–723. [Google Scholar] [CrossRef]
- Enayati, A.A.; Eslah, F. Modeling beech (Fagus orientalis) particleboard properties based on resin content and board density. J. Indian Acad. Wood Sci. 2014, 11, 45–49. [Google Scholar] [CrossRef]
- Iwakiri, S.; Trianoski, R.; Chies, D.; Tavares, E.L.; França, M.C.; Lau, P.C.; Iwakiri, V.T. Use of residues of forestry exploration of Pinus taeda for particleboard manufacture. Rev. Árvore 2017, 41, e410304. [Google Scholar] [CrossRef]
- Karlinasari, L.; Hermawan, D.; Maddu, A.; Martiandi, B.; Hadi, Y.S. Development of particleboard from tropical fast-growing species for acoustic panel. J. Trop. For. Sci. 2012, 24, 64–69. [Google Scholar]
- Oliveira, S.L.; Mendes, R.F.; Mendes, L.M.; Freire, T.P. Particleboard panels made from sugarcane bagasse: Characterization for use in the furniture industry. Mater. Res. 2016, 19, 914–922. [Google Scholar] [CrossRef]
- Essoua, E.G.G.; Blanchet, P.; Landry, V.; Beauregard, R. Pine wood treated with a citric acid and glycerol mixture: Biomaterial performance improved by a biobyproduct. Bioresources 2016, 11, 3049–3072. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Esteves, B.; Nunes, L.; Domingos, I. Life cycle assessment of thermally treated and untreated maritime pine boards: A Portuguese case study. In Proceedings of the Seventh European Conference on Wood Modification, ECWM7, Lisbon, Portugal, 10–12 March 2014. [Google Scholar]
- Essoua, E.G.G.; Beauregard, R.; Amor, B.; Blanchet, P.; Landry, V. Evaluation of environmental impacts of citric acid and glycerol outdoor softwood treatment: Case-study. J. Clean. Prod. 2017, 164, 1507–1518. [Google Scholar] [CrossRef]
- Citric Acid Market. Citric Acid Market by form (Anhydrous and Liquid), Application (Food, Pharmaceuticals, and Cosmetics), Function (Acidulant, Antioxidant, Preservative, and Sequestrant), and by Region (North America, Europe, Asia-Pacific, and Row)—Global Forecast to 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/citric-acid-market-185568353.html (accessed on 23 July 2020).
- Lazarevic, D.; Kautto, P.; Antikainen, R. Finland’s woodframe multi-storey construction innovation system: Analysing motors of creative destruction. For. Policy Econ. 2020, 110, 101861. [Google Scholar] [CrossRef]
- Domenech, T.; Bahn-Walkowiak, B. Transition towards a resource efficient circular economy in Europe: Policy lessons from the EU and the member states. Ecol. Econ. 2019, 155, 7–19. [Google Scholar] [CrossRef]
- Wilts, H.; O’Brien, M. A policy mix for resource efficiency in the EU: Key instruments, challenges and research needs. Ecol. Econ. 2019, 155, 59–69. [Google Scholar] [CrossRef]
- Heräjärvi, H.; Kunttu, J.; Hurmekoski, E.; Hujala, T. Outlook for modified wood use and regulations in circular economy. Holzforschung 2020, 74, 334–343. [Google Scholar] [CrossRef]
- Sommerhuber, P.F.; Welling, J.; Krause, A. Substitution potentials of recycled HDPE and wood particles from post-consumer packaging waste in wood-plastic composites. Waste Manag. 2015, 46, 76–85. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Rodrigues, C.; De Carvalho, J.C.; Medeiros, A.B.P.; Soccol, C.R. Production and application of citric acid. In Current Developments in Biotechnology and Bioengineering; Larroche, C., Sanroman, M., Dr, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 557–575. [Google Scholar]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crop. Prod. 2013, 50, 346–351. [Google Scholar] [CrossRef]

| Advantages | Disadvantages |
|---|---|
| Reduction in water absorption (WA) | No improvement in weathering resistance |
| Improved resistance against termites and fungi | Reduction in modulus of rupture (MOR) |
| Improved modulus of elasticity (MOE) and compression strength | Reduction in impact strength |
| Better dimensional stability | Yellowing of the treated wood |
| Materials Source | Products | Adhesive Solution | Reference |
|---|---|---|---|
| Acacia mangium bark powder | Wood based molding | Citric acid | [57] |
| Acacia mangium bark powder | Wood based molding | Citric acid | [60] |
| Bamboo materials | Particleboard | Citric acid | [61] |
| Teak | Particleboard | Critic acid and sucrose | [62] |
| Recycled wood particles | Particleboard | Citric acid and sucrose | [63] |
| Recycled wood particles | Particleboard | Citric acid and sucrose | [64] |
| Petung (Dendrocalamus asper) | Particleboard | Citric acid and starch | [65] |
| Sweet sorghum bagasse | Particleboard | Citric acid | [66] |
| Sweet sorghum bagasse | Particleboard | Citric acid | [67] |
| Recycled wood particles | Particleboard | Tannin, sucrose and citric acid | [68] |
| Nipa fronds | Particleboard | Maltodextrin, sucrose and citric acid | [69] |
| Nipa fronds | Particleboard | Maltodextrin and citric acid | [70] |
| alang-alang (Imperata cylindrica) | Particleboard | Citric acid | [71] |
| New Giant Reed (Arundo Donax L.) | Particleboard | Citric acid | [72] |
| Sugarcane bagasse | Particleboard | Citric acid and sucrose | [73] |
| Pineapple (Ananas comosus (L.) Merr.) leaves | Medium density fiberboard | Citric acid and sucrose | [74] |
| Elephant dung fibers | Fiberboard | Citric acid | [75] |
| Poplar (Populus tomentosa Carr) | Plywood | Citric acid and sucrose | [76] |
| Poplar (Populus tomentosa Carr) | Plywood | Citric acid and sucrose | [77] |
| Poplar Veneer | Wood veneer panels | Citric acid | [58] |
| Studies | Reference |
|---|---|
| Board density | |
| Bending strength of particleboard increased proportionately as the density increased from 400 kg/m3 to 800 kg/m3 and maintained an almost constant value at 1000 kg/m3. 800 kg/m3 is the optimum density for particleboard. TS increased with increasing density but maintained at <12%. WA decreased as the board density increased. Board density of 800 kg/m3 is required to obtained optimum water resistance. | [64] |
| Press temperature and time | |
| Bending strength was low when pressed at 140 and 160 °C, but increased significantly when pressed at 180 °C and remained constant at temperature above 180 °C. The internal bonding (IB) strength increased significantly when pressed at temperature up to 200 °C and started to decreased when pressed at 220 and 240 °C. A press temperature of 200 °C or higher is required to obtain optimum dimensional stability. | [64] |
| The IB and bending strength of the bamboo particleboard increased along with pressing temperature and the maximum IB was recorded when 200 °C was used and decreased sharply when 220 °C was used. TS and WA displayed the same trend. The IB and bending strength of the bamboo particleboard increased when the pressing time increased from 2 to 10 min and started to decreased when pressed at 15 min. TS and WA decreased along with increasing pressing time. No significant different was detected between pressing time of 10 and 15 min. A pressing time of 10 min is optimum for particleboard production | [67] |
| Pre-drying treatment | |
| Pre-drying treatment time of 6 h and 12 h at 80 °C has reduced the TS from 26.1% to 13.3% and 7.7%, respectively. MOR, MOE and IB of the particleboard produced was higher when pre-drying treatment of 12 h was applied compared to that of without pre-drying treatment or after 6 h pre-drying treatment. | [71] |
| Particleboard made from pre-dried particles at 80 °C for 12 h has higher bending strength. | [66] |
| Citric acid content | |
| Particleboard bonded with 20 wt % CA has significantly lower TS compared to that of the particleboard bonded with 10 and 15% CA. | [71] |
| Bending strength of the particleboard increased as the CA content increased from 0 to 20 wt % but decreased when 30 wt % were applied. As for IB strength, 30 wt % resulted in the best result. TS and WA decreased along with increasing CA content. | [66] |
| The most optimum IB value was obtained when the particleboard was bonded with 15 wt % CA. Any addition of CA content did not bring significant improvement in IB. | [62] |
| Materials | Density (kg/m3) | Type of Resin | Resin Content (wt %) | MOR (MPa) | MOE (MPa) | IB (MPa) | TS (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Recycled wood particles | 800 | Citric acid | 20 | 10.7 | 3300 | 0.32 | - | [63] |
| 800 | Citric acid: sucrose (25:75) | 20 | 20.1 | 4400 | 1.13 | - | ||
| Sweet sorghum bagasse | 800 | Citric acid | 20 | 21.8 | 5200 | 0.89 | 10.1 | [67] |
| 800 | PF | 12 | 32.9 | 4500 | 0.78 | 20.6 | ||
| 800 | pMDI | 8 | 34.1 | 4600 | 1.33 | 23.1 | ||
| New Giant Reed | 700 | Citric acid | 5 | 3.91 | 1019 | 0.27 | 38.99 | [72] |
| 700 | Citric acid | 10 | 4.99 | 1245 | 0.22 | 28.03 | ||
| Rubberwood | 700 | MUF | 8 | 14.3 | 2152 | 1.3 | 38.2 | [83] |
| Oil palm trunk | 700 | UF | 8 | 11.18 | 1843 | 1.01 | 14.99 | [84] |
| Poplar | 700 | UF | 10 | 14.57 | 2015 | 1.32 | 31.26 | [82] |
| Eucalyptus | 720 | pMDI | 4 | 10.4 | 1651 | 1.45 | 26.95 | [85] |
| Date palm trunk | 700 | PF | 10 | 18 | 2970 | 0.95 | 17.6 | [86] |
| 700 | MUF | 10 | 17.6 | 2890 | 0.9 | 19.8 | ||
| Beech | 720 | UF | 8 | 16.3 | 3261 | - | 18.2 | [87] |
| Pine | 720 | UF | 8 | 10.3 | 1913 | 0.51 | 64.71 | [88] |
| Acacia mangium | 730 | MDI | 12 | 15.2 | - | 0.8 | - | [89] |
| Eucalyptus | 620 | UF | 10 | 17.1 | 2869 | 0.4 | 15.6 | [90] |
| Pine | 620 | UF | 10 | 13.6 | 2450 | 0.47 | 10 | |
| Sugarcane bagasse | 620 | UF | 10 | 15.3 | 2295 | 0.26 | 11.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Md Tahir, P.; Lum, W.C.; Tan, L.P.; Bawon, P.; Park, B.-D.; Osman Al Edrus, S.S.; Abdullah, U.H. A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers 2020, 12, 1692. https://doi.org/10.3390/polym12081692
Lee SH, Md Tahir P, Lum WC, Tan LP, Bawon P, Park B-D, Osman Al Edrus SS, Abdullah UH. A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers. 2020; 12(8):1692. https://doi.org/10.3390/polym12081692
Chicago/Turabian StyleLee, Seng Hua, Paridah Md Tahir, Wei Chen Lum, Li Peng Tan, Paiman Bawon, Byung-Dae Park, Syeed SaifulAzry Osman Al Edrus, and Ummi Hani Abdullah. 2020. "A Review on Citric Acid as Green Modifying Agent and Binder for Wood" Polymers 12, no. 8: 1692. https://doi.org/10.3390/polym12081692
APA StyleLee, S. H., Md Tahir, P., Lum, W. C., Tan, L. P., Bawon, P., Park, B.-D., Osman Al Edrus, S. S., & Abdullah, U. H. (2020). A Review on Citric Acid as Green Modifying Agent and Binder for Wood. Polymers, 12(8), 1692. https://doi.org/10.3390/polym12081692









