Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis Methods
2.2.1. Synthesis of Butoxyethanol
2.2.2. Synthesis of Phenoxyethanol
2.2.3. The synthesis of Butoxyethyl Phenoxyethyl Adipate (BEPEA)
2.3. Methods of Analysis
3. Results and Discussion
3.1. Synthesis of Ethoxylated Alcohols
3.2. The Synthesis of Butoxyethyl Phenoxyethyl Adipate (BEPEA)
- 1—basic formulation (PVC 7059M—100 parts by mass, plasticizer DOP—42 parts by mass, stabilizer calcium stearate—3 parts by mass)
- 2—basic formulation containing 42 parts by weight of a mixture of plasticizers DOP:BEPEA in a ratio of 35:7
- 3—basic formulation containing, instead of the BEPEA plasticizer, a plasticizing composition with a zinc compound.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uilki, C.; Sammers, D.; Daniels, C. Polyvinyl Chloride; Profession: St. Petersburg, Russia, 2007; 728p. [Google Scholar]
- Holden, G.; Kriheldorf, H.R.; Kuirk, R.P. Thermoplastic Elastomers; Profession: St. Petersburg, Russia, 2011; 720p. [Google Scholar]
- Wickson, E.J.; Grossman, R.F.; Kuirk, R.P. The Development of Compositions Based on PVC; Scientific foundations and technologies: St. Petersburg, Russia, 2009; 608p. [Google Scholar]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Brazel, C.S.; Rosen, S.L. Fundamental Principles of Polymeric Materials; Wiley: New York, USA, 2012; 432p. [Google Scholar]
- Fadina, Y.I. Analysis of the Russian polymer market and its further development. Bus. Educ. Knowl. Econ. 2017, 1, 99–101. [Google Scholar]
- Jakoby, R. Marketing and Sales in the Chemical Industry in Plastics and Rubbers, 2nd ed.; Wiley-VCH: New York, NY, USA, 2002; 177p. [Google Scholar]
- Godwin, A.D. Applied Plastics Engineering Handbook, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 533–553. [Google Scholar]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Savicheva, J.N.; Gareeva, N.B.; Shaikhullin, I.R. The influence of nanoadditives in the synthesis of eco-friendly polyester plasticizers. Nanotechnologies Constr. 2020, 12, 21–26. [Google Scholar]
- European Commission Health & Consumer protection directorate-general, Directorate C-Scientific Opinions, C2. Opinion on Medical Devices Containing DEHP plasticized PVC; Neonates and Other Groups Possibly at Risk from DEHP Toxicity. 2002. Doc.SANCO/SCMPMD/2002/0010 Final. Available online: https://ec.europa.eu/health/ph_risk/committees/scmp/documents/out43_en.pdf (accessed on 15 July 2020).
- European Commission Directive. Off. J. Eur. Union. 1999, L315, 46.
- Technical Expert. Available online: http://docs.cntd.ru/document/8308082 (accessed on 15 July 2020).
- Available online: http://www.wno.org/rio-declaration-on-environment-and-development (accessed on 15 July 2020).
- Vijayendrana, R.; Benecke, H.; Elhard, J.D.; Mcginnis, V.D.; Ferris, K.F. Environmentally friendly plasticizers for polyvinyl chloride(PVC) resins. Soc. Plast. Eng. Annu. Tech. Conf. 2001, 3, 2978. [Google Scholar]
- Mazitova, A.K.; Aminova, G.K.; Nafikova, R.F.; Deberdeev, R.Y. Basic Polyvinyl Chloride Compositions for Construction Purposes; USPTU: Ufa, Russia, 2013; 130p. [Google Scholar]
- Smith, V.A.; Dilman, A.D. Fundamentals of Modern Organic Synthesis; Binom: Moscow, Russia, 2015; 752p. [Google Scholar]
- James, C.; Duncan, C. Handbook of Green Chemistry and Technology; Blackwell Sci. Ltd.: Oxford, London, UK, 2002; 560p. [Google Scholar]
- Serio, M.; Tesser, R.L.; Pengmei, S.E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Mazitova, A.K.; Vikhareva, I.N.; Maskova, A.R.; Gareeva, N.B.; Shaikhullin, I.R. Study of the effect of additives on biodegradation of PVC materials. Nanotechnologies Constr. 2020, 12, 94–99. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Maireles Torres, P. Transesterification of ethyl butyrate with methanol using MgO/CaO catalysts. J. Mol. Catal. A Chem. 2009, 300, 19–24. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Modern Catalysis and Chemical Kinetics; Intellect: Moscow, Russia, 2010; 504p. [Google Scholar]
- Vikhareva, I.N.; Builova, E.A.; Gatiyatullina, D.R.; Arslanov, V.R.; Gilem’yanov, D.A.; Mazitova, A.K. Synthesis and properties of adipic acid esters. Bashkir Chem. J. 2019, 26, 33–36. [Google Scholar]
- Vikhareva, I.N.; Silantieva, E.A.; Zaripov, I.I.; Builova, E.A.; Kamaletdinov, F.F. Synthesis of monochloroacetic esters. Bashkir Chem. J. 2018, 25, 67. [Google Scholar] [CrossRef]
- Bolotina, L.I.; Kucenko, A.I. Improvement of the production technology of complex ester plasticizers. Chem. Ind. 1974, 1, 10. [Google Scholar]
- Hadis, M.; Ümit, Ö. Zinc Oxide: Fundamentals, Materials and Device Technology; WILEY-VCH: Weinheim, Germany, 2009; 488p. [Google Scholar]
- Shargh, H.; Hosseini, M. Solvent-Free and One-Step Beckmann Rearrangement of Ketones and Aldehydes by Zinc Oxide. Synthesis 2002, 1057. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Moeini, F. One-Pot Multi-Component Route to Propargylamines Using Zinc Oxide Under Solvent-Free Conditions. Comb. Chem. High Throughput Screen. 2004, 17, 439–449. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43. [Google Scholar] [CrossRef]
- Kim, Y.J.; Varma, R.S. Microwave-assisted preparation of cyclic ureas from diamines in the presence of ZnO. Tetrahedron Lett. 2004, 45, 7205. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M. ZnO/CH3COCl: A new and highly efficient catalyst for dehydration of aldoximes into nitriles under solvent-free condition. Synthesis 2005, 05, 787–790. [Google Scholar] [CrossRef]
- Matsuo, J.-I.; Kitagawa, H.; Iida, D.; Mukaiyama, T. A Mild and Efficient Oxidation of Alcohols with N-tert-Butylphenylsulfinimidoyl Chloride in the Coexistence of Zinc Oxide. Chem. Lett. 2001, 30, 150–151. [Google Scholar] [CrossRef]
- Minsker, K.S.; Fedoseeva, G.T. Destruction and Stabilization of Polyvinyl Chloride; Chemistry: Moscow, Russia, 1979; 272p. [Google Scholar]
- Degradation and Stabilization of PVC; Owen, J., Ed.; Elsivier Publ.: New York, NY, USA, 1984; 320p. [Google Scholar]
- Guide to the Development of Compositions Based on PVC; Grossman, R.F., Ed.; Scientific Bases and Technologies: St. Petersburg, Russia, 2009; 608p. [Google Scholar]
- Thomas, N.L. Calcium/zinc stabilisers for PVC pressure pipe. Plast. Rubber Compos. Process. Appl. 1993, 19, 263–271. [Google Scholar]
- Frye, A.H.; Horst, R.W. The mechanism of poly(vinyl chloride) stabilization by barium, cadmium, and zinc carboxylates. I. Infrared studies. J. Polym. Sci. 1959, 40, 419–431. [Google Scholar] [CrossRef]
- Frye, A.H.; Horst, R.W. The mechanism of polyvinyl chloride stabilization by barium, cadmium, and zinc carboxylates. II. Radioactive tracer studies. J. Polym. Sci. 1960, 45, 1–12. [Google Scholar] [CrossRef]
- Interstate Standard 8728-88. Plasticizers. Specifications; IPK Publishing house’s standards of quality: Moscow, Russia, 2003; 11p.
- Interstate Standard 18329-2014. Liquid Resins and Plasticizers. Methods for Determination of Density; FSA STANDARTINFORM: Moscow, Russia, 2015; 8p.
- Interstate Standard 18995. 2-73 Liquid Chemical Products. Method for Determination of Refractive Index; IPK Publishing house’s standards of quality: Moscow, Russia, 1998; 2p.
- Interstate Standard 14041-91. Plastics. Determination of the Tendency of Compounds and Products Based on Vinyl Chloride Homopolymers and Copolymers to Evolve Hydrogen Chloride and Any Other Acidic Products at Elevated Temperatures. Congo Red Method; Standards publishing house: Moscow, Russia, 1992; 7p.
- Interstate Standard 14332-78. Suspension Polyvinylchloride. Specifications; IPK Publishing house’s standards of quality: Moscow, Russia, 1998; 17p.
- Interstate Standard 9998-86. Polyvinylchloride Films for Household Use. General Specifications; IPK Publishing house’s standards of quality: Moscow, Russia, 2002; 5p.
- Miller, S.A.; Bann, B.; Thrower, R.D. The reaction between phenol and ethylene oxide. J. Chem. Soc. 1950, 1, 3623–3628. [Google Scholar] [CrossRef]
- Staude, F. The lithium phenoxide catalized edition of propylene oxide to phenol. Polym. J. 1971, 2, 468–478. [Google Scholar] [CrossRef]
- Eidus, Y.T. On the synthesis of carboxylic acids under acid catalysis from carbon monoxide, olefins and acylating compounds. J. Org. Chem. 1968, 4, 1214–1219. [Google Scholar]
- Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Timofeev, A.A.; Builova, E.A.; Distanov, R.S. Investigation of the effect of the amount of additives on the properties of adipic acid esters. Nanotechnologies Constr. 2019, 11, 437–446. [Google Scholar] [CrossRef]

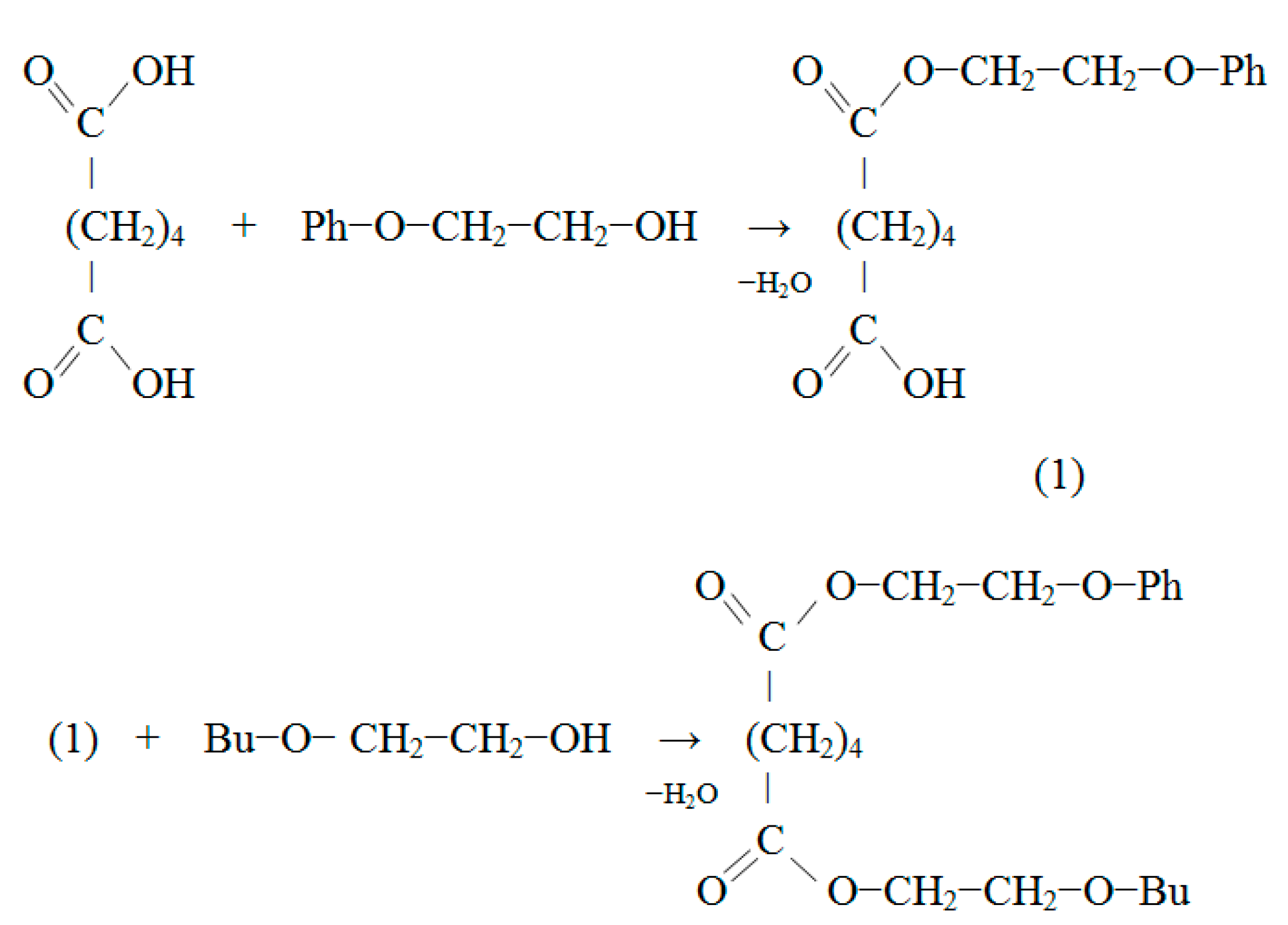
| Indicators | Butoxyethanol | Phenoxyethanol |
|---|---|---|
| Density, d204 | 0.9648 | 1.1007 |
| Refractive index, n 20d | 1.4267 | 1.5314 |
| Molecular weight | 118 | 138 |
| Ester | Indicators | |||
|---|---|---|---|---|
| Molecular Weight | Acid Number, mg KOH/g | Ester Number, mg KOH/g | d204 | |
| BEPEA | 407 | 0.1 | 274 | 1.0042 |
| Sample | Characteristics | ||||
|---|---|---|---|---|---|
| Temperature, °C | Δm, % | ||||
| Beginning | Maximal Value | End | at 180 °C | at 200 °C | |
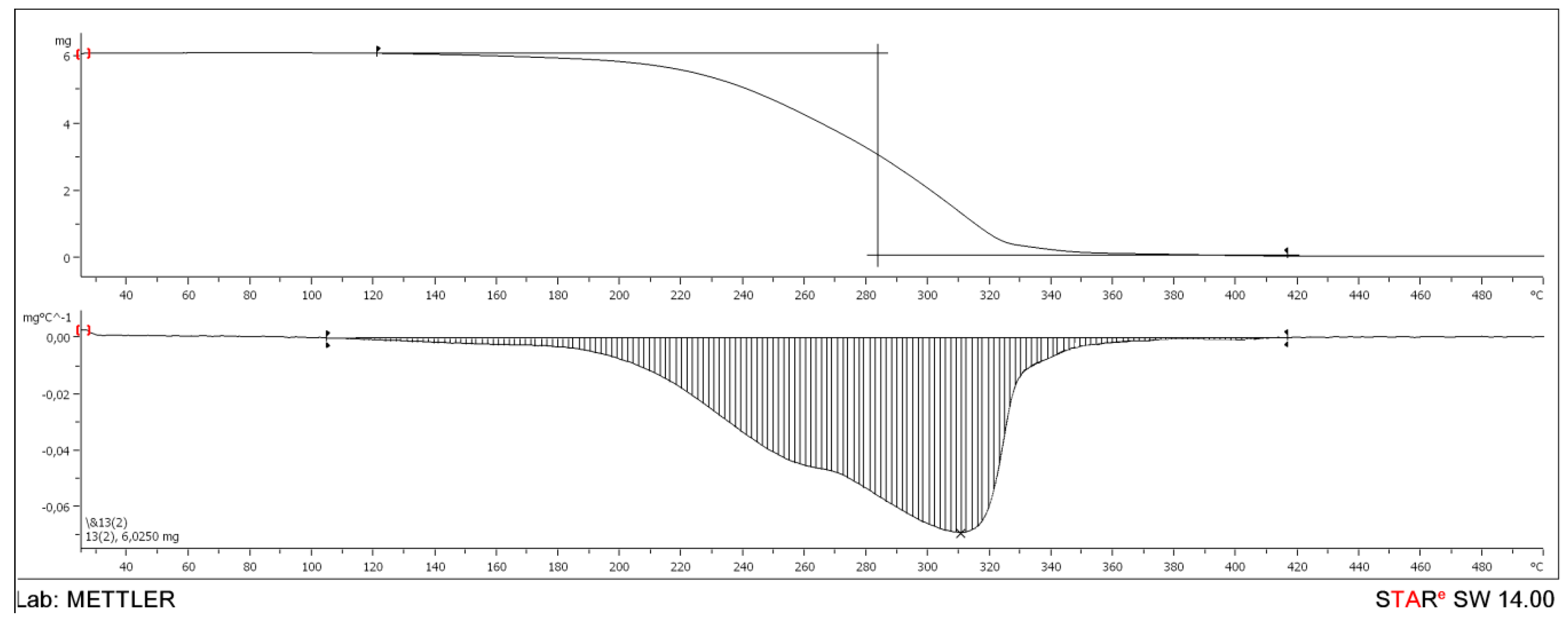
| BEPEA | 121.47 | 310.67 | 417.16 | 1.1 | 1.9 |
| DOP | 134.07 | 284.83 | 497.89 | 1.0 | 1.9 |
| Sample | Endotherm Characteristics | Exotherm Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | ΔHm, J/g | Temperature, °C | ΔHcr, J/g | |||||
| Beginning | Maximal Value | End | Beginning | Maximal Value | End | |||
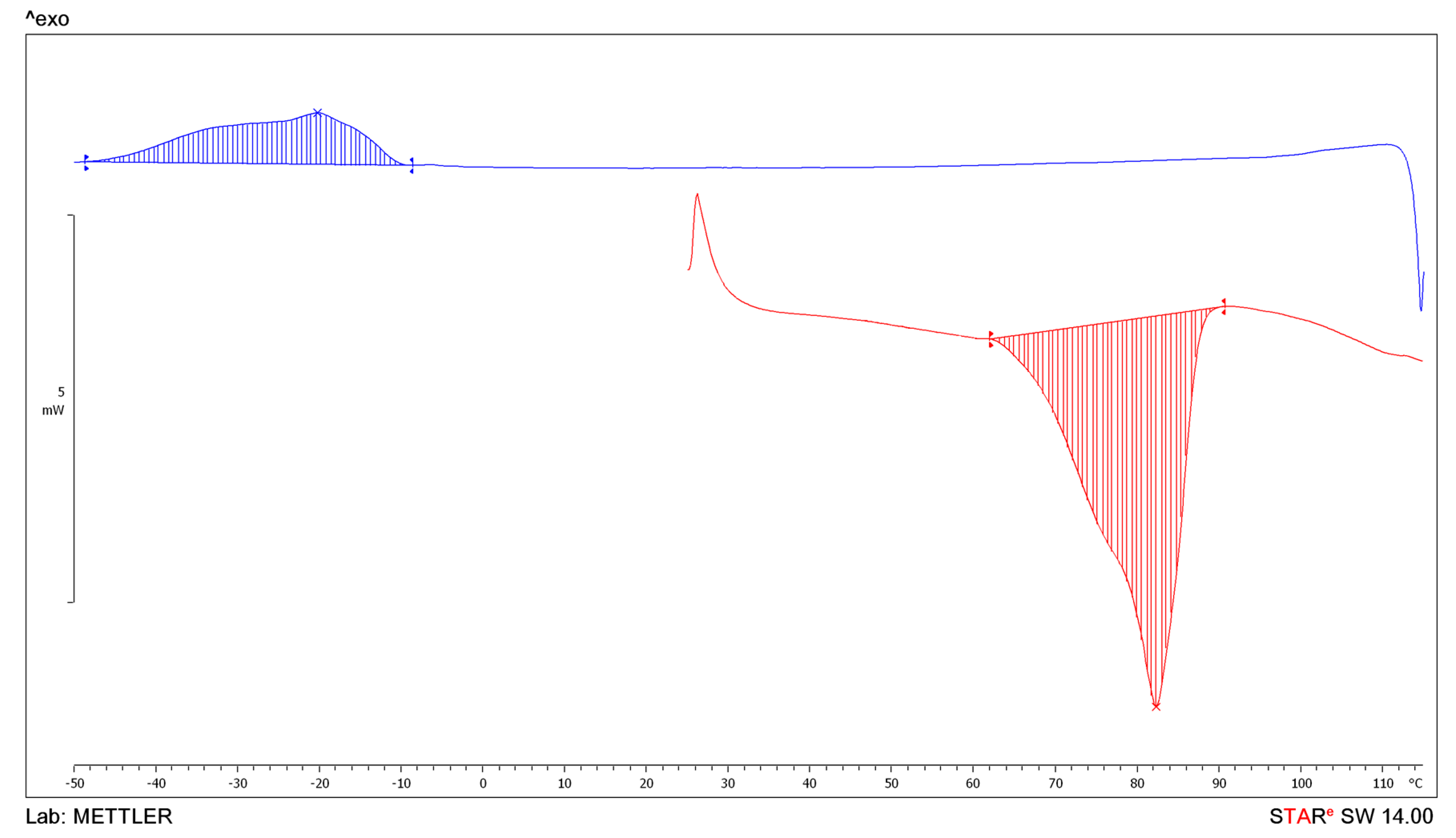
| BEPEA | 73.89 | 82.33 | 87.42 | −66.4 | −10.58 | −20.17 | −44.35 | 34.4 |
| Plasticizer | Tc, °C |
|---|---|
| DOP | 113 |
| BEPEA | 172 |
| Mixture DOP + BEPEA | 119 |
| Name of Indicator | PVC Composition | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Tensile stress, MPa | 16.1 | 14.0 | 14.2 |
| Elongation at break,% | 225 | 232 | 234 |
| Color stability at 180 °C, min | 25 | 30 | 45 |
| Thermostability at 160 °C, min | 80 | 85 | 97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazitova, A.K.; Vikhareva, I.N.; Aminova, G.K.; Savicheva, J.N. Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride. Polymers 2020, 12, 1728. https://doi.org/10.3390/polym12081728
Mazitova AK, Vikhareva IN, Aminova GK, Savicheva JN. Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride. Polymers. 2020; 12(8):1728. https://doi.org/10.3390/polym12081728
Chicago/Turabian StyleMazitova, Aliya K., Irina N. Vikhareva, Guliya K Aminova, and Juliya N. Savicheva. 2020. "Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride" Polymers 12, no. 8: 1728. https://doi.org/10.3390/polym12081728
APA StyleMazitova, A. K., Vikhareva, I. N., Aminova, G. K., & Savicheva, J. N. (2020). Application of Zinc Oxide to Obtain and Modify Properties of Adipate Plasticizer of Polyvinyl Chloride. Polymers, 12(8), 1728. https://doi.org/10.3390/polym12081728





